A Comparative Analyses of Granulometry, Mineral Composition and Major and Trace Element Concentrations in Soils Commonly Ingested by Humans
Abstract
:1. Introduction
2. Description of Soils in the Study Area

3. Experimental Section
3.1. Collection of Geophagic Soil Samples
3.2. Determination of Granulometric Properties of Geophagic Soils
3.3. Mineralogical Analyses of the Soil Samples
3.4. Determination of Major and Trace Element Oxides in Soil Samples
3.5. Statistical Analyses
4. Results
4.1. Description of Soil Samples
4.2. Granulometric Properties of Soil Samples
Particle Size Characteristics
| Country from Where Samples Were Collected | Sampling Site | Sample I.D. | Specific Surface Area/Volume (micron-1) | Sand * (%) | Clay * (%) | Silt * (%) | Derived Diameters (µm) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| D(v,0.1) | D(v,0.5) | D(v,0.9) | D[3,2] | |||||||
| South Africa | Eastern Cape | EN1 | 0.25 | 73 | 2 | 25 | 17,62 | 124,41 | 296,79 | 23,74 |
| EN4 | 0.32 | 70 | 2 | 28 | 11,21 | 99,41 | 449,58 | 18,87 | ||
| ECM6 | 0.54 | 51 | 4 | 44 | 4,89 | 52,53 | 201,12 | 10,64 | ||
| ECM7 | 0.57 | 59 | 4 | 36 | 5,42 | 86,11 | 330,27 | 11,40 | ||
| ECM8 | 1.74 | 5 | 14 | 80 | 1,41 | 10,74 | 43,96 | 3,53 | ||
| ECK9 | 0.07 | 88 | 0 | 12 | 43,32 | 132,94 | 369,51 | 70,74 | ||
| ECK10 | 1.10 | 24 | 10 | 66 | 2,12 | 20,47 | 104,53 | 5,21 | ||
| ECK12 | 1.13 | 21 | 10 | 69 | 1,96 | 14,75 | 79,70 | 4,89 | ||
| ECK13 | 0.33 | 69 | 3 | 28 | 10,70 | 91,41 | 252,14 | 16,69 | ||
| ECK14 | 1.82 | 12 | 17 | 70 | 1,35 | 8,44 | 67,40 | 3,52 | ||
| ECK15 | 1.16 | 18 | 10 | 72 | 2,00 | 20,31 | 70,92 | 5,17 | ||
| ECK16 | 0.57 | 54 | 4 | 41 | 5,08 | 60,61 | 290,66 | 11,05 | ||
| ECK17 | 0.39 | 57 | 3 | 40 | 9,91 | 64,59 | 308,75 | 15,78 | ||
| FSB18 | 1.39 | 11 | 13 | 76 | 1,68 | 12,98 | 54,76 | 4,43 | ||
| Bloemfontein | FSB19 | 1.10 | 12 | 9 | 79 | 2,10 | 17,69 | 54,55 | 5,40 | |
| FSB20 | 1.01 | 11 | 8 | 80 | 2,59 | 21,21 | 54,47 | 6,20 | ||
| FSB21 | 1.57 | 9 | 15 | 76 | 1,42 | 9,32 | 46,62 | 3,68 | ||
| FSB22 | 0.56 | 47 | 4 | 49 | 5,58 | 45,96 | 238,90 | 11,25 | ||
| FSB23 | 0.80 | 17 | 6 | 77 | 3,27 | 25,15 | 59,39 | 7,21 | ||
| FSB25 | 0.38 | 60 | 3 | 37 | 7,50 | 62,05 | 218,64 | 13,94 | ||
| FSB26 | 2.18 | 6 | 18 | 76 | 1,13 | 7,03 | 35,69 | 2,78 | ||
| Eastern Cape | ECB27 | 0.69 | 43 | 5 | 52 | 3,74 | 31,20 | 279,44 | 8,11 | |
| Swaziland | Mahlanya | SZ28 | 1.19 | 18 | 9 | 73 | 2,19 | 16,90 | 155,30 | 5,11 |
| Manzini | SZ29 | 0.73 | 32 | 5 | 62 | 4,19 | 31,39 | 284,39 | 7,88 | |
| SZ30 | 1.40 | 12 | 12 | 76 | 1,61 | 12,64 | 55,77 | 3,98 | ||
| Nsingweni | SZ31 | 0.50 | 38 | 3 | 58 | 5,46 | 37,27 | 371,22 | 11,17 | |
| Ezulwini | SZ32 | 1.02 | 12 | 7 | 81 | 2,72 | 16,11 | 54,90 | 5,81 | |
