Aniline Induces Oxidative Stress and Apoptosis of Primary Cultured Hepatocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.2.1. Isolation of Rat Hepatocytes
2.2.2. Culture Conditions and Procedures
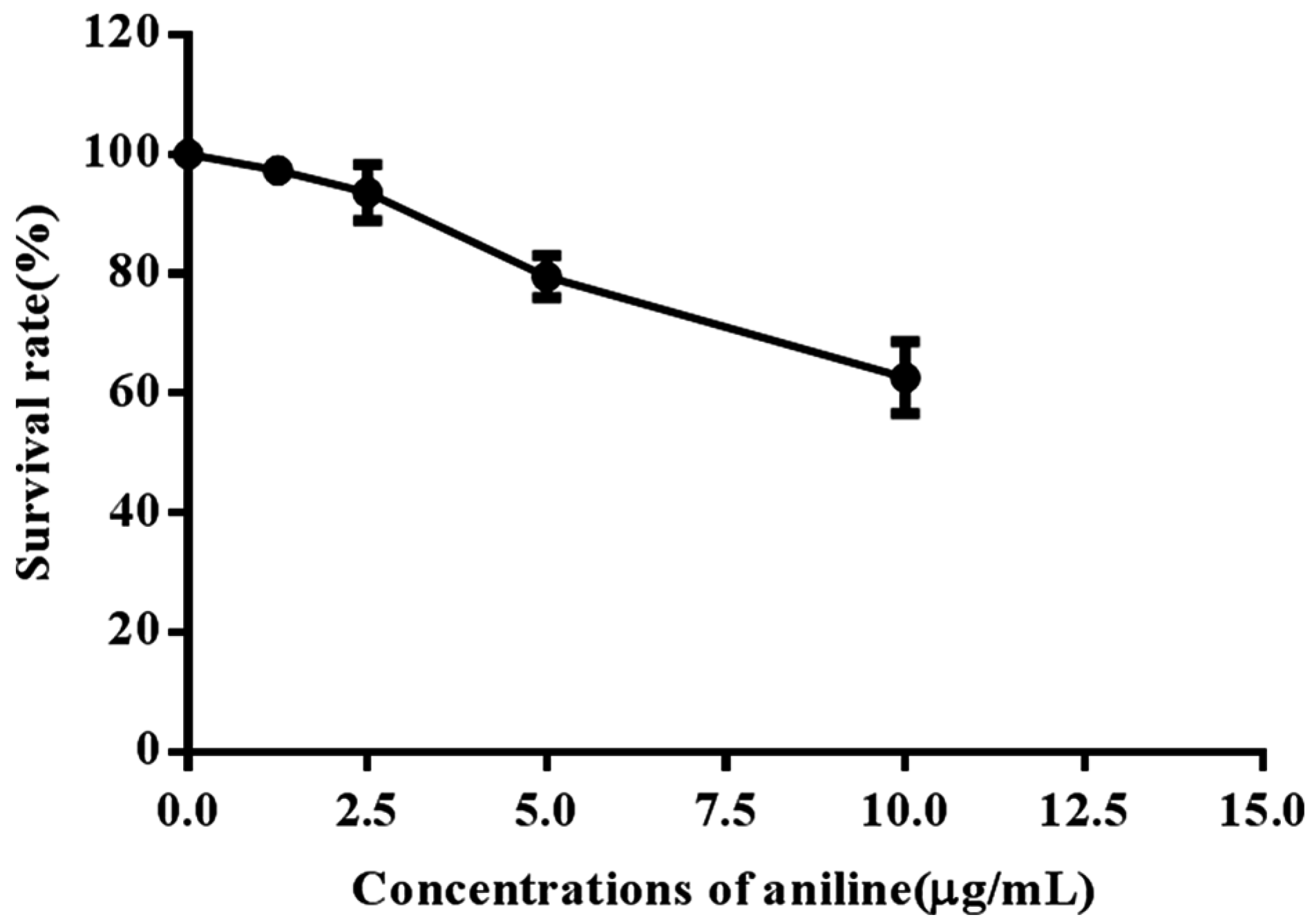
2.3. Measurement of Cell Viability by MTT Assay
2.4. Detection of ROS
2.5. Determination of Intracellular Total Glutathione (GSH)
2.6. Detection of Superoxide Dismutase (SOD)
2.7. Measurement of Catalase (CAT) Activity
2.8. Membrane Lipid Peroxidation Assay
2.9. Detection of Mitochondrial Membrane Potential
2.10. DNA Damage Determination by Comet Assay
2.11. Assessment of Apoptosis
2.12. Statistical Analysis
3. Results and Discussion
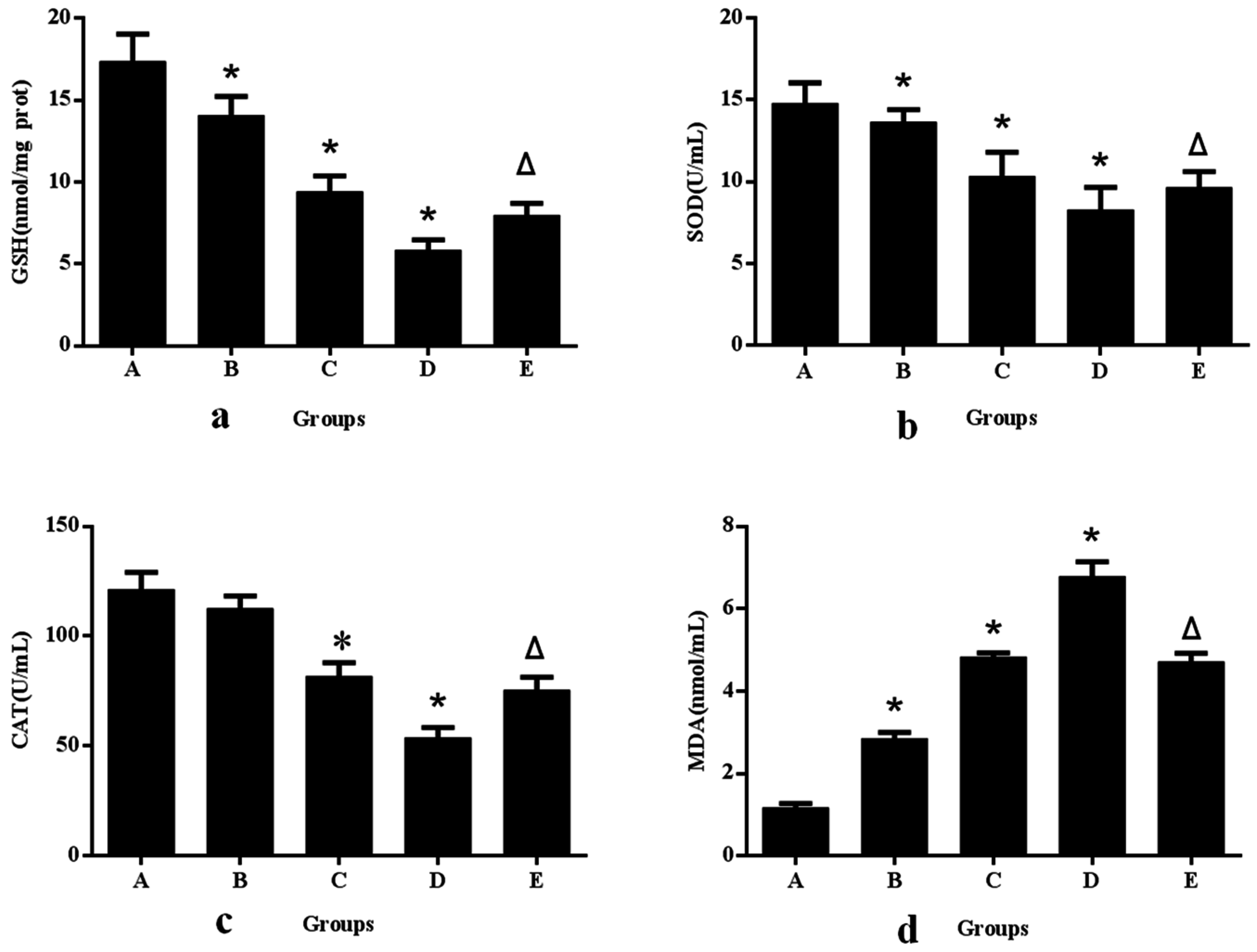
3.1. Aniline-Induced Generation of ROS and Oxidative Changes
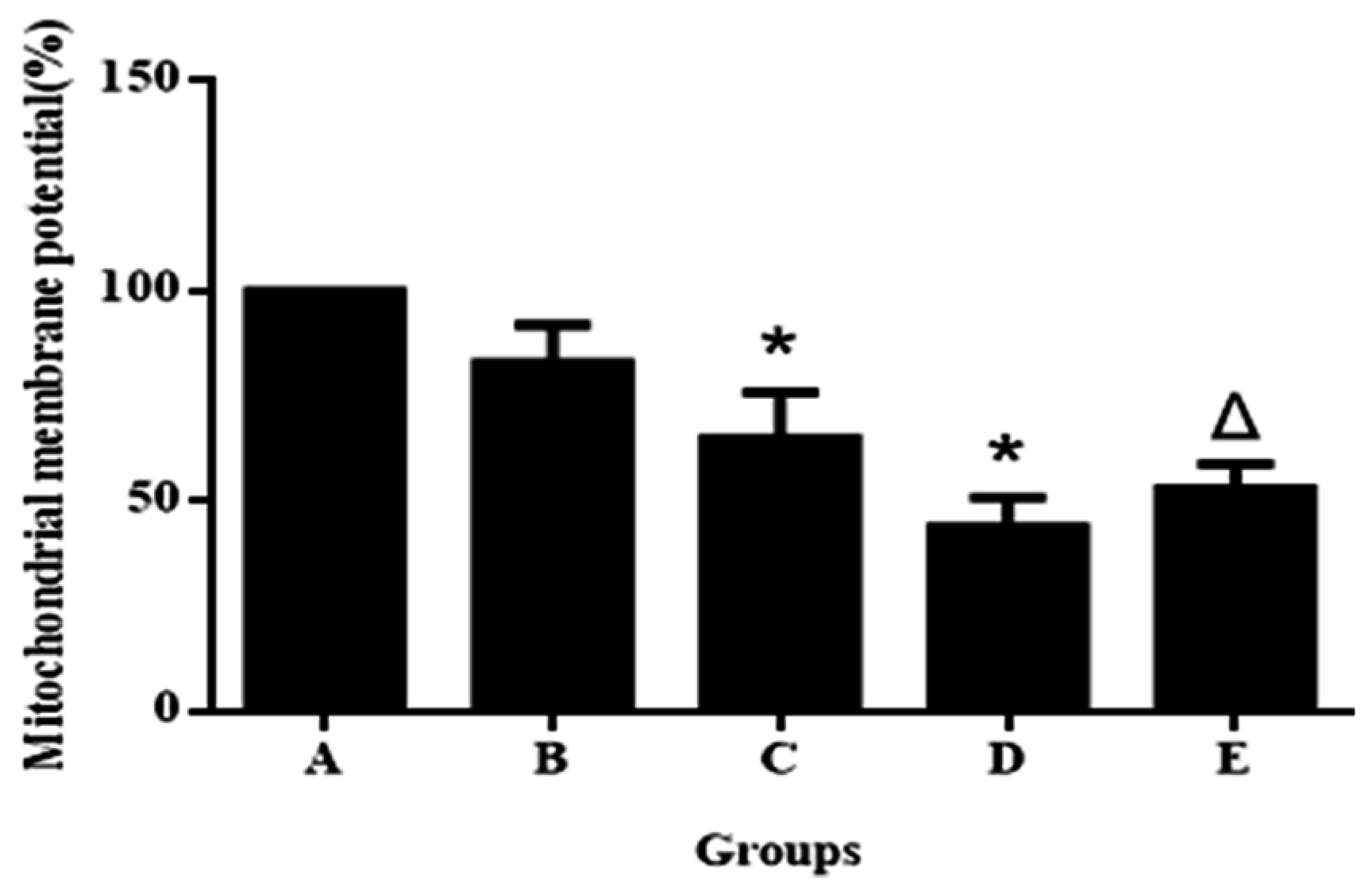
3.2. Aniline Causes Loss of Mitochondrial Permeability
3.3. Aniline-Induced DNA Injury in Hepatocytes
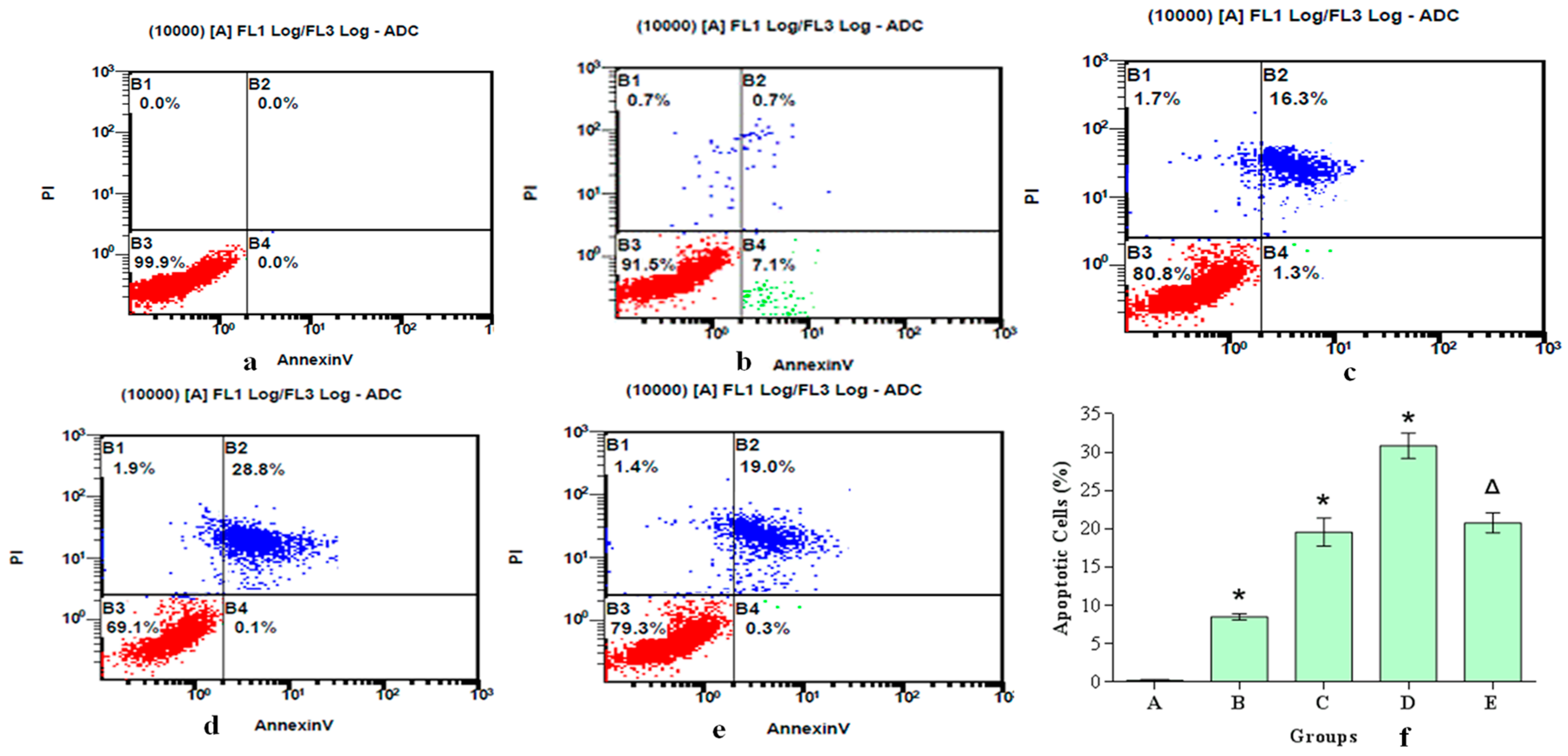
3.4. ROS Generation and DNA Injury Play a Role in Analine-Induced Apoptosis in Hepatocytes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holm, J.B.; Chalmey, C.; Modick, H.; Jensen, L.S.; Dierkes, G.; Weiss, T.; Jensen, B.A.; Nørregård, M.M.; Borkowski, K.; Styrishave, B.; et al. Aniline is rapidly converted into paracetamol impairing male reproductive development. Toxicol. Sci. 2015, 148, 288–298. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, F.; Campanella, L.; Samperi, R.; Bachi, A. Mass spectrometric identication of hemoglobin modications induced by nitrosobenzene. Ecotoxicol. Environ. Saf. 2009, 72, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, J.; Soman, K.V.; Ansari, G.A.S.; Khan, M.F. Aniline-induced nitrosative stress in rat spleen: Proteomic identication of nitrated proteins. Toxicol. Appl. Pharmacol. 2011, 255, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, H.; Boor, P.J.; Ramanujam, V.M.S.; Ansari, G.A.S.; Khan, M.F. Up-regulation of hemeoxygenase-1 in rats pleen after aniline exposure. Free Radic. Biol. Med. 2010, 48, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Goodall, C.M.; Lijinsky, W. Oncogenicity tests of p-nitroso-N,N-dimethylaniline and p-nitroso-N,N-diethylaniline in NZR rats and NZO mice. Pathology 1976, 8, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Goodall, C.M.; Moore, C.M.; Stephens, O.B. Mutagenicity of para-nitroso-dimethyl- and diethyl-anilines. Xenobiotica 1986, 16, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, R.F.; Mertsch, K.; Rohde, E.; Baeger, I.; Grigor’ev, I.A.; Blasig, I.E. Cytotoxicity of spin trapping compounds. FEBS Lett. 1997, 418, 73–75. [Google Scholar] [CrossRef]
- Bus, J.S.; Popp, J.A. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally-related compounds. Food Chem. Toxicol. 1987, 25, 619–626. [Google Scholar] [CrossRef]
