Do Nanoparticle Physico-Chemical Properties and Developmental Exposure Window Influence Nano ZnO Embryotoxicity in Xenopus laevis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and NPs Used
2.2. NP Characterization
2.3. Characterization of NP Suspensions
2.4. FETAX Assay
2.5. Experimental Design
2.6. Superoxide Dismutase Enzymatic Activity
2.7. Light and Electron Microscopy Analyses
2.8. Data Collection and Statistical Analysis
3. Results
3.1. NP Physical and Chemical Characteristics
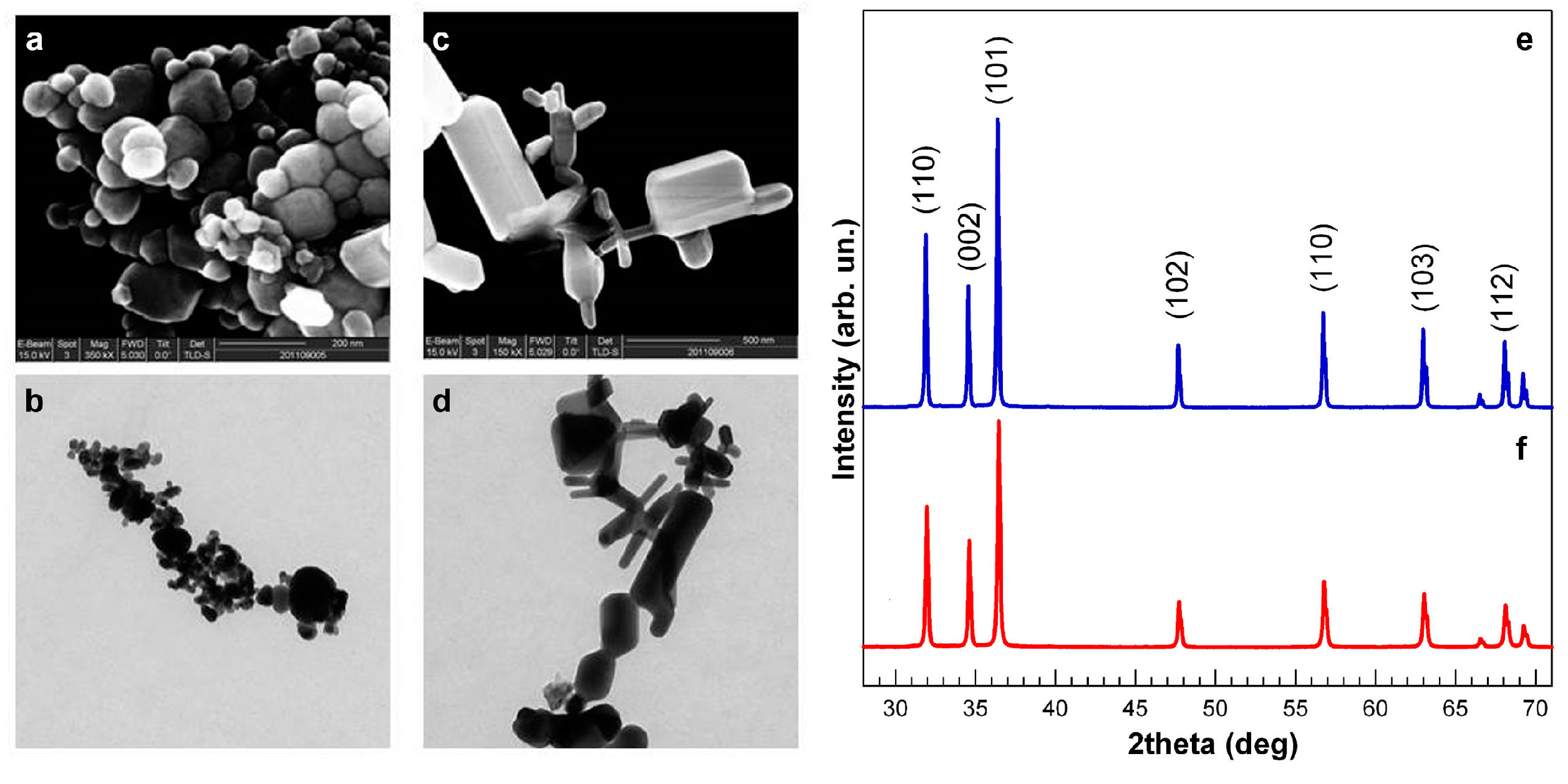
3.2. Comparative Embryotoxicity of Differently-Sized and -Shaped ZnONPs

3.3. Influence of Polymer Surface Coating on ZnONP Embryotoxicity
| sZno (mg/L) | bZnO (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 1 | 10 | 50 | 100 | 1 | 10 | 50 | 100 | |
| Living Larvae | 247 | 247 | 249 | 258 | 173 | 243 | 249 | 257 | 169 |
| Malformation | |||||||||
| Severe n (%) | 3 (1.2) | 5 (2.0) | 8 (3.2) | 4 (1.6) | 7 (4.0) | 1 (0.4) | 3 (1.2) | 9 (3.5) | 2 (1.2) |
| Gut n (%) | 4 (1.6) | 35 (14.2) b | 66 (26.5) b | 116 (45.0) b,c | 79 (45.7) b,c | 29 (11.9) b | 71 (28.5) b | 70 (27.2) b | 48 (28.4) b |
| Edema n(%) Cardiac | 0 | 0 | 2 (0.8) | 31 (12.0) b | 4 (2.3) a | 2 (0.8) | 32 (12.9) b | 12 (4.7) b | 0 |
| Abdominal | 8 (3.2) | 15 (6.1) | 34 (13.7) b | 90 (34.9) b | 39 (22.5) b | 19 (7.8) a | 70 (28.1) b | 57 (22.2) b | 47 (27.8) b |
| Dorsal Flexure n (%) | 0 | 0 | 2 (0.8) | 13 (5.0) b | 1 (0.6) | 0 | 0 | 8 (3.1) b | 16 (9.5) b |

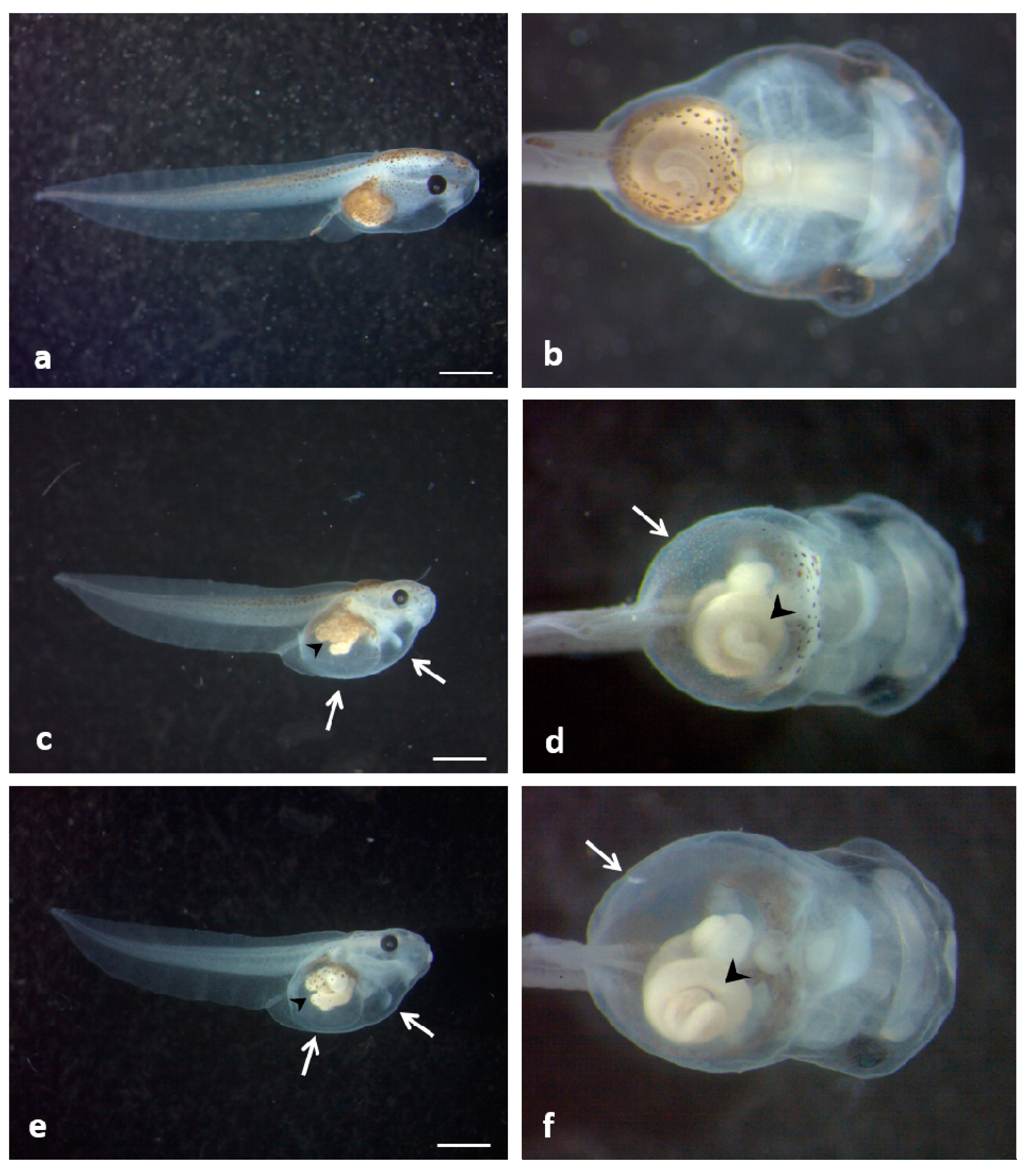
3.4. Ingestion-Dependent Toxicity of ZnONPs

3.5. Oxidative Stress Responses

3.5. Histological and Ultrastructural Effects of ZnONPs on Small Intestine
4. Discussion

4.1. Comparative Toxicity of Differently-Sized and -Shaped ZnONPs
4.2. Effects of Polymer Surface Coating
4.3. Route of Exposure, nZnOs Target Organs and the Sensitive Developmental Window
5. Conclusions
Supplementary
| Hydrodinamic Diameter (nm) | Zeta Potential (mV) | Average Crystalline Size (nm) | |
|---|---|---|---|
| sZnO | 819 | +3.7 | 70 |
| bZno | 579 | −12.8 | 98 |
| PVP-sZnO | 355 | +15.3 | - |
| PEG-sZnO | 237 | +19.6 | - |
| PVP-bZnO | 454 | −10.5 | - |
| PEG-bZnO | 685 | +3.8 | - |

Acknowledgements
Author Contributions
Conflicts of Interest
References
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, M.; Kim, D. In vitro toxicity of zinc oxide nanoparticles: A review. J. Nanopart. Res. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Gerberding, J.L. Toxicological Profile for Zinc; Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005; pp. 11–118. [Google Scholar]
- Skjolding, L.M.; Winther-Nielsen, M.; Baun, A. Trophic transfer of differently functionalized zinc oxide nanoparticles from crustaceans (Daphnia magna) to zebrafish (Danio rerio). Aquat. Toxicol. 2014, 157, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ates, M.; Arslan, Z.; Demir, V.; Daniels, J.; Farah, I.O. Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environ. Toxicol. 2015, 30, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rincker, M.J.; Hill, G.M.; Link, J.E.; Meyer, A.M.; Rowntree, J.E. Effects of dietary zinc and iron supplementation on mineral excretion, body composition, and mineral status of nursery pigs. J. Anim. Sci. 2005, 83, 2762–2774. [Google Scholar] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Santo, N.; Fascio, U.; Torres, F.; Guazzoni, N.; Tremolada, P.; Bettinetti, R.; Mantecca, P.; Bacchetta, R. Toxic effects and ultrastructural damages to Daphnia magna of two differently sized ZnO nanoparticles: Does size matter? Water Res. 2014, 53, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total Environ. 2014, 476–477, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanopart. Res. 2010, 12, 1645–1654. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Santo, N.; Fascio, U.; Moschini, E.; Freddi, S.; Chirico, G.; Camatini, M.; Mantecca, P. Nano-sized CuO, TiO2 and ZnO affect Xenopus laevis development. Nanotoxicology 2012, 6, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Nations, S.; Wages, M.; Cañas, J.E.; Maul, J.; Theodorakis, C.; Cobb, G.P. Acute effects of Fe2O3, TiO2, ZnO and CuO nanomaterials on Xenopus laevis. Chemosphere 2011, 83, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Nations, S.; Long, M.; Wages, M.; Canas, J.; Maul, J.D.; Theodorakis, C.; Cobb, G.P. Effects of ZnO nanomaterials on Xenopus laevis growth and development. Ecotoxicol. Environ. Saf. 2011, 74, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Moschini, E.; Santo, N.; Fascio, U.; Del Giacco, L.; Freddi, S.; Camatini, M.; Mantecca, P. Evidence and uptake routes for Zinc oxide nanoparticles through the gastrointestinal barrier in Xenopus laevis. Nanotoxicology 2014, 8, 728–744. [Google Scholar] [PubMed]
- American Society for Testing and Materials. Standard Guide for Conducting the Frog Embryo Teratogenesis Assay-Xenopus (FETAX); ASTM: Pennsylvania, PA, USA, 1998. [Google Scholar]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus Laevis (Daudin). North Holland Publishing Co.: Amsterdam, Netherlands, 1956. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Dawson, D.A.; Bantle, J.A. Development of a reconstituted water medium and preliminary validation of the frog embryo teratogenesis assay—Xenopus (FETAX). J. Appl. Toxicol. 1987, 7, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health Tox. Hazard. Subst. Environ. Eng. 2008, 43, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; Wang, M.; Ji, Z.; Zink, J.I.; Madler, L.; Castranova, V.; Nel, A.E. Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136–137, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Lin, C.-C.; Meng, P.-J. Zinc oxide nanoparticles alter hatching and larval locomotor activity in zebrafish (Danio rerio). J. Hazard. Mater. 2014, 277, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.B.; Ladner, D.A.; Higgins, C.P.; Westerhoff, P.; Ranville, J.F. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol. Chem. 2012, 31, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mantecca, P.; Moschini, E.; Bonfanti, P.; Fascio, U.; Perelshtein, I.; Lipovsky, A.; Chirico, G.; Bacchetta, R.; Del Giacco, L.; Colombo, A.; Gedanken, A. Toxicity evaluation of a new Zn-doped Cuo nanocomposite with highly effective antibacterial properties. Toxicol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.H.; Himmelreich, U.; Nuytten, N.; De Cuyper, M. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Li, Y.; Kudera, S.; Della Sala, F.; Zanella, M.; Parak, W.J.; Cingolani, R.; Manna, L.; Gigli, G. Blue light emitting diodes based on fluorescent CdSe/ZnS nanocrystals. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.; Turney, T.W. Reducing ZnO nanoparticle cytotoxicity by surface modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfanti, P.; Moschini, E.; Saibene, M.; Bacchetta, R.; Rettighieri, L.; Calabri, L.; Colombo, A.; Mantecca, P. Do Nanoparticle Physico-Chemical Properties and Developmental Exposure Window Influence Nano ZnO Embryotoxicity in Xenopus laevis? Int. J. Environ. Res. Public Health 2015, 12, 8828-8848. https://doi.org/10.3390/ijerph120808828
Bonfanti P, Moschini E, Saibene M, Bacchetta R, Rettighieri L, Calabri L, Colombo A, Mantecca P. Do Nanoparticle Physico-Chemical Properties and Developmental Exposure Window Influence Nano ZnO Embryotoxicity in Xenopus laevis? International Journal of Environmental Research and Public Health. 2015; 12(8):8828-8848. https://doi.org/10.3390/ijerph120808828
Chicago/Turabian StyleBonfanti, Patrizia, Elisa Moschini, Melissa Saibene, Renato Bacchetta, Leonardo Rettighieri, Lorenzo Calabri, Anita Colombo, and Paride Mantecca. 2015. "Do Nanoparticle Physico-Chemical Properties and Developmental Exposure Window Influence Nano ZnO Embryotoxicity in Xenopus laevis?" International Journal of Environmental Research and Public Health 12, no. 8: 8828-8848. https://doi.org/10.3390/ijerph120808828
APA StyleBonfanti, P., Moschini, E., Saibene, M., Bacchetta, R., Rettighieri, L., Calabri, L., Colombo, A., & Mantecca, P. (2015). Do Nanoparticle Physico-Chemical Properties and Developmental Exposure Window Influence Nano ZnO Embryotoxicity in Xenopus laevis? International Journal of Environmental Research and Public Health, 12(8), 8828-8848. https://doi.org/10.3390/ijerph120808828







