Variation in the Effect of Particulate Matter on Pulmonary Function in Schoolchildren in Western Japan and Its Relation with Interleukin-8
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants

2.2. Monitoring of PEF
2.3. Measurement of Air Pollutant Levels
2.4. Preparation of the PM
2.5. IL-8 Promoter-Luciferase Gene Reporter Assay
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Children
| 2012 | 2013 | |
|---|---|---|
| Number | 399 | 384 |
| Boy/Girl | 205/194 | 194/190 |
| Height (cm) | 132.3 ± 5.9 | 137.7 ± 7.0 |
| Boy | 132.2 ± 5.5 | 136.9 ± 6.3 |
| Girl | 132.4 ± 6.4 | 138.5 ± 7.7 |
| Weight (kg) | 29.5 ± 5.8 | 32.4 ± 6.6 |
| Boy | 29.6 ± 6.2 | 32.3 ± 6.8 |
| Girl | 29.3 ± 5.4 | 32.6 ± 6.4 |
| Allergic disease (number) | ||
| Asthma | 38 | 45 |
| Allergic rhinitis | 78 | 74 |
| Allergic conjunctivitis | 8 | 15 |
| Atopic dermatitis | 44 | 36 |
| Food allergy | 19 | 20 |
3.2. PEF
| Year | Exposure Metric | IQR | All Children | ||
|---|---|---|---|---|---|
| Change in PEF Value (L/min) | 95%CI | p Value | |||
| 2012 | SPM | 14.0 μg/m3 | −2.16 | −2.88, −1.43 | <0.0001 |
| PM2.5 | 10.7 μg/m3 | −2.58 | −3.59, −1.57 | <0.0001 | |
| 2013 | SPM | 14.0 μg/m3 | −0.81 | −1.68, 0.06 | 0.068 |
| PM2.5 | 10.7 μg/m3 | −0.55 | −1.30, 0.19 | 0.146 | |
| Year | Exposure Metric | IQR | Children without Asthma | ||
| Change in PEF Value (L/min) | 95%CI | p Value | |||
| 2012 | SPM | 14.0 μg/m3 | −2.06 | −2.81, −1.30 | <0.0001 |
| PM2.5 | 10.7 μg/m3 | −2.46 | −3.51, −1.41 | <0.0001 | |
| 2013 | SPM | 14.0 μg/m3 | −0.44 | −1.37, 0.47 | 0.337 |
| PM2.5 | 10.7 μg/m3 | −0.29 | −1.07, 0.49 | 0.464 | |
| Year | Exposure Metric | IQR | Children with Asthma | ||
| Change in PEF Value (L/min) | 95%CI | p Value | |||
| 2012 | SPM | 14.0 μg/m3 | −3.11 | −5.70, −0.54 | 0.018 |
| PM2.5 | 10.7 μg/m3 | −3.69 | −7.28, −0.10 | 0.044 | |
| 2013 | SPM | 14.0 μg/m3 | −3.41 | −6.11, −0.70 | 0.014 |
| PM2.5 | 10.7 μg/m3 | −2.42 | −4.70, −0.13 | 0.039 | |


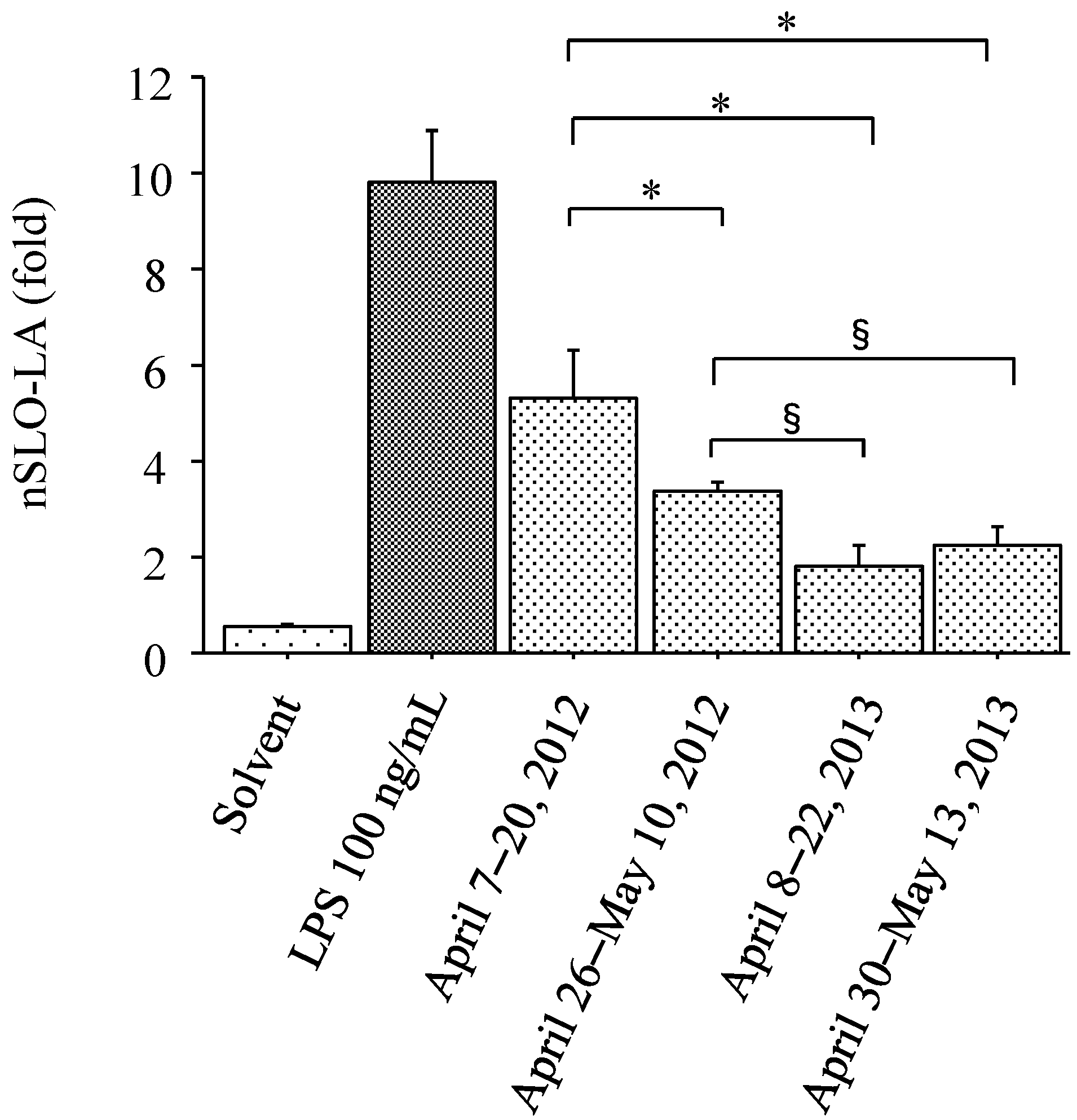
3.3. IL-8 Promoter Activity in THP-G8 Cells
3.4. Supplementary Results
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflict of Interest
Abbreviations
| ADS | Asian dust storm |
| ANOVA | analysis of variance |
| CI | confidence intervals |
| IL | interleukin |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| IQR | interquartile range |
| LPS | lipopolysaccharide |
| nSLO-LA | normalized stable luciferase orange luciferase activity |
| PEF | peak expiratory flow |
| PM | particulate matter |
| PM10 | particulate matter smaller than 10 μm |
| PM2.5 | particulate matter smaller than 2.5 μm |
| PM0.5 | particulate matter smaller than 0.5 μm |
| PTA | Parent Teacher Association |
| SD | standard deviation |
| SLO | stable luciferase orange |
| SLR | stable luciferase red |
| SPM | suspended particle matter |
References
- Beelen, R.; Hoek, G.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.H.; et al. Natural cause mortality and long-term exposure to particle components: An analysis of 19 European cohorts within the multi-center ESCAPE project. Environ. Health Perspect. 2015, 123, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; el Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Almazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Ward, D.J.; Ayres, J.G. Particulate air pollution and panel studies in children: A systematic review. Occup. Environ. Med. 2004. [Google Scholar] [CrossRef]