- Goodman, D.G.; Ward, J.M.; Reichardt, W.D. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4′-sulfonyldianiline, or D & C red No. 9. J. Natl. Cancer Inst. 1984, 73, 265–273. [Google Scholar] [PubMed]
- Hatakeyama, S.; Kovacs, K.; Yeghiayan, E.; Blascheck, J.A. Aniline-induced changes in the corpora lutea of rats. Am. J. Obstet. Gynecol. 1971, 109, 469–476. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Aniline and aniline hydrochloride. In IARC Monographs on the Evaluation of the Caecinogenic Risk of Chemical to Humans; IARC: Lyon, France, 1982; Volume 27, pp. 39–61. [Google Scholar]
- Jenkins, F.P.; Robinson, J.A.; Gellatly, J.B.; Salmond, G.W. The no-effect dose of aniline in human subjects and a comparison of aniline toxicity in man and the rat. Food Cosmet. Toxicol. 1972, 10, 671–679. [Google Scholar] [CrossRef]
- Okazaki, Y.; Yamashita, K.; Sudo, M.; Tsuchitani, M.; Narama, I.; Yamaguchi, R.; Tateyama, S. Neurotoxicity induced by a single oral dose of aniline in rats. J. Vet. Med. Sci. 2001, 63, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Z. Health status of workers exposed to aniline. Occup. Health 2004, 20, 35–36. [Google Scholar]
- Yang, Z.L. Effects of aniline and living factors on health status of workers. Chin. J. Med. Writ. 2003, 10, 1364–1365. [Google Scholar]
