Agricultural and Management Practices and Bacterial Contamination in Greenhouse versus Open Field Lettuce Production
Abstract
:1. Introduction
2. Experimental Section
2.1. Selection of Lettuce Production Farms
| Farm 1 | Farm 2 | Farm 3 | Farm 4 | Farm 5 | Farm 6 | Farm 7 | Farm 8 | |
|---|---|---|---|---|---|---|---|---|
| Type | Greenhouse | Greenhouse | Greenhouse | Greenhouse | Open field | Open field | Open field | Open field |
| Size | 2.5 ha lettuce | 1.75 ha lettuce | 0.95 ha lettuce | 1.8 ha lettuce | 12 ha lettuce | 5 ha open field | 20 ha 1, 2.25 ha 2 lettuce | 120 ha 1, 6 ha 2 lettuce |
| Personnel (approximate) | 5 | 4 | 3 | 3 | 6 | 2 | 6 | 8 |
| Period of production | Whole year | Whole year | September–April | Whole year | April–September | April–October | April–September | May–September |
| Marketing | Auction | Auction | Auction | Fresh-cut processing Auction | Fresh-cut processing | Auction | Auction | Fresh-cut processing Auction |
2.2. Interview on Good Agricultural Practices and Checklist Concerning Water Management
2.3. Microbiological Surveys
| Greenhouse Farms | Open Field Farms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence | Med | Min | Max | n | Prevalence | Med | Min | Max | ||
| lettuce | TPAC | 144 | 100% | 6.3 | 5.0 | 8.5 | 120 | 100% | 6.0 | 5.0 | 7.2 |
| E. coli | 144 | 1.4% | 0.7 | 0.7 | 0.7 | 120 | 10% | 1.0 | 0.7 | 2.0 | |
| Pathogens | 48 | 8.4% | 40 | 10% | |||||||
| seedling | TPAC | 12 | 100 % | 6.2 | 5.1 | 6.9 | 11 | 100% | 5.6 | 4.6 | 6.3 |
| E. coli | 12 | 0 % | 0.7 | 0.7 | 0.7 | 11 | 9.1% | 1.4 | 1.4 | 1.4 | |
| seedling soil | TPAC | 28 | 100% | 9.0 | 7.0 | 9 | 29 | 100% | 8.0 | 6.1 | 9.3 |
| E. coli | 28 | 92.9% | 1.7 | 0.7 | 3.7 | 29 | 100% | 2.2 | 1.4 | 3.9 | |
| Pathogens | 12 | 0% | 11 | 0% | |||||||
| soil | TPAC | 144 | 100% | 7.2 | 6.3 | 8.3 | 132 | 100% | 7.1 | 6.0 | 8.9 |
| E. coli | 144 | 38.2% | 1.2 | 0.7 | 2.9 | 132 | 34.8% | 1.2 | 0.7 | 3.2 | |
| Pathogens | 48 | 4.2% | 44 | 9% | |||||||
| water source | TPAC | 35 | 100% | 5.0 | 2.7 | 7.2 | 33 | 100% | 5.9 | 4.8 | 7.1 |
| E. coli | 35 | 48.6% | 1.0 | 0 | 1.9 | 33 | 0% | 2 | 1.0 | 3.6 | |
| Coliforms | 35 | 31.4% | 1.0 | 0 | 3.5 | 33 | 0% | 2.3 | 1.0 | 4.1 | |
| Enterococcus | 35 | 45.8% | 1.3 | 0 | 2.5 | 33 | 0% | 1.9 | 0.6 | 3.6 | |
| Pathogens | 35 | 20% | 33 | 54% | |||||||
| water tap | TPAC | 36 | 100% | 5.3 | 2.3 | 7.8 | 5 | 100% | 6.7 | 5.8 | 7.7 |