| Manzini | SZ33 | 0.32 | 62 | 2 | 36 | 8,27 | 71,74 | 687,35 | 16,86 | |
| Nsingweni | SZ34 | 0.42 | 50 | 2 | 47 | 6,55 | 49,18 | 405,35 | 13,58 | |
| Elangeni Mt | SZ35 | 0.98 | 22 | 8 | 70 | 3,12 | 23,13 | 313,14 | 6,49 | |
| Nsingweni | SZ36 | 1.4 | 15 | 10 | 75 | 1,89 | 16,12 | 67,90 | 4,28 | |
| SZ37 | 1.7 | 4 | 15 | 81 | 1,40 | 8,86 | 34,54 | 3,49 | ||
| Elangeni Mt | SZ38 | 0.5 | 31 | 3 | 66 | 7,11 | 31,75 | 129,90 | 11,62 | |
| Manzini | SZ39 | 1.06 | 12 | 8 | 80 | 2,52 | 16,59 | 53,86 | 5,32 | |
| DRC | Kinshasa | DRC-1 | 1 | 5 | 4 | 91 | 2,62 | 7,55 | 25,14 | 5,69 |
| DRC-2 | 1.15 | 4 | 5 | 91 | 2,48 | 6,77 | 26,74 | 5,30 | ||
| DRC-3 | 21.9 | 2 | 57 | 41 | 0,10 | 0,75 | 13,33 | 0,26 | ||
| DRC-4 | 1.1 | 1 | 4 | 95 | 2,54 | 6,97 | 18,86 | 5,31 | ||
| DRC-5 | 16.79 | 7 | 45 | 49 | 0,11 | 3,57 | 36,46 | 0,35 | ||
| DRC-6 | 0.61 | 35 | 3 | 62 | 3,39 | 28,93 | 172,37 | 10,13 | ||
| DRC-7 | 15.14 | 7 | 42 | 51 | 0,11 | 3,39 | 38,54 | 0,35 | ||
| DRC-8 | 3.67 | 5 | 25 | 70 | 0,17 | 5,36 | 34,78 | 0,64 | ||
| Lubumbashi | DRC-9 | 19.1 | 1 | 59 | 40 | 0,09 | 0,46 | 14,44 | 0,25 | |
| DRC-10 | 9.87 | 0 | 26 | 74 | 0,15 | 7,12 | 21,48 | 0,56 | ||
| DRC-11 | 0.95 | 0 | 2 | 98 | 2,88 | 8,18 | 25,10 | 6,16 | ||
| DRC-12 | 17.9 | 2 | 56 | 41 | 0,10 | 0,46 | 22,26 | 0,26 | ||
| DRC-13 | 0.7 | 2 | 3 | 95 | 3,36 | 14,50 | 35,48 | 8,30 | ||
| DRC-14 | 15.85 | 3 | 51 | 47 | 0,10 | 1,99 | 15,91 | 0,30 | ||
| DRC-15 | 5.1 | 1 | 39 | 60 | 0,12 | 3,11 | 17,54 | 0,37 | ||

4.3. Major and Trace Element Oxide Concentrations of Soil Samples
| Country | Sample | Oxide Concentrations (Wt%) | (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | Al2O3 | Fe2O3(t) | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cr2O3 | Total | CIA | CIW | ||
| South Africa | EN1 | 77.68 | 1.11 | 5.39 | 5.87 | 0.06 | 0.44 | 2.10 | 0.53 | 0.76 | 0.10 | 0.02 | 99.96 | 61.38 | 67.19 |
| EN4 | 74.14 | 1.01 | 11.13 | 4.77 | 0.05 | 0.61 | 0.17 | 0.37 | 1.31 | 0.04 | 0.01 | 100.15 | 85.76 | 95.38 | |
| ECM6 | 77.17 | 0.51 | 10.03 | 3.34 | 0.04 | 0.46 | 1.13 | 1.58 | 1.15 | 0.08 | 0.01 | 100.43 | 72.27 | 78.77 | |
| ECM7 | 90.34 | 0.51 | 4.77 | 1.84 | 0.05 | 0.20 | 0.34 | 0.40 | 0.87 | 0.04 | 0.01 | 101.98 | 74.76 | 86.54 | |
| ECM8 | 42.76 | 1.41 | 26.48 | 17.20 | 0.05 | 0.48 | 0.03 | 0.02 | 0.39 | 0.04 | 0.04 | 100.15 | 98.34 | 99.81 | |
| ECM9 | 71.71 | 1.78 | 8.96 | 6.86 | 0.14 | 1.41 | 2.53 | 1.05 | 0.92 | 0.10 | 0.03 | 100.43 | 66.59 | 71.47 | |
| ECK10 | 63.69 | 0.72 | 18.55 | 7.08 | 0.07 | 1.29 | 0.03 | 0.10 | 2.74 | 0.06 | 0.01 | 100.32 | 86.58 | 99.29 | |
| ECK12 | 66.81 | 0.80 | 14.09 | 9.55 | 0.05 | 0.81 | 0.21 | 0.38 | 1.68 | 0.08 | 0.02 | 100.32 | 86.11 | 95.98 | |
| ECK13 | 86.09 | 0.38 | 4.64 | 2.42 | 0.05 | 0.17 | 0.24 | 0.79 | 0.96 | 0.07 | 0.01 | 100.34 | 70.01 | 81.85 | |
| ECK14 | 72.23 | 0.93 | 16.71 | 2.65 | 0.01 | 0.38 | <0.01 | <0.01 | 2.29 | 0.04 | 0.01 | 100.22 | 87.85 | 99.85 | |
| ECK15 | 71.41 | 1.05 | 10.00 | 7.29 | 0.06 | 0.63 | 0.28 | 0.28 | 1.35 | 0.41 | 0.02 | 99.81 | 83.99 | 94.71 | |
| ECK16 | 75.14 | 0.85 | 8.72 | 3.92 | 0.08 | 0.58 | 0.85 | 0.92 | 1.27 | 0.12 | 0.02 | 100.62 | 74.13 | 83.12 | |
| ECK17 | 83.38 | 1.39 | 5.72 | 4.11 | 0.05 | 0.29 | 0.11 | 0.23 | 0.69 | 0.03 | 0.01 | 100.64 | 84.66 | 94.37 | |
| FSB18 | 73.87 | 0.97 | 15.86 | 1.80 | 0.00 | 0.48 | 0.02 | 0.22 | 2.33 | 0.03 | 0.01 | 100.17 | 86.05 | 98.51 | |
| FSB19 | 66.39 | 0.84 | 18.60 | 4.73 | 0.01 | 0.63 | <0.01 | <0.10 | 3.20 | 0.06 | 0.01 | 100.22 | 84.88 | 99.40 | |
| FSB20 | 68.55 | 0.76 | 17.44 | 4.02 | 0.03 | 1.08 | <0.01 | 0.04 | 2.56 | 0.09 | 0.01 | 100.14 | 87.00 | 99.73 | |
| FSB21 | 65.99 | 0.72 | 17.10 | 6.73 | 0.01 | 0.86 | 0.15 | 0.08 | 3.19 | 0.07 | 0.01 | 100.12 | 83.33 | 98.65 | |
| FSB22 | 66.81 | 0.85 | 17.47 | 4.30 | 0.02 | 0.98 | 0.28 | 0.76 | 3.50 | 0.07 | 0.01 | 99.60 | 79.36 | 94.37 | |
| FSB23 | 69.11 | 0.75 | 17.27 | 3.91 | 0.03 | 1.06 | 0.01 | 0.05 | 2.51 | 0.09 | 0.01 | 100.24 | 87.04 | 99.63 | |
| FSB24 | 69.85 | 0.87 | 17.28 | 3.11 | 0.01 | 0.85 | 0.33 | 0.07 | 1.56 | 0.03 | 0.01 | 100.64 | 89.76 | 97.70 | |
| FSB25 | 73.62 | 0.78 | 14.61 | 3.07 | 0.02 | 0.85 | 0.05 | 0.79 | 2.79 | 0.12 | 0.01 | 100.22 | 80.10 | 94.54 | |
| FSB26 | 43.46 | 1.74 | 25.57 | 17.63 | 0.07 | 0.42 | <0.01 | 0.01 | 0.15 | 0.05 | 0.03 | 100.16 | 99.34 | 99.94 | |
| FSB27 | 66.60 | 0.87 | 15.05 | 5.57 | 0.06 | 1.51 | 0.89 | 2.30 | 1.64 | 0.03 | 0.02 | 99.60 | 75.73 | 82.52 | |
| Swaziland | SZ28 | 57.24 | 0.97 | 24.27 | 8.47 | 0.04 | 0.18 | <0.01 | <0.01 | 0.16 | 0.02 | 0.01 | 100.40 | 99.28 | 99.92 |
| SZ29 | 49.31 | 0.72 | 18.04 | 19.58 | 0.05 | 0.48 | 1.20 | 2.10 | 1.00 | 0.16 | 0.01 | 99.80 | 80.74 | 84.52 | |
| SZ30 | 46.16 | 0.60 | 26.55 | 14.38 | 0.13 | 0.83 | <0.01 | 0.03 | 0.35 | 0.03 | 0.03 | 100.08 | 98.56 | 99.85 | |
| SZ31 | 59.33 | 0.51 | 25.02 | 2.90 | 0.03 | 0.53 | <0.01 | 4.46 | 2.03 | 0.03 | 0.00 | 100.16 | 79.39 | 84.85 | |
| SZ32 | 68.34 | 0.50 | 16.62 | 1.58 | 0.02 | 0.27 | <0.01 | 0.02 | 1.34 | 0.02 | 0.00 | 100.11 | 92.38 | 99.81 | |
| SZ33 | 43.10 | 1.57 | 28.09 | 20.82 | 0.08 | 0.24 | <0.01 | <0.01 | 0.11 | 0.03 | 0.03 | 100.02 | 99.53 | 99.93 | |
| SZ34 | 55.21 | 0.09 | 29.15 | 1.65 | 0.01 | 0.49 | <0.00 | 4.71 | 3.37 | 0.01 | 0.00 | 100.07 | 78.29 | 86.09 | |
| SZ35 | 70.38 | 0.15 | 18.61 | 2.06 | 0.03 | 0.11 | <0.01 | 0.02 | 3.83 | 0.02 | 0.01 | 100.41 | 82.81 | 99.83 | |
| SZ36 | 48.23 | 0.03 | 27.78 | 12.18 | 0.00 | 0.03 | <0.01 | <0.01 | 0.05 | 0.05 | 0.04 | 100.11 | 99.74 | 99.93 | |
| SZ37 | 45.74 | 0.03 | 38.57 | 0.84 | 0.00 | 0.25 | <0.01 | <0.01 | 0.74 | 0.05 | 0.01 | 99.81 | 98.08 | 99.95 | |
| SZ38 | 72.07 | <0.01 | 18.56 | 1.35 | 0.09 | 0.01 | <0.01 | 0.09 | 2.88 | 0.02 | 0.01 | 100.33 | 86.13 | 99.44 | |
| SZ39 | 46.27 | 1.58 | 24.92 | 15.13 | 0.07 | 0.26 | <0.01 | <0.01 | 0.12 | 0.05 | 0.01 | 99.99 | 99.43 | 99.92 | |
| DRC | DRC1 | 54.74 | 0.81 | 28.09 | 1.35 | 0.01 | 0.76 | 0.02 | 1.13 | 1.02 | 0.07 | 0.01 | 100.43 | 92.82 | 96.05 |
| DRC2 | 53.94 | 0.80 | 29.32 | 1.64 | 0.01 | 0.89 | 0.04 | <0.01 | 1.19 | 0.08 | 0.01 | 100.26 | 95.92 | 99.82 | |
| DRC3 | 46.52 | 1.25 | 32.55 | 6.48 | 0.02 | 0.16 | <0.01 | 0.01 | 0.21 | 0.07 | 0.01 | 100.08 | 99.29 | 99.94 | |
| DRC4 | 51.48 | 0.89 | 30.21 | 1.09 | 0.01 | 0.71 | 0.03 | 1.02 | 0.79 | 0.06 | 0.01 | 99.57 | 94.28 | 96.65 | |
| DRC5 | 53.76 | 1.29 | 23.04 | 13.12 | 0.02 | 0.02 | <0.01 | <0.01 | 0.92 | 0.21 | 0.02 | 100.04 | 96.08 | 99.91 | |
| DRC6 | 72.29 | 1.08 | 18.98 | 0.48 | 0.00 | 0.08 | <0.01 | <0.01 | 0.04 | 0.05 | 0.01 | 100.42 | 99.66 | 99.89 | |
| DRC7 | 54.11 | 1.30 | 23.42 | 12.45 | 0.02 | 0.05 | <0.01 | <0.01 | 0.95 | 0.21 | 0.02 | 100.20 | 96.03 | 99.91 | |
| DRC8 | 61.02 | 1.18 | 26.70 | 0.72 | 0.00 | 0.74 | <0.01 | <0.01 | 1.74 | 0.02 | 0.02 | 100.41 | 93.81 | 99.93 | |
| DRC9 | 43.95 | 1.41 | 32.94 | 7.70 | 0.02 | 0.11 | <0.01 | <0.01 | 0.14 | 0.06 | 0.02 | 100.25 | 99.53 | 99.94 | |
| DR10 | 60.91 | 1.43 | 22.13 | 4.50 | 0.00 | 1.00 | <0.01 | 0.36 | 2.43 | 0.03 | 0.02 | 100.45 | 88.75 | 98.35 | |
| DR11 | 60.47 | 0.07 | 0.65 | 0.40 | 0.02 | 32.64 | 0.02 | <0.01 | 0.13 | 0.02 | <0.001 | 98.99 | 80.63 | 95.75 | |
| DR12 | 44.07 | 1.69 | 35.60 | 4.32 | 0.01 | 0.15 | <0.01 | <0.01 | 0.05 | 0.04 | 0.02 | 100.56 | 99.81 | 99.94 | |
| DR13 | 53.92 | 2.57 | 27.25 | 3.76 | 0.01 | 0.61 | 0.04 | <0.01 | 1.01 | 0.06 | 0.01 | 99.99 | 96.25 | 99.82 | |
| DR14 | 46.46 | 1.31 | 32.97 | 6.41 | 0.02 | 0.18 | <0.01 | <0.01 | 0.23 | 0.08 | 0.01 | 100.56 | 99.26 | 99.94 | |
| DR15 | 47.42 | 1.33 | 36.20 | 1.53 | 0.01 | 0.28 | <0.01 | <0.01 | 0.21 | 0.06 | 0.01 | 101.45 | 99.36 | 99.94 | |
| Certified | 45.42 | 1.54 | 16.62 | 9.73 | 0.18 | 8.15 | 10.93 | 3.65 | 0.70 | 0.26 | 0.07 | 99.75 | 52.10 | 53.27 | |
| Results | 44.34 | 1.55 | 16.44 | 9.76 | 0.18 | 8.29 | 11.10 | 3.31 | 0.68 | 0.22 | 0.07 | 98.79 | 52.13 | 53.28 | |
4.4. Mineral Composition of the Soil Samples
| Sample Code | Mineral Abundance (Wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Magnetite | Anatase | Hematite/Goethite | Microcline/Rutile | Plagioclase | Quartz | Kaolinite/Chlorite | Kaolinite | Muscovite | Smectite | Il/Sm Interstratification | |
| EN1 | - | - | Trace | - | 4 | 89 | 1 | - | 2 | - | 2 |
| EN2 | - | - | 2 | - | - | 87 | 2 | - | 2 | - | 7 |
| EN3 | - | - | - | - | 14 | 76 | 1 | - | 4 | - | 5 |
| EN4 | - | - | - | - | 2 | 89 | 2 | - | 3 | 1 | 3 |
| EN5 | - | - | - | - | 10 | 72 | 1 | - | 8 | - | 8 |
| ECM6 | - | - | - | - | 7 | 84 | 1 | - | 2 | 1 | 4 |
| ECM7 | - | - | - | 1 | 2 | 93 | - | - | 1 | - | 3 |
| ECM8 | - | - | 3 | - | - | 33 | - | 47 | - | - | 17 |
| ECK9 | - | - | - | - | 18 | 73 | - | - | 1 | - | 4 |
| ECK10 | 2 | 1 | - | - | - | 70 | 5 | - | 15 | 2 | 5 |
| ECK11 | 1 | 3 | - | - | 3 | 61 | 1 | - | 16 | 5 | 9 |
| ECK12 | 1 | - | 1 | - | 3 | 80 | 2 | - | 5 | 2 | 5 |
| ECK13 | - | - | - | 2 | 5 | 93 | - | - | 1 | - | Trace |
| ECK14 | 1 | 1 | - | 2 | - | 73 | - | 7 | 3 | - | 13 |
| ECK15 | 1 | - | - | - | 1 | 88 | 1 | - | 3 | - | 5 |
| ECK16 | 1 | - | - | - | 5 | 88 | - | - | 2 | - | 4 |
| ECK17 | Trace | - | - | Trace | 2 | 93 | - | - | 1 | - | 2 |
| FSB18 | - | 1 | - | 1 | 2 | 75 | 7 | - | 3 | - | 10 |
| FSB19 | - | 1 | - | 1 | 1 | 68 | - | 7 | 5 | - | 17 |
| FSB20 | - | 1 | - | 1 | - | 69 | 6 | - | 6 | 4 | 13 |
| FSB21 | - | 1 | 1 | 1 | - | 71 | 3 | - | 6 | - | 17 |
| FSB22 | - | 2 | - | 1 | 6 | 61 | 3 | - | 15 | - | 13 |
| FSB23 | - | 1 | - | 1 | - | 73 | 5 | - | 5 | 2 | 13 |
| FSB24 | - | - | - | 2 | 1 | 74 | - | 6 | 1 | 2 | 14 |
| FSB25 | - | - | - | 3 | 7 | 79 | - | 2 | 7 | 2 | - |
| FSB26 | - | - | 6 | - | - | 52 | - | 29 | - | 3 | 9 |
| FSB27 | - | - | - | - | 12 | 79 | - | - | 4 | 1 | - |
| Sample Code | Mineral Abundance (Wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Hematite/ Goethite | Microcline/ Rutile | Plagioclase | Quartz | Kaolinite | Muscovite | Il/Sm Interstratification | |
| SZ28 | 1 | Trace | - | 76 | 23 | - | - |
| SZ29 | 6 | 4 | 22 | 60 | - | - | 8 |
| SZ30 | 2 | 1 | - | 47 | 45 | - | - |
| SZ31 | - | 2 | 30 | 30 | 20 | 18 | - |
| SZ32 | 5 | - | - | 13 | 80 | - | - |
| SZ33 | - | 1 | Trace | 79 | 16 | 3 | - |
| SZ34 | - | - | 37 | 1 | 22 | 40 | - |
| SZ35 | - | 10 | - | 77 | 11 | 1 | - |
| SZ36 | 14 | - | - | 49 | 37 | - | - |
| SZ37 | - | - | 1 | - | 94 | 6 | - |
| SZ38 | - | 8 | - | 77 | 13 | 2 | 1 |
| SZ39 | 3 | 2 | - | 42 | 54 | - | - |
| Sample | Mineral Abundance (Wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siderite | Halite | Alunite | Gibbsite | Goethite/Hematite | Anatase | Microcline/Rutile | Plagioclase | Quartz | Kaolinite | Mica | Smectite | I/S Interstratification | |
| DRC-1 | - | 4 | - | 3 | - | 2 | 1 | - | 35 | 51 | 3 | 2 | - |
| DRC-2 | - | - | - | 2 | - | 2 | 1 | 1 | 35 | 52 | 3 | 4 | - |
| DRC-3 | - | - | - | - | 3 | - | - | - | 12 | 82 | 2 | 2 | - |
| DRC-4 | - | 3 | - | 5 | - | 2 | - | - | 29 | 57 | 1 | 2 | - |
| DRC-5 | - | - | 1 | - | 3 | 1 | - | 1 | 21 | 59 | 14 | - | - |
| DRC-6 | - | - | - | 2 | - | 3 | - | - | 53 | 43 | - | - | - |
| DRC-7 | - | - | 1 | - | 3 | 2 | 1 | - | 22 | 56 | 15 | - | - |
| DRC-8 | - | - | - | - | - | 3 | - | - | 41 | 46 | 10 | - | - |
| DRC-9 | 6 | - | - | - | 2 | 2 | - | - | 8 | 82 | - | - | - |
| DRC-10 | - | - | - | - | 1 | - | 3 | - | 67 | 10 | 5 | - | 14 |
| DRC-11 | - | - | - | - | - | - | - | - | 0 | - | - | 1 | - |
| DRC-12 | 4 | - | - | - | 1 | 4 | - | - | 6 | 86 | - | - | - |
| DRC-13 | - | - | - | - | - | 3 | 5 | - | 57 | 23 | - | 4 | 8 |
| DRC-14 | - | - | - | - | 2 | 4 | - | - | 35 | 59 | - | - | - |
| DRC-15 | - | - | - | - | - | 2 | - | - | 11 | 87 | - | - | - |
4.5. Extent of Weathering of Soil Samples
5. Discussion
5.1. Granulometric Properties of Soil Samples

5.2. Mineralogical Properties of the Soils
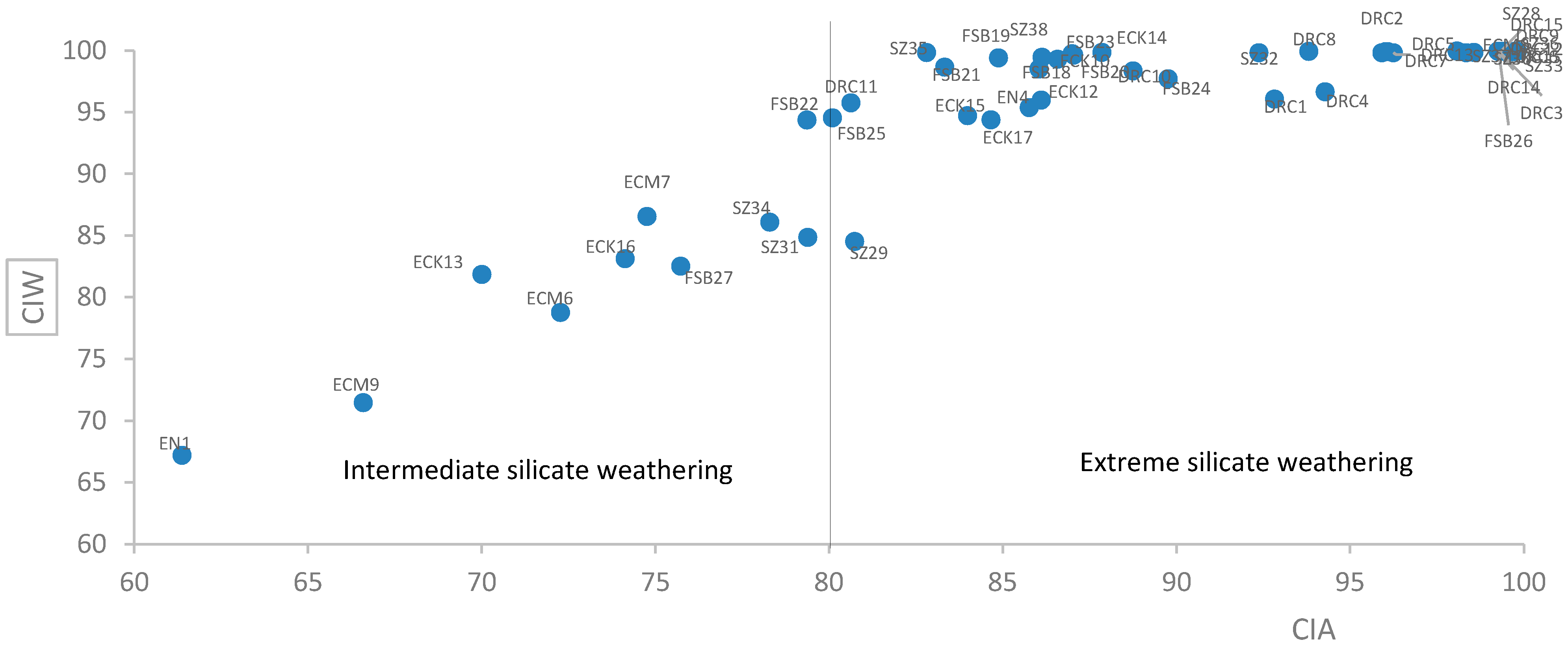
5.3. Major and Trace Element Oxides in Soil Samples
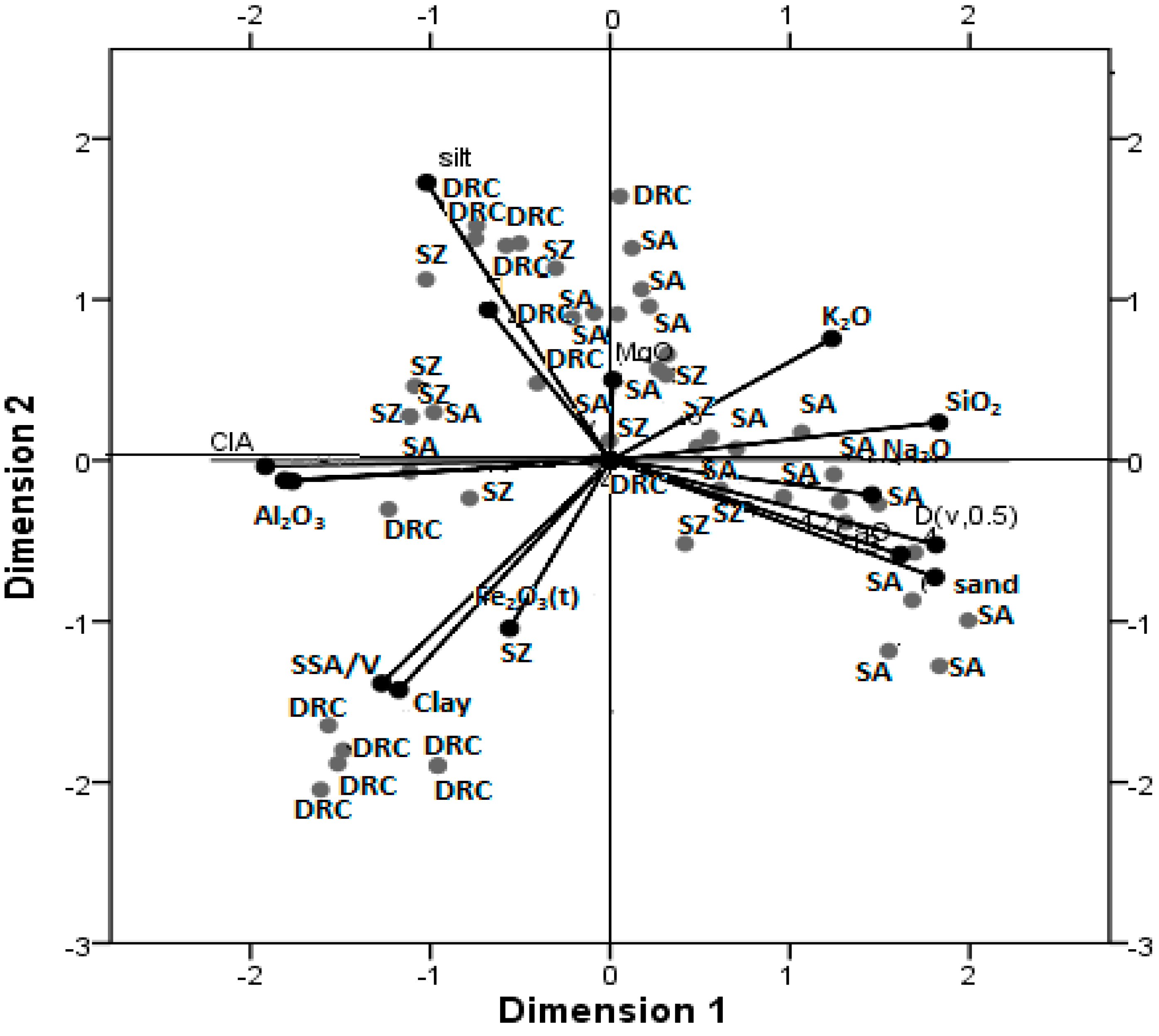
5.4. Association of Geophagic Soils
5.5. Possible Health Implication of Ingesting These Soils
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Trace Elements in Human Nutrition and Health; World Health Organisation: Geneva, Switzerland, 1996. [Google Scholar]
- Songca, S.P.; Ngole, V.M.; Ekosse, G.E.; De Jager, L. Demographic characteristics associated with consumption of geophagic clays among ethnic groups in the Free State and Limpopo provinces. Indilinga: Afr. J. Indigen. Knowl. Syst. 2010, 9, 110–123. [Google Scholar]
- Henry, J.M.; Cring, F.D. Geophagy: An Anthropological perspective. In Soils and Human Health; Brevik, E.C., Burgess, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 179–199. [Google Scholar]
- Dean, J.R.; Deary, M.E.; Gbefa, B.K.; Scott, W.C. Characterization and analysis of persistent organic pollutants and major, minor and trace elements in calabash chalk. Chemosphere 2004, 57, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Hanson, H.C. Mineral Licks, Geophagy and Biogeochemistry of North American Ungulates; Iowa State University Press: Ames, IA, USA, 1985. [Google Scholar]
- Johns, T.; Duquette, M. Detoxification and mineral supplementation as functions of geophagy. Am. J. Clin. Nutr. 1991, 53, 448–456. [Google Scholar] [PubMed]
- Mahaney, W.C.; Hancock, V.G.R.; Aufreiter, S.; Huffman, M.A. Geochemistry and clay mineralogy of termite mound soil and the role of geophagy in chimpanzees of the Mahale Mountains, Tanzania. Primates 1996, 37, 121–134. [Google Scholar] [CrossRef]
- Aufreiter, S.; Hancock, R.G.V.; Mahaney, W.C.; Stambolic-Robb, A.; Sanmugadas, K. Geochemistry and mineralogy of soils eaten by humans. Int. J. Food Sci. Nutr. 1997, 48, 293–305. [Google Scholar] [CrossRef]
- Olivier, M.A. Soil and human health: A review. Eur. J. Soil Sci. 1997, 48, 573–592. [Google Scholar] [CrossRef]
- Hooda, P.S. Soil ingestion affects the potential bioavailability of Cu, Mn, and Zn. In Proceedings of the 7th International Conference on the Biogeochemistry of Trace Elements, Uppsala, Sweden, 15 June–19 June 2003; pp. 8–11.
- Wilson, M.J. Clay mineralogical and related characteristics of geophagic materials. J. Chem. Ecol. 2003, 29, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Dominy, N.J.; Davoust, E.; Minekus, M. Adaptive function of soil consumption: An in vitro study modelling the human stomach and small intestine. J. Exp. Biol. 2004, 207, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, J.D.; Duffey, S.S.; Munn, C.A.; Tell, L.A. Biochemical functions of geophagy in parrots: Detoxification of dietary toxins and cytoprotective effects. J. Chem. Ecol. 1999, 25, 897–922. [Google Scholar] [CrossRef]
- Wang, P.; Afriyie-Gyawu, E.; Tang, Y.; Johnson, N.M.; Xu, L.; Tang, L.; Huebner, H.J.; Ankrah, N.A.; Ofori-Adjei, D.; Ellis, W.; Jolly, P.E.; Williams, J.H.; Wang, J.S.; Phillips, T.D. NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II. Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2008, 25, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Afriyie-Gyawu, E.; Ankrah, N.A.; Huebner, H.J.; Ofosuhene, M.; Kumi, J.; Johnson, N.M.; Tang, L.; Xu, L.; Jolly, P.E.; Ellis, W.O.; Ofori-Adjei, D.; Williams, J.H.; Wang, J.S.; Phillips, T.D. Novasil clay intervention in Ghanaina at risk of aflatoxins: I. Study design and clinical outcomes. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2008, 25, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Severance, H.W.; Holt, T.; Patrone, N.A.; Chapman, L. Profound muscle weakness and hypokalemia due to clay ingestion. S. Med. J. 1998, 18, 272–274. [Google Scholar] [CrossRef]
- Hooda, P.; Henry, J. Geophagia and human nutrition. In Consuming the Inedible: Neglected Dimensions of Food Choice; MacClancy, J., Henry, J., MacBeth, H., Eds.; Berghahn Books: New York, NY, USA, 2007; pp. 89–98. [Google Scholar]
- Brouillard, M.Y.; Rateau, J.G. Smectitie and kaolin on bacterial enterotoxins. Gastroen. Clin. Biol. 1989, 13, 18–24. (in French). [Google Scholar]
- Glickman, L.T.; Camara, A.O.; Glickman, N.W.; Mccabe, G.P. Nematode intestinal parasite of children in rural Guinea, Africa: Prevalence and relationship to geophagia. Int. J. Epidemiol. 1999, 28, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Saathoff, E.; Olsen, A.; Kvalsvig, J.D.; Geissler, P.W. Geophagy and its association with geohelminth infections in rural schoolchildren from northern KwaZulu Natal—South Africa. Trans. Roy. Soc. Trop. Med. Hyg. 2002, 96, 485–490. [Google Scholar] [CrossRef]
- Kawai, K.; Saathoff, E.; Antelman, G.; Msamanga, G.; Fawzi, W.W. Geophagy (soil-eating) in relation to anaemia and helminth infection among HIV-infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 2009, 80, 36–43. [Google Scholar] [PubMed]
- Shigova, W.; Moturi, W. Geophagia as a risk factor for diarrhea. J. Infect. Develop. Countries 2009, 3, 94–98. [Google Scholar]
- Seim, G.L.; Ahn, C.I.; Bodis, M.S.; Luwedde, F.; Miller, D.D.; Hillier, S.; Tako, E.; Glahn, R.P.; Young, S.L. Bioavailability of iron in geophagic earths and clay minerals, and their effect on dietary iron absorption using an in vitro digestion/Caco-2 cell model. Food Funct. 2013, 4, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Pebsworth, P.A.; Seim, G.L.; Huffman, M.A.; Glahn, R.P.; Tako, F.; Young, S.L. Soil consumed by Chacma baboons is low in bioavailable iron and high in clay. J. Chem. Ecol. 2013, 39, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Ekosse, G.E.; de Jager, L.; Ngole, V. Traditional mining and mineralogy of geophagic clays from Limpopo and Free State provinces, South Africa. Afr. J. Biotechnol. 2010, 9, 8058–8067. [Google Scholar]
- Ngole, V.M.; Ekosse, G.E. Physico-chemistry, mineralogy and geochemistry of geophagic clayey soils from Eastern Cape, South Africa, and their nutrient bioaccessibility. J. Sci. Res. Essays 2012, 7, 1319–1331. [Google Scholar]
- Abrahams, P.W. Geophagy (soil consumption) and iron supplementation in Uganda. Trop. Med. Int. Health 1997, 2, 617–623. [Google Scholar]
- Mahaney, W.; Hancock, R.G.V.; Inoue, M. Geochemistry and clay mineralogy of soils eaten by Japanese macaques. Primates 1993, 34, 85–91. [Google Scholar]
- Kutalek, R.; Wewalka, G.; Gundacker, C.; Auer, H.; Wilson, J.; Haluza, D.; Huhulescu, S.; Hillier, S.; Sager, M.; Prinz, A. Geophagy and potential health implications: Geohelminths, microbes and heavy metals. Trans. Roy. Soc. Trop. Med. Hyg. 2010, 104, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Callahan, G.N. Eating dirt. Emerg. Infect. Dis. 2003, 9, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.J.P.; Dexter, P.B.; Darton-Hill, I. The impact of consuming iron from non-food sources on iron status in developing countries. Public Health Nutr. 2000, 3, 375–383. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Andrews, P.; Boz, B. Effect of taphonomic processes on dental microwear. Am. J. Phys. Anthropol. 1999, 108, 359–373. [Google Scholar] [CrossRef]
- Prince, R.J.; Luoba, A.I.; Adhiambo, P.; Ng’uono, J.; Geissler, P.W. Geophagy is common among Luo woman in western Kenya. Trans. Roy. Soc. Trop. Med. Hyg. 1999, 93, 515–516. [Google Scholar]
- Hunter, J.M.; de Kleine, R. Geophagy in Central America. Geogr. Rev. 1984, 74, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Remmelzwaal, A.; Masuku, B.S. Characterization and Correlation of the Soils of Swaziland. In Land Use Planning for Rational Utilization of Land and Water Resources Project SWA 89/001; FAO/UNDP/Govt. of Swaziland: Mbanbane, Swaziland, 1994. [Google Scholar]
- Kasongo, R.K.; Verdoodt, A.P.; Kanyankagote, P.; Baert, G.; Van Ranst, E. Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments. Soil Use Manage. 2011, 27, 94–102. [Google Scholar]
- Mandiringana, O.T.; Mnkeni, P.N.S.; Mkile, Z.; van Averbeke, W.; van Ranst, E.; Verplancke, H. Minerology and fertility status of selected soils of the Eastern Cape Province, South Africa. Commun. Soil Sci. Plant Anal. 2005, 36, 2431–2446. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, Technical Paper, No. 9; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 2002; p. 19. [Google Scholar]
- Council for Geosciences. Guide to the Services of the CGS Analytical Laboratory. Available online: http://196.33.85.14/cgs_inter/images/stories/Lab_Guide/Services_of_the_CGS_Analytical_Laboratory.pdf (accessed on 18 March 2011).
- Brime, C. The accuracy of X-ray diffraction methods for determining mineral mixtures. Miner. Mag. 1985, 49, 531–538. [Google Scholar] [CrossRef]
- Bish, D.L.; Reynolds, R.C., Jr. Sample preparation for X-ray diffraction. In Reviews in Mineralogy: Modern Powder Diffraction 20; Bish, D.L., Post, J.E., Eds.; Mineralogical Society America: Washington, DC, USA, 1989; pp. 73–99. [Google Scholar]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. 378. [Google Scholar]
- Fitton, G. X-ray fluorescence spectrometry. In Modern Analytical Geochemistry: An Introduction to Quantitative Chemical Analysis Techniques for Earth, Environmental and Material Sciences; Gill, R., Ed.; Addison Wesley Longman Ltd.: Harlow, UK, 1997; pp. 135–153. [Google Scholar]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of luttites. Nature 1982, 291, 715–717. [Google Scholar]
- Harnois, L. The CIW index: A new chemical index of weathering. Sediment. Geol. 1988, 55, 319–322. [Google Scholar] [CrossRef]
- Zhou, D.; Chang, T.; Davis, J.C. Dual Extraction of R-Mode and Q-Mode Factor Solutions. Math. Geol. 1983, 15, 581–606. [Google Scholar] [CrossRef]
- Abrahams, P.W.; Parsons, J.A. Geophagy in the Tropics: An Appraisal of Three Geophagical Materials. Environ. Geochem. Health 1997, 19, 19–22. [Google Scholar]
- Mahaney, W.C.; Milner, M.W.; Mulyono, H.S.; Hancock, R.G.V.; Aufreiter, S.; Reich, M.; Wink, M. Mineral and chemical analyses of soils eaten by humans in Indonesia. Int. J. Environ. Health 2000, 10, 93–109. [Google Scholar] [CrossRef]
- Kikouama, O.J.R.; Konan, K.L.; Bonnet, J.P.; Baldé, L.; Yagoubi, N. Physicochemical characterization of edible clays and release of trace elements. Appl. Clay Sci. 2009, 43, 135–141. [Google Scholar] [CrossRef]
- Young, S.L.; Wilson, M.J.; Hillier, S.; Delbos, E.; Ali, S.M.; Stoltzfus, R.J. Differences and Commonalities in Physical, Chemical and Mineralogical Properties of Zanzibari Geophagic Soils. J. Chem. Ecol. 2010, 36, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.M. Phase changes which occur on heating kaolin clays. In The X-Ray Identification and Crystal Structures of Clay Minerals; Brown, G., Ed.; Mineralogical Society Publishing: London, UK, 1972; pp. 132–142. [Google Scholar]
- Cornell, R.M.; Chwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurence and Uses, 2nd ed.; VCH Weinheim: Berlin, Germany, 1996. [Google Scholar]
- Goldberg, K.; Humayun, M. The applicability of the Chemical Index of Alteration as a paleoclimatic indicator: An example from the Permian of the Paraná Basin, Brazil. Paleogeography, Paleoclimate, Paleoecol. 2010, 29, 175–183. [Google Scholar] [CrossRef]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. Forest Res. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Drew, L.J.; Grunsky, E.C.; David, M.; Sutphin, D.M.; Woodruff, L.G. Multivariate analysis of the geochemistry and mineralogy of soils along two continental-scale transects in North America. Sci. Total Environ. 2010, 409, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Grunsky, E.C.; Smee, B.W. Enhancements in the interpretation of geochemical data using multivariate methods and digital topography. Can. Inst. Min. Met. 2003, 96, 39–43. [Google Scholar]
- Lohn, J.W.G.; Austin, R.C.T.; Winslet, M.C. Unusual causes of small-bowel obstruction. J. Roy. Soc. Med. 2000, 93, 365–368. [Google Scholar] [PubMed]
- Haydel, S.E.; Remenih, C.M.; Williams, L.B. Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial Pathogens. J. Antimicrob. Chemother. 2008, 61, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Rateau, J.G.; Morgant, G.; Droy-Priot, M.T.; Parier, J.L. A histological, enzymatic and water-electrolyte study of the action of smectite, a mucoprotective clay, on experimental infectious diarrhoea in the rabbit. Cur. Med. Res. Opin. 1982, 8, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C.; Burgess, L.C. Soils and human health: An overview. In Soils and Human Health; Brevik, E.C., Burgess, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 29–56. [Google Scholar]
- Abrahams, P.W.; Follansbee, M.H.; Hunt, A.; Smith, B.; Wragg, J. Iron nutrition and possible lead toxicity: an appraisal of geophagy undertaken by pregnant women of UK Asian communities. Appl. Geochem. 2006, 21, 98–108. [Google Scholar] [CrossRef] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngole-Jeme, V.M.; Ekosse, G.-I.E. A Comparative Analyses of Granulometry, Mineral Composition and Major and Trace Element Concentrations in Soils Commonly Ingested by Humans. Int. J. Environ. Res. Public Health 2015, 12, 8933-8955. https://doi.org/10.3390/ijerph120808933
Ngole-Jeme VM, Ekosse G-IE. A Comparative Analyses of Granulometry, Mineral Composition and Major and Trace Element Concentrations in Soils Commonly Ingested by Humans. International Journal of Environmental Research and Public Health. 2015; 12(8):8933-8955. https://doi.org/10.3390/ijerph120808933
Chicago/Turabian StyleNgole-Jeme, Veronica M., and Georges-Ivo E. Ekosse. 2015. "A Comparative Analyses of Granulometry, Mineral Composition and Major and Trace Element Concentrations in Soils Commonly Ingested by Humans" International Journal of Environmental Research and Public Health 12, no. 8: 8933-8955. https://doi.org/10.3390/ijerph120808933
APA StyleNgole-Jeme, V. M., & Ekosse, G.-I. E. (2015). A Comparative Analyses of Granulometry, Mineral Composition and Major and Trace Element Concentrations in Soils Commonly Ingested by Humans. International Journal of Environmental Research and Public Health, 12(8), 8933-8955. https://doi.org/10.3390/ijerph120808933