- Jia, L.; Wang, Y.J.; Dong, S.Y.; Shang, L.; Na, X.L. Combined toxicity of nitrobenzene and aniline in primary culture of rat hepatocytes. Carcinog. Teratog. Mutagen. 2012, 24, 283–285. (In Chinese) [Google Scholar]
- Bian, G.P.; Liu, R.X.; Shi, B.Z.; Jiao, H.H. DNA damages in mouse hepatocytes and lymphocytes induced by aniline and their repair dynamics. Acta Lab. Anim. Sci. Sin. 2016, 24, 139–144. (In Chinese) [Google Scholar]
- Qu, J.K.; Liu, Y.D.; Song, X.F.; Li, T.Y. The acute toxic effect of aniline on the liver of mice. Lab. Anim. Comp. Med. 2012, 32, 538–540. (In Chinese) [Google Scholar]
- Khan, M.F.; Boor, P.J.; Gu, Y.; Alcock, N.W.; Ansari, G.A. Oxidative stress in the splenotoxicity of aniline. Fund. Appl. Toxicol. 1997, 35, 22–30. [Google Scholar] [CrossRef]
- Ma, H.; Wang, J.; Abdel-Rahman, S.Z.; Boor, P.J.; Khan, M.F. Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline. Toxicol. Appl. Pharmacol. 2008, 233, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, W.H.; Lim, J.H.; Song, E.H.; Song, J.; Choi, K.Y.; Jung, M.H. Mitochondrial dysfunction: Glucokinase downregulation lowers interaction of glucokinase with mitochondria, resulting in apoptosis of pancreatic β-cells. Cell Signal. 2009, 21, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.C.; Strasser, A. BH3-only proteins-essential initiators of apoptotic cell death. Cell 2000, 103, 839–842. [Google Scholar] [CrossRef]