| E. coli | 36 | 19.4% | 1.1 | 0 | 1.7 | 5 | 0% | 2 | 1.5 | 2.1 | |
| Coliforms | 36 | 27.7% | 0.7 | 0 | 2.1 | 5 | 0% | 2.1 | 1.5 | 2.7 | |
| Enterococcus | 36 | 33.3% | 0.9 | 0 | 2.3 | 5 | 0% | 2.0 | 1.7 | 2.7 | |
| Pathogens | 36 | 2.8% | 5 | 20% | |||||||
| wash water | TPAC | 4 | 100% | 5.5 | 4.3 | 6.4 | 7 | 100% | 6.3 | 5.7 | 7.7 |
| E. coli | 4 | 75% | 0 | 0 | 0.3 | 7 | 71.4% | 0.9 | 0.8 | 1.5 | |
| Coliforms | 4 | 75% | 0.1 | 0 | 0.3 | 7 | 71.4% | 1.2 | 0.9 | 1.45 | |
| Enterococcus | 4 | 50% | 0.2 | 0 | 0.5 | 7 | 71.4% | 0.6 | 0.3 | 1.0 | |
| Pathogens | 4 | 0% | 7 | 57% | |||||||
| n a | Pathogen | PCR Screening b | Confirmed by Culture | |||
|---|---|---|---|---|---|---|
| GREENHOUSE FARM | Lettuce | 48 | Campylobacter spp. | 8.4% | ||
| Soil | 48 | EHEC | 4.2% | vt1, eae | ||
| Soil | 48 | EHEC | 4.2% | vt2, eae | ||
| Water source | 35 | Salmonella spp. | 2.9% | |||
| Water source | 35 | EHEC | 2.9% | vt1, vt2, eae | ||
| Water source | 35 | Campylobacter spp. | 20% | |||
| Water tap | 36 | Campylobacter spp. | 2.8% | |||
| OPEN FIELD FARM | Lettuce | 40 | Campylobacter spp. | 10% | ||
| Soil | 44 | Salmonella spp. | 2.4% | |||
| Soil | 44 | EHEC | 6.8% | vt2, eae | O157 | |
| Soil | 44 | EHEC | 6.8% | vt1, vt2, eae | O103, O157 | |
| Soil | 44 | EHEC | 6.8% | vt1, eae | O26 | |
| Water source | 33 | EHEC | 15.2% | vt1, eae | ||
| Water source | 33 | EHEC | 15.2% | vt1, eae | O111 | |
| Water source | 33 | EHEC | 15.2% | vt1, eae | O26 | |
| Water source | 33 | EHEC | 15.2% | vt1, vt2, eae | ||
| Water source | 33 | EHEC | 15.2% | vt1, eae | ||
| Water tap | 5 | Campylobacter spp. | 20% | |||
| Wash water | 7 | Campylobacter spp. | 57.1% | |||
2.4. Data Processing and Statistical Methods
3. Results
3.1. Context, Organization & Management Practices of Lettuce Production Farms


3.2. Agricultural Practices (Control and Assurance Activities)

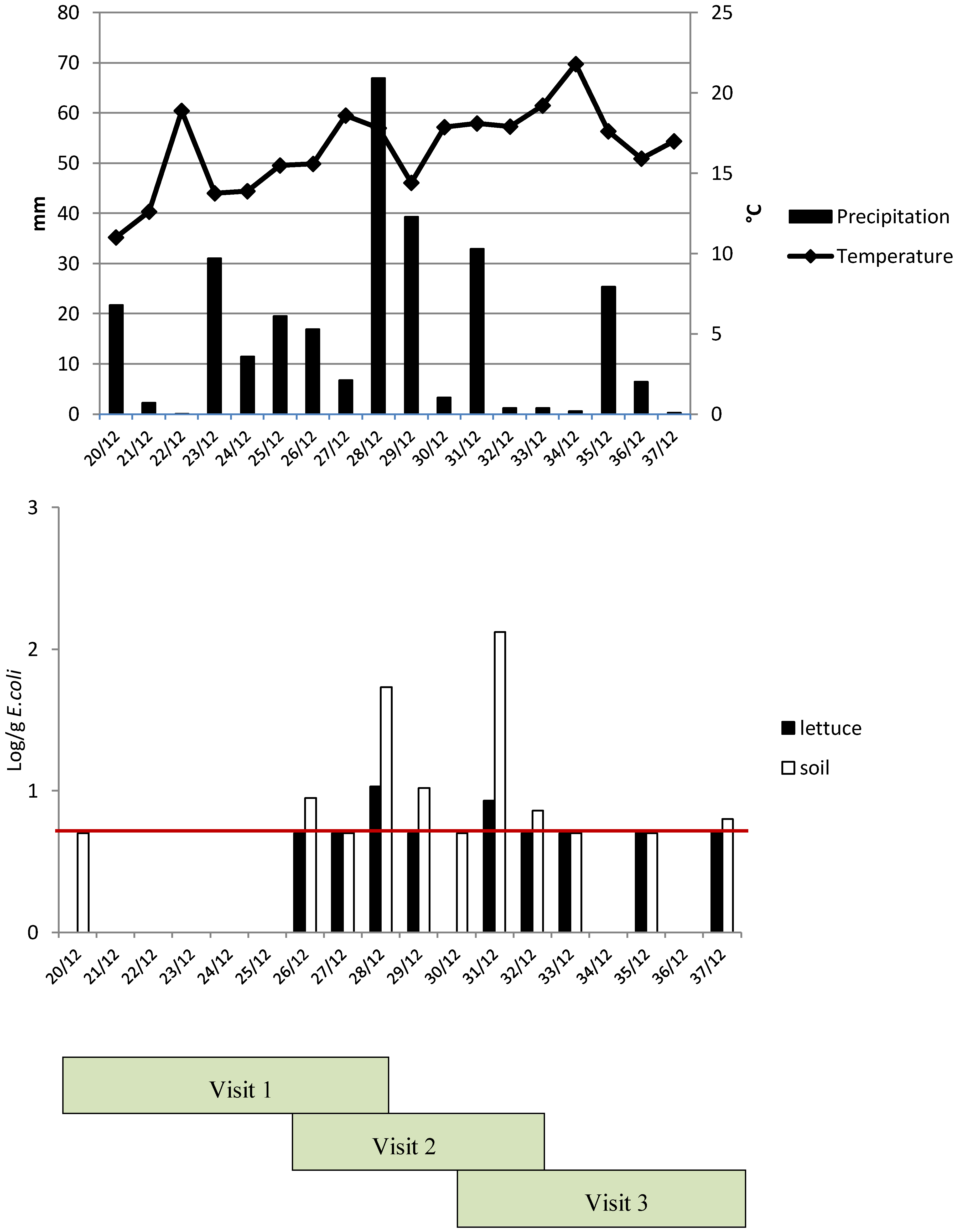
3.3. Microbiological Data on Lettuce Production: Greenhouse versus Open Field Farms

3.4. Impact of Agricultural Practices and Management Systems on Microbial Quality


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanning, I.B.; Johnson, M.G.; Ricke, S.C. Precut prepackaged lettuce: A risk for listeriosis? Foodborne Pathog. Dis. 2008, 5, 731–746. [Google Scholar]
- Hanning, I.B.; Nutt, J.D.; Ricke, S.C. Salmonellosis outbreaks in the United States due to fresh produce: Sources and potential intervention measures. Foodborne Pathog. Dis. 2009, 6, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Sivapalasingam, S.; Friedman, C.R.; Cohen, L.; Tauxe, R.V. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Protect. 2004, 67, 2342–2353. [Google Scholar]
- Strawn, L.K.; Schneider, K.R.; Danyluk, M.D. Microbial safety of tropical fruits. Crit. Rev.Food Sci. 2011, 51, 132–145. [Google Scholar] [CrossRef]
- Nygård, K.; Lassen, J.; Vold, L.; Aavitsland, P. E-Alert: Outbreak of Salmonella Thompson Infections Caused by Contaminated Ruccola (Rocket) Salad. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2594 (accessed on 25 November 2004).
- Nygård, K.; Lassen, J.; Vold, L.; Andersson, Y.; Fisher, I.; Lofdahl, S.; Threlfall, J.; Luzzi, I.; Peters, T.; Hampton, M.; et al. Outbreak of Salmonella Thompson infections linked to imported rucola lettuce. Foodborne Pathog. Dis. 2008, 5, 165–173. [Google Scholar] [PubMed]
- Soderstrom, A.; Lindberg, A.; Andersson, Y. EHEC O157 Outbreak in Sweden from Locally Produced Lettuce, August-September. 2005. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2794 (accessed on 22 September 2005).
- Takkinen, J.; Nakari, U.; Johansson, T.; Niskanen, T.; Siitonen, A.; Kuusi, M. A Nationwide Outbreak of Multiresistant Salmonella Typhimurium. var Copenhagen DT104B Infection in Finland due to Contaminated Lettuce from Spain. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2734 (accessed on 30 June 2005).
- Wendel, A.M.; Johnson, D.H.; Sharapov, U.; Grant, J.; Archer, J.R.; Monson, T.; Koschmann, C.; Davis, J.P. Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, August-September 2006: the Wisconsin investigation. Clin. Infect. Dis. 2009, 48, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA J. 2013, 11. [Google Scholar] [CrossRef]
- Erickson, M.C.; Webb, C.C.; Diaz-Perez, J.C. Surface and internalized Escherichia coli O157:H7 on field-grown spinach and lettuce treated with spray-contaminated irrigation water. J. Food Protect. 2010, 73, 1023–1029. [Google Scholar]
- CFERT. Investigation of an Escherichia coli O157:H7 Outbreak Associated with Dole Pre-Packaged Spinach. Available online: http://www.cdc.gov/nceh/ehs/Docs/Investigation_of_an_E_Coli_Outbreak_Associated_with_Dole_Pre-Packaged_Spinach.pdf (accessed on 6 October 2014).
- Jay, M.T.; Cooley, M.; Carychao, D. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerg. Infect. Dis. 2007, 13, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards of Fresh-cut Fruits and Vegetables; Food and Drug Administration: Silver Spring, MD, USA, 2008. [Google Scholar]
- Franz, E.; van Bruggen, A.H. Ecology of E. coli O157:H7 and Salmonella enterica in the primary vegetable production chain. Crit. Rev. Microbiol. 2008, 34, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; O’Brien, S.J.; Adak, G.K. A national outbreak of multi-resistant Salmonella enterica serovar Typhimurium definitive phage type (DT) 104 associated with consumption of lettuce. Epidemiol. Infect. 2003, 130, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Survival of Escherichia coli O157:H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol. 2005, 22, 63–70. [Google Scholar] [CrossRef]
- Suslow, T.V.; Oria, M.P.; Beuchat, L.R.; Garrett, E.H.; Parish, M.E.; Harris, L.J.; Farber, J.N.; Busta, F.F. Production practices as risk factors in microbial food safety of fresh and fresh-cut produce. Compr. Rev. Food Sci. F. 2003, 2, 38–77. [Google Scholar] [CrossRef]
- Gu, G.; Luo, Z.; Cevallos-Cevallos, J.M.; Adams, P.; Vellidis, G.; Wright, A.; van Bruggen, A.H.C. Factors affecting the occurrence and population density of Campylobacter jejuni in irrigation ponds on produce farms in the Suwannee River Watershed. Can. J. Microbiol. 2013, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, G.S.; Froseth, R.B.; Solemdal, L.; Jarp, J.; Wasteson, Y.; Rorvik, L.M. Influence of bovine manure as fertilizer on the bacteriological quality of organic Iceberg lettuce. J. Appl. Microbiol. 2004, 96, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.; Odumeru, J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Protect. 2004, 67, 2839–2849. [Google Scholar]
- Alam, M.J.; Zurek, L. Seasonal prevalence of Escherichia coli O157:H7 in beef cattle feces. J. Food Protect. 2006, 69, 3018–3020. [Google Scholar]
- Khaitsa, M.L.; Bauer, M.L.; Lardy, G.P.; Doetkott, D.K.; Kegode, R.B.; Gibbs, P.S. Fecal shedding of Escherichia coli O157:H7 in North Dakota feedlot cattle in the fall and spring. J. Food Protect. 2006, 69, 1154–1158. [Google Scholar]
- Oporto, B.; Esteban, J.I.; Aduriz, G.; Juste, R.A.; Hurtado, A. Escherichia coli O157:H7 and non-O157 Shiga toxin-producing E. coli in healthy cattle, sheep and swine herds in northern Spain. Zoonoses Public Health 2008, 55, 73–81. [Google Scholar] [PubMed]
- Franz, E.; Klerks, M.M.; De Vos, O.J.; Termorshuizen, A.J.; van Bruggen, A.H.C. Prevalence of Shiga toxin-producing Escherichia coli stx1, stx2, eaeA and rfbE genes and survival of E. coli O157:H7 in manure from organic and low-input conventional dairy farms. Appl. Environ. Microbiol. 2007, 73, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Lewis Ivey, M.L.; LeJeune, J.T.; Miller, S.A. Vegetable producers’ perceptions of food safety hazards in the Midwestern USA. Food Control 2012, 26, 453–465. [Google Scholar] [CrossRef]
- Tsiodras, S.; Kelesidis, T.; Kelesidis, I.; Bauchinger, U.; Falagas, M.E. Human infections associated with wild birds. J. Infect. 2008, 56, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Brackett, R.E. Incidence, contributing factors, and control of bacterial pathogens in produce. Postharvest Biol. Tec. 1999, 15, 305–311. [Google Scholar] [CrossRef]
- Guber, A.K.; Shelton, D.R.; Pachepsky, Y.A.; Sadeghi, A.M.; Sikora, L.J. Rainfall-induced release of fecal coliforms and other manure constituents: Comparison and modeling. Appl. Environ. Microbiol. 2006, 72, 7531–7539. [Google Scholar] [CrossRef]
- Holvoet, K.; Sampers, I.; Seynnaeve, M.; Uyttendaele, M. Relationships among hygiene indicators and enteric pathogens in irrigation water, soil and lettuce and the impact of climatic conditions on contamination in the lettuce primary production. Int. J. Food Microbiol. 2014, 171, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.K.; McIntyre, D.; Noble, R.T. Characterizing fecal contamination in stormwater runoff in coastal North Carolina, USA. Water Res. 2010, 44, 4186–4194. [Google Scholar] [CrossRef] [PubMed]
- Van Boxstael, S.; Habib, I.; Jacxsens, L.; De Vocht, M.; Baert, L.; Van De Perre, E.; Rajkovic, A.; Lopez-Galvez, F.; Sampers, I.; Spanoghe, P.; et al. Food safety issues in fresh produce: Bacterial pathogens, viruses and pesticide residues indicated as major concerns by stakeholders in the fresh produce chain. Food Control 2013, 32, 190–197. [Google Scholar]
- World Health Organization. Code of Hygienic Practices for Fresh Fruits and Vegetables; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2007. [Google Scholar]
- Commission Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: https://www.fsai.ie/uploadedFiles/Reg852_2004%286%29.pdf (accessed on 6 October 2014).
- Kher, S.V.; De Jonge, J.; Wentholt, M.T.A.; Deliza, R.; de Andrade, J.C.; Cnossen, H.J.; Luijckx, N.B.L.; Frewer, L.J. Consumer perceptions of risks of chemical and microbiological contaminants associated with food chains: A cross-national study. Int. J. Consumer Stud. 2013, 37, 73–83. [Google Scholar] [CrossRef]
- Definitieve Resultaten van de Landbouwtelling van Mei. 2010. Available online: http://statbel.fgov.be/nl/modules/pressrelease/statistieken/economie/recensement_agricole_de_mai_2010.jsp (accessed on 6 October 2014).
- Martins, C.; Tosstorff, G. Large farms in Europe: Less than 1 % of European farms occupy 20 % of the Utilised Agricultural Area. Available online: http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-SF-11-018/EN/KS-SF-11-018-EN.PDF (accessed on 6 October 2014).
- Kirezieva, K.; Jacxsens, L.; Uyttendaele, M.; Van Boekel, M.A.J.S.; Luning, P.A. Assessment of Food Safety Management Systems in the global fresh produce chain. Food Res. Int. 2013, 52, 230–242. [Google Scholar] [CrossRef]
- Kirezieva, K.; Nanyunja, J.; Jacxsens, L.; van der Vorst, J.G. A.J.; Uyttendaele, M.; Luning, P.A. Context factors affecting design and operation of Food Safety Management Systems in the fresh produce chain. Trends Food Sci. Tech. 2013, 32, 108–127. [Google Scholar] [CrossRef]
- IKKB. IKKB Standaard Voor de Primaire Plantaardige Productie. Available online: http://www.vegaplan.be/index.php?id=41&L=4 (accessed on 6 October 2014).
- Posse, B.; De Zutter, L.; Heyndrickx, M.; Herman, L. Novel differential and confirmation plating media for Shiga toxin-producing Escherichia coli serotypes O26, O103, O111, O145 and sorbitol-positive and -negative O157. Fems Microbiol. Lett. 2008, 282, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005: On Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin Andamending Council Directive 91/414/EEC. Available online: http://faolex.fao.org/docs/pdf/eur50711.pdf (accessed on 6 October 2014).
- Buchholz, U.; Bernard, H.; Werber, D.; Böhmer, M.M.; Remschmidt, C.; Wilking, H.; Deleré, Y.; an der Heiden, M.; Adlhoch, C.; Dreesman, J.; et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. New Engl. J. Med. 2011, 365, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, M.; Cauberghe, V.; Sas, B.; Uyttendaele, M. Analyzing consumers’ reactions to news coverage of the 2011 Escherichia coli O104:H4 outbreak, using the Extended Parallel Processing Model. J. Food Protect. 2013, 76, 473–481. [Google Scholar]
- Pielaat, A.; Wijnands, L.M.; Fitz-James, I.; van Leusden, F.M. Survey Analysis of Microbial Contamination of Fresh Produce and Ready-to-Eat Salads, and the Associated Risk to Consumers in the Netherlands; RIVM Report 330371002; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2008. [Google Scholar]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2005R2073:20071227:EN:PDF (accessed on 6 October 2014).
- EFSA. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2011; Scientific Report of EFSA; European Food Safety Authority (EFSA): Parma, Italy, 2011. [Google Scholar]
- Casteel, M.J.; Sobsey, M.D.; Mueller, J.P. Fecal contamination of agricultural soils before and after hurricane-associated flooding in North Carolina. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2006, 41, 173–184. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J. A Word or Two about Gardening: Biohazards in the Yard: Playing it Safe. Available online: http://miami-dade.ifas.ufl.edu/old/programs/urbanhort/urbanhortpublications.htm (accessed on 6 October 2014).
- Casati, S.; Gioria-Martinoni, A.; Gaia, V. Commercial potting soils as an alternative infection source of Legionella pneumophila and other Legionella species in Switzerland. Clin. Microbiol. Infect. 2009, 15, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Pravinkumar, S.J.; Edwards, G.; Lindsay, D. A cluster of Legionnaires’ disease caused by Legionella longbeachae linked to potting compost in Scotland, 2008–2009. Eurosurveillance 2010, 15, 4–6. [Google Scholar]
- Santamaria, J.; Toranzos, G.A. Enteric pathogens and soil: A short review. Int. Microbiol. 2003, 6, 5–9. [Google Scholar] [PubMed]
- Semenov, A.V.; van Bruggen, A.H.C.; van Overbeek, L.; Termorshuizen, A.J.; Semenov, A.M. Influence of temperature fluctuations on Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in cow manure. Fems Microbiol. Ecol. 2007, 60, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Warriner, K.; Huber, A.; Namvar, A.; Fan, W.; Dunfield, K. Chapter 4 recent advances in the microbial safety of fresh fruits and vegetables. Adv. Food Nutr. Res. 2009, 57, 155–208. [Google Scholar]
- Venglovsky, J.; Sasakova, N.; Placha, I. Pathogens and antibiotic residues in animal manures and hygienic and ecological risks related to subsequent land application. Bioresource. Technol. 2009, 100, 5386–5391. [Google Scholar] [CrossRef]
- Johannessen, G.S.; Bengtsson, G.B.; Heier, B.T.; Bredholt, S.; Wasteson, Y.; Rorvik, L.M. Potential uptake of Escherichia coli O157:H7 from organic manure into crisphead lettuce. Appl. Environ. Microbiol. 2005, 71, 2221–2225. [Google Scholar] [PubMed]
- Fong, T.T.; Mansfield, L.S.; Wilson, D.L.; Schwab, D.J.; Molloy, S.L.; Rose, J.B. Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, South Bass Island, Ohio. Environ. Health Perspect. 2007, 115, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Prudham, S. Poisoning the well: Neoliberalism and the contamination of municipal water in Walkerton, Ontario. Geoforum 2004, 35, 343–359. [Google Scholar] [CrossRef]
- Richardson, H.Y.; Nichols, G.; Lane, C.; Lake, I.R.; Hunter, P.R. Microbiological surveillance of private water supplies in England—The impact of environmental and climate factors on water quality. Water Res. 2009, 43, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- D’hooghe, J.; Wustenberghs, H.; Lauwers, L. Inschatting van het Watergebruik in de Landbouw op Basis van Nieuwe en Geactualiseerde Kengetallen per Landbouwactiviteit. Available online: http://www.milieurapport.be/Upload/Main/MiraData/MIRA-T/02_THEMAS/02_14/2007-04-WATERGEBRUIK%20LANDBOUW.PDF (accessed on 6 October 2014).
- Bichai, F.; Polo-Lopez, M.I.; Ibanez, P.F. Solar disinfection of wastewater to reduce contamination of lettuce crops by Escherichia coli in reclaimed water irrigation. Water Res. 2012, 46, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- Norton-Brandão, D.; Scherrenberg, S.M.; van Lier, J.B. Reclamation of used urban waters for irrigation purposes—A review of treatment technologies. J. Environ. Manage. 2013, 122, 85–98. [Google Scholar] [CrossRef]
- Cottyn, B.; Heylen, K.; Heyrman, J.; Vanhouteghem, K.; Pauwelyn, E.; Bleyaert, P.; van Vaerenbergh, J.; Höfte, M.; De Vos, P.; Maes, M. Pseudomonas cichorii as the causal agent of midrib rot, an emerging disease of greenhouse-grown butterhead lettuce in Flanders. Syst. Appl. Microbiol. 2009, 32, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Pauwelyn, E.; Vanhouteghem, K.; Cottyn, B.; De Vos, P.; Maes, M.; Bleyaert, P.; Hofte, M. Epidemiology of Pseudomonas cichorii, the cause of lettuce midrib rot. J. Phytopathol. 2011, 159, 298–305. [Google Scholar] [CrossRef]
- Cottyn, B.; Baeyen, S.; Pauwelyn, E.; Verbaendert, I.; De Vos, P.; Bleyaert, P.; Höfte, M.; Maes, M. Development of a real-time PCR assay for Pseudomonas cichorii, the causal agent of midrib rot in greenhouse-grown lettuce, and its detection in irrigating water. Plant Pathol. 2011, 60, 453–461. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hallam, N.B.; West, J.R.; Forster, C.F.; Simms, J. The potential for biofilm growth in water distribution systems. Water Res. 2001, 35, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Szewzyk, U.; Szewzyk, R.; Manz, W.; Schleifer, K.H. Microbiological safety of drinking water. Annu. Rev. Microbiol. 2000, 54, 81–127. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, C.; Alum, A.; Suarez-Rey, E.M.; Choi, C.Y.; Oron, G.; Gerba, C.P. Bacteriophages MS2 and PRD1 in turfgrass by subsurface drip irrigation. J. Environ. Eng. 2003, 129, 852–857. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Stagnitti, F.; Premier, R.; Boland, A.-M.; Hale, G. Quantitative microbial risk assessment models for consumption of raw vegetables irrigated with reclaimed water. Appl. Environ. Microbiol. 2006, 72, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- Oron, G.; Demalach, Y.; Hoffman, Z.; Manor, Y. Effect of effluent quality and application method on agricultural productivity and environmental control. Water Sci. Technol. 1992, 26, 1593–1601. [Google Scholar]
- Song, I.; Stine, S.W.; Choi, C.Y.; Gerba, C.P. Comparison of crop contamination by microorganisms during subsurface drip and furrow irrigation. J. Environ. Eng. 2006, 132, 1243–1248. [Google Scholar]
- Verbeten, E. Irrigation in Arid and Semi-Arid Environments. Available online: http://www.heindehaas.com/IMAROM/IMAROM%20working%20papers/IMAROM%20Working%20Paper%2001%20(Verbeten).pdf (accessed on 6 October 2014).