- Neas, L.M.; Dockery, D.W.; Koutrakis, P.; Tollerud, D.J.; Speizer, F.E. The association of ambient air pollution with twice daily peak expiratory flow rate measurements in children. Am. J. Epidemiol. 1995, 141, 111–122. [Google Scholar] [PubMed]
- Pope, C.A., III; Dockery, D.W. Acute health effects of PM10 pollution on symptomatic and asymptomatic children. Am. Rev. Respir. Dis. 1992, 145, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Romeo, E.; de Sario, M.; Weiland, S.K.; Forastiere, F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: A systematic review and meta-analysis. Environ. Health Perspect. 2010, 118, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Pan, X.C.; Kim, S.Y.; Park, K.; Park, E.J.; Jin, X.; Yi, S.M.; Kim, Y.H.; Park, C.H.; Song, S.; et al. Asian dust storm and pulmonary function of school children in seoul. Sci. Total Environ. 2010, 408, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Roemer, W.; Hoek, G.; Brunekreef, B.; Haluszka, J.; Kalandidi, A.; Pekkanen, J. Daily variations in air pollution and respiratory health in a multicentre study: The PEACE project. Pollution effects on asthmatic children in Europe. Eur. Respir. J. 1998, 12, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Bilos, C.; Colombo, J.C.; Skorupka, C.N.; Presa, M.J.R. Sources, distribution and variability of airborne trace metals in La Plata City area, Argentina. Environ. Pollut. 2001, 111, 149–158. [Google Scholar] [CrossRef]
- Sweet, C.W.; Vermette, S.J.; Landsberger, S. Sources of toxic trace elements in urban air in Illinois. Environ. Sci. Technol. 1993, 27, 2502–2510. [Google Scholar] [CrossRef]
- Sullivan, R.; Woods, I. Using emission factors to characterise heavy metal emissions from sewage sludge incinerators in Australia. Atmos. Environ. 2000, 34, 4571–4577. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Selzle, K.; Pöschl, U. Hazardous components and health effects of atmospheric aerosol particles: reactive oxygen species, soot, polycyclic aromatic compounds and allergenic proteins. Free Radic. Res. 2012, 46, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Hetland, R.B.; Cassee, F.R.; Låg, M.; Refsnes, M.; Dybing, E.; Schwarze, P.E. Cytokine release from alveolar macrophages exposed to ambient particulate matter: Heterogeneity in relation to size, city and season. Part. Fibre Toxicol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Vargas, M.P.; Guzman-Grenfell, A.M.; Blanco-Jimenez, S.; Sepulveda-Sanchez, J.D.; Bernabe-Cabanillas, R.M.; Cardenas-Gonzalez, B.; Ceballos, G.; Hicks, J.J. Airborne particulate matter PM2.5 from Mexico city affects the generation of reactive oxygen species by blood neutrophils from asthmatics: An in vitro approach. J. Occup. Med. Toxicol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Sandström, T.; Frew, A.J.; Stenfors, N.; Nördenhall, C.; Salvi, S.; Blomberg, A.; Helleday, R.; Söderberg, M. Health effects of acute exposure to air pollution. Part I: Healthy and asthmatic subjects exposed to diesel exhaust. Res. Rep. Health Eff. Inst. 2003, 112, 1–30. [Google Scholar] [PubMed]
- Watanabe, M.; Noma, H.; Kurai, J.; Sano, H.; Saito, R.; Abe, S.; Kimura, Y.; Aiba, S.; Oshimura, M.; Yamasaki, A.; et al. Decreased pulmonary function in school children in western Japan after exposures to Asian desert dusts and its association with interleukin-8. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Fujimoto, G.; Yoshimura, I.; Nitta, H.; Ono, M. Effects of particulate matter on daily mortality in 13 Japanese cities. J. Epidemiol. 2003, 13, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E., Jr. The size of suspended particulate matter in air. Science 1972, 178, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Yamagami, S.; Fujishima, H.; Noma, H.; Kamei, Y.; Goto, M.; Kondo, A.; Matsubara, M. Sensitization to Asian dust and allergic rhinoconjunctivitis. Environ. Res. 2014, 132, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kimura, Y.; Saito, R.; Nakajima, Y.; Ohmiya, Y.; Yamasaki, K.; Aiba, S. An in vitro test to screen skin sensitizers using a stable THP-1-drived IL-8 reporter cell line, THP-G8. Toxicol. Sci. 2011, 124, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Pope, C.A., III; Dockery, D.W.; Spengler, J.D.; Raizenne, M.E. Respiratory health and PM10 pollution: A daily time series analysis. Am. Rev. Respir. Dis. 1991, 144, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Roemer, W.; Hoek, G.; Brunekreef, B. Effect of ambient winter air pollution on respiratory health of children with chronic respiratory symptoms. Am. Rev. Respir. Dis. 1993, 147, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.E.; Chow, J.C.; Claiborn, C.; Fusheng, W.; Engelbrecht, J.; Watson, J.G. Monitoring of particulate matter outdoors. Chemosphere 2002, 49, 1009–1043. [Google Scholar] [CrossRef]
- Zaady, E.; Offer, Z.Y.; Shachak, M. The content and contributions of deposited aeolian organic matter in a dry land ecosystem of the Negev desert, Israel. Atmos. Environ. 2001, 35, 769–776. [Google Scholar] [CrossRef]
- Gong, H.J., Jr.; Linn, W.S.; Sioutas, C.; Terrell, S.L.; Clark, K.W.; Anderson, K.R.; Terrell, L.L. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient fine particles in Los Angeles. Inhal. Toxicol. 2003, 15, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, N.; Nordenhäll, C.; Salvi, S.S.; Mudway, I.; Soderberg, M.; Blomberg, A.; Helleday, R.; Levin, J.O.; Holgate, S.T.; Kelly, F.J.; et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur. Respir. J. 2004, 23, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Boylen, C.E.; Wong, R.S.; Smirk, M.N.; Gutiérrez, L.; Woodward, R.C.; Siah, W.S.; Devine, B.; Maley, F.; Cook, A. Variability and consistency in lung inflammatory responses to particles with a geogenic origin. Respirology 2014, 19, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Shadie, A.M.; Bucknall, M.P.; Rutlidge, H.; Garthwaite, L.; Herbert, C.; Halliburton, B.; Parsons, K.S.; Wark, P.A. Differential injurious effects of ambient and traffic-derived particulate matter on airway epithelial cells. Respirology 2015, 20, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.Y.; Chiba, M. A numerical study of the concentrations of dust source regions to the global dust budget. Global. Planet. Change 2006, 52, 88–104. [Google Scholar] [CrossRef]
- Onishi, K.; Kurosaki, Y.; Otani, S.; Yoshida, A.; Sugimoto, N.; Kurozawa, Y. Atmospheric transport route determines components of Asian dust and health effects in Japan. Atmos. Environ. 2012, 49, 94–102. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2015; National Institutes of Health: Bethesda, MD, USA, 2015. [Google Scholar]
- Wiesch, D.G.; Meyers, D.A.; Bleecker, E.R. Genetics of asthma. J. Allergy Clin. Immunol. 1999, 104, 895–901. [Google Scholar] [CrossRef]
- Gergen, P.J.; Mullally, D.I.; Evans, R. National survey of prevalence of asthma among children in the United States, 1976 to 1980. Pediatrics 1988, 81, 1–7. [Google Scholar] [PubMed]
- Schwarze, P.E.; Øvrevik, J.; Låg, M.; Refsnes, M.; Nafstad, P.; Hetland, R.B.; Dybing, E. Particulate matter properties and health effects: Consistency of epidemiological and toxicological studies. Hum. Exp. Toxicol. 2006, 25, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Iosifidis, T.; Perks, K.; Ditcham, W.G.; Devadason, S.G.; Siah, W.S.; Devine, B.; Maley, F.; Cook, A. The concentration of iron in real-world geogenic PM10 is associated with increased inflammation and deficits in lung function in mice. PLoS ONE 2014, 9, e90609. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Noma, H.; Kurai, J.; Sano, H.; Kitano, H.; Saito, R.; Kimura, Y.; Aiba, S.; Oshimura, M.; Shimizu, E. Variation in the Effect of Particulate Matter on Pulmonary Function in Schoolchildren in Western Japan and Its Relation with Interleukin-8. Int. J. Environ. Res. Public Health 2015, 12, 14229-14243. https://doi.org/10.3390/ijerph121114229
Watanabe M, Noma H, Kurai J, Sano H, Kitano H, Saito R, Kimura Y, Aiba S, Oshimura M, Shimizu E. Variation in the Effect of Particulate Matter on Pulmonary Function in Schoolchildren in Western Japan and Its Relation with Interleukin-8. International Journal of Environmental Research and Public Health. 2015; 12(11):14229-14243. https://doi.org/10.3390/ijerph121114229
Chicago/Turabian StyleWatanabe, Masanari, Hisashi Noma, Jun Kurai, Hiroyuki Sano, Hiroya Kitano, Rumiko Saito, Yutaka Kimura, Setsuya Aiba, Mitsuo Oshimura, and Eiji Shimizu. 2015. "Variation in the Effect of Particulate Matter on Pulmonary Function in Schoolchildren in Western Japan and Its Relation with Interleukin-8" International Journal of Environmental Research and Public Health 12, no. 11: 14229-14243. https://doi.org/10.3390/ijerph121114229
APA StyleWatanabe, M., Noma, H., Kurai, J., Sano, H., Kitano, H., Saito, R., Kimura, Y., Aiba, S., Oshimura, M., & Shimizu, E. (2015). Variation in the Effect of Particulate Matter on Pulmonary Function in Schoolchildren in Western Japan and Its Relation with Interleukin-8. International Journal of Environmental Research and Public Health, 12(11), 14229-14243. https://doi.org/10.3390/ijerph121114229