- Cook, J.R.; Langlet, F.; Kido, Y.; Accili, D. Pathogenesis of selective insulin resistance in isolated hepatocytes. J. Biol. Chem. 2015, 290, 13972–13980. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Levitz, S.M.; Diamond, R.D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis. 1985, 152, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, X.; Chang, H. Proliferation inhibition and apoptosis of breast cancer MCF-7 cells under the influence of colchicine. J. BUON 2016, 3, 570–575. [Google Scholar]
- Yi, J.; Yang, J.; He, R.; Gao, F.; Sang, H.; Tang, X.; Ye, R.D. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004, 64, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Zhang, X.; Jin, L.; Chen, J.; Du, B.; Pang, Q. Protective effects of caffeic acid phenethyl ester against acute radiation-induced hepatic injury in rats. Environ. Toxicol. Pharmacol. 2015, 39, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V.; Obidin, A.B.; Marinov, B.S. Modulation of superoxide dismutase by electron donors and acceptors. FEBS Lett. 1987, 211, 211–214. [Google Scholar] [CrossRef]
- Huang, X.; Qin, J.; Lu, S. Magnesium isoglycyrrhizinate protects hepatic L02 cells from ischemia/reperfusion induced injury. Int. J. Clin. Exp. Pathol. 2014, 7, 4755–4764. [Google Scholar] [PubMed]