- Amoah, P.; Drechsel, P.; Abaidoo, R.C.; Ntow, W.J. Pesticide and pathogen contamination of vegetables in Ghana’s urban markets. Arch. Environ. Con. Tox. 2006, 50, 1–6. [Google Scholar]
- Lapidot, A.; Yaron, S. Transfer of Salmonella enterica serovar Typhimurium from contaminated irrigation water to parsley is dependent on curli and cellulose, the biofilm matrix components. J. Food Protect. 2009, 72, 618–623. [Google Scholar]
- Melloul, A.A.; Hassani, L.; Rafouk, L. Salmonella contamination of vegetables irrigated with untreated wastewater. World J. Microbiol. Biot. 2001, 17, 207–209. [Google Scholar] [CrossRef]
- Solomon, E.B.; Pang, H.J.; Matthews, K.R. Persistence of Escherichia coli O157:H7 on lettuce plants following spray irrigation with contaminated water. J. Food Protect. 2003, 66, 2198–2202. [Google Scholar]
- Greene, S.K.; Daly, E.R.; Talbot, E.A.; Demma, L.J.; Holzbauer, S.; Patel, N.J.; Hill, T.A.; Walderhaug, M.O.; Hoekstra, R.M.; Lynch, M.F.; et al. Recurrent multistate outbreak of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol. Infect. 2008, 136, 157–165. [Google Scholar] [CrossRef] [PubMed]
- James, J. Overview of Microbial Hazards in Fresh Fruit and Vegetables Operations, in Microbial Hazard Identification in Fresh Fruit and Vegetables; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Jawahar, P.; Ringler, C. Water quality and food safety: A review and discussion of risks. Water Policy 2009, 11, 680–695. [Google Scholar] [CrossRef]
- Allende, A.; Selma, M.V.; Lopez-Galvez, F.; Villaescusa, R.; Gil, M.I. Impact of wash water quality on sensory and microbial quality, including Escherichia coli cross-contamination, of fresh-cut escarole. J. Food Protect. 2008, 71, 2514–2518. [Google Scholar]
- Holvoet, K.; Jacxsens, L.; Sampers, I.; Uyttendaele, M. Insight into the prevalence and distribution of microbial contamination to evaluate water management in the fresh produce processing industry. J. Food Protect. 2012, 75, 671–681. [Google Scholar] [CrossRef]
- Luo, Y.G. Fresh-cut produce wash water reuse affects water quality and packaged product quality and microbial growth in Romaine lettuce. Hortscience 2007, 42, 1413–1419. [Google Scholar]
- Koninklijk Besluit Betreffende De Kwaliteit Van Voor Menselijke Consumptie Bestemd Water dat in de Voedingsmiddeleninrichtingen Verpakt Wordt of dat Voor De Fabricage En/of Het in Handel Brengen van Voedingsmiddelen Wordt Gebruikt. Available online: http://www.afsca.be/autocontrole-fr/informations/_documents/2007-06-15_AR_14-01-2002_MB_19-03-2002.pdf (accessed on 6 October 2014).
- Fonseca, J.M.; Fallon, S.D.; Sanchez, C.A.; Nolte, K.D. Escherichia coli survival in lettuce fields following its introduction through different irrigation systems. J. Appl. Microbiol. 2011, 110, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Moyne, A.L.; Sudarshana, M.R.; Blessington, T.; Koike, S.T.; Cahn, M.D.; Harris, L.J. Fate of Escherichia coli O157:H7 in field-inoculated lettuce. Food Microbiol. 2011, 28, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Ottoson, J.R.; Nyberg, K.; Lindqvist, R.; Albihn, A. Quantitative microbial risk assessment for Escherichia coli O157 on lettuce, based on survival data from controlled studies in a climate chamber. J. Food Protect. 2011, 74, 2000–2007. [Google Scholar] [CrossRef]
- Yaun, B.R.; Sumner, S.S.; Eifert, J.D.; Marcy, J.E. Response of Salmonella and Escherichia coli O157:H7 to UV energy. J. Food Protect. 2003, 66, 1071–1073. [Google Scholar]
- Penner, J.E.; Lister, D.; Griggs, D.J.; Dokken, D.J.; McFarland, M. Aviation and the Global Atmosphere: A Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 1999. [Google Scholar]
- Cevallos-Cevallos, J.M.; Danyluk, M.D.; Gu, G.Y.; Vallad, G.E.; van Bruggen, A.H.C. Dispersal of Salmonella Typhimurium by rain splash onto tomato plants. J. Food Protect. 2012, 75, 472–479. [Google Scholar] [CrossRef]
- Franz, E.; Semenov, A.V.; Termorshuizen, A.J.; de Vos, O.J.; Bokhorst, J.G.; van Bruggen, A.H.C. Manure-amended soil characteristics affecting the survival of E. coli O157:H7 in 36 Dutch soils. Environ. Microbiol. 2008, 10, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Girardin, H.; Morris, C.E.; Albagnac, C.; Dreux, N.; Glaux, C.; Nguyen-The, C. Behaviour of the pathogen surrogates Listeria innocua and Clostridium sporogenes during production of parsley in fields fertilized with contaminated amendments. Fems Microbiol. Ecol. 2005, 54, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ntahimpera, N.; Wilson, L.L.; Ellis, M.A.; Madden, L.V. Comparison of rain effects on splash dispersal of three Colletotrichum species infecting strawberry. Phytopathology 1999, 89, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Pietravalle, S.; van den Bosch, F.; Welham, S.J.; Parker, S.R.; Lovell, D.J. Modelling of rain splash trajectories and prediction of rain splash height. Agr. Forest Meteorol. 2001, 109, 171–185. [Google Scholar] [CrossRef]
Appendix: Water Management Questionnaire
Part 1: Water Sources
- ○
- Borehole water - closed wells
- ○
- Surface water
- ○
- Canal
- ○
- Creek
- ○
- River
- ○
- Collected open well
- ○
- River transfer
- ○
- Rain
- ○
- Waste water
- ○
- Municipal waste water
- ○
- Industrial waste water
- ○
- Others
- ○
- Drainage water
- ○
- …
- ○
- Yes
- ○
- Annual
- ○
- Semestrial
- ○
- Monthly
- ○
- Weekly
- ○
- No
- ○
- Potable water
- ○
- Borehole water
- ○
- Irrigation water
- ○
- treated
- ○
- untreated
- ○
- River
- ○
- Other: …
Part 2: Preventive Measurements
- ○
- Yes
- ○
- No
- ○
- Yes
- ○
- No
- ○
- Yes
- ○
- No
- ○
- Yes
- ○
- No
- ○
- Through lining of canals and well heads
- ○
- Redirection of contaminated water with diversion dikes, gradients, inlet/outlet control structures
- ○
- Other actions: …
Part 3: Irrigation Method/Water Treatment System
- ○
- None
- ○
- Water filtration
- ○
- Chemical sanitizers
- ○
- Chlorine
- ○
- H2O2
- ○
- Others: …
- ○
- Coagulation + flocculation
- ○
- UV
- ○
- Others: …
- ○
- Furrow/flood irrigation
- ○
- Sprinkler/spray irrigation
- ○
- Drip irrigation
- ○
- Manual irrigation
- ○
- Yes
- ○
- No
- ○
- Disinfected
- ○
- Maintained
- ○
- Cleaned
- ○
- Not maintained
- ○
- Same day as harvest
- ○
- 1 day before harvest
- ○
- 2–4 days before harvest
- ○
- 5–7 days before harvest
- ○
- More than 7 days before harvest
- ○
- Depending on the weather
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holvoet, K.; Sampers, I.; Seynnaeve, M.; Jacxsens, L.; Uyttendaele, M. Agricultural and Management Practices and Bacterial Contamination in Greenhouse versus Open Field Lettuce Production. Int. J. Environ. Res. Public Health 2015, 12, 32-63. https://doi.org/10.3390/ijerph120100032
Holvoet K, Sampers I, Seynnaeve M, Jacxsens L, Uyttendaele M. Agricultural and Management Practices and Bacterial Contamination in Greenhouse versus Open Field Lettuce Production. International Journal of Environmental Research and Public Health. 2015; 12(1):32-63. https://doi.org/10.3390/ijerph120100032
Chicago/Turabian StyleHolvoet, Kevin, Imca Sampers, Marleen Seynnaeve, Liesbeth Jacxsens, and Mieke Uyttendaele. 2015. "Agricultural and Management Practices and Bacterial Contamination in Greenhouse versus Open Field Lettuce Production" International Journal of Environmental Research and Public Health 12, no. 1: 32-63. https://doi.org/10.3390/ijerph120100032
APA StyleHolvoet, K., Sampers, I., Seynnaeve, M., Jacxsens, L., & Uyttendaele, M. (2015). Agricultural and Management Practices and Bacterial Contamination in Greenhouse versus Open Field Lettuce Production. International Journal of Environmental Research and Public Health, 12(1), 32-63. https://doi.org/10.3390/ijerph120100032