- Durak, I.; Güven, T.; Birey, M.; Oztürk, H.S.; Kurtipek, O.; Ye, M.; Dikmen, B.; Canbolat, O.; Kavutcu, M.; Kaçmaz, M. Halothane hepatotoxicity and hepatic free radical metabolism in guinea pigs; the effects of vitamin E. Can. J. Anaesth. 1996, 43, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, W.; Ben, Y.; Jiang, J.; Tan, C.; Xu, Z.; Wang, X.; Bai, C. Preventive effects of curcumin and dexamethasone on lung transplantation-associated lung injury in rats. Crit. Care Med. 2008, 36, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Chan, G.C.; Ye, J.; Lian, Q.; Chen, J.; Yang, M. Thrombopoietin protects cardiomyocytes from iron-overload induced oxidative stress and mitochondrial injury. Cell. Physiol. Biochem. 2015, 36, 2063–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, D.; Huang, J.; Liu, W.; Guo, Y.; Xiao, W.; Wang, N. Astragaloside IV prevents acute kidney injury in two rodent models by inhibiting oxidative stress and apoptosis pathways. Apoptosis 2013, 18, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jia, L.; Zhou, H.M.; Liu, Y.; Zhong, L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Li, J.Q.; Shao, L.M.; Yang, Q.; Liu, B.H.; Wu, T.F.; Wu, P.; Yi, W.; Chen, Q.X. Puerarin inhibits proliferation and induces apoptosis in human glioblastoma cell lines. Int. J. Clin. Exp. Med. 2015, 8, 10132–10142. [Google Scholar] [PubMed]
- Tabuenca, J.M. Toxic-allergic syndrome caused by ingestion of rapeseed oil denatured with aniline. Lancet 1981, 2, 567–568. [Google Scholar] [CrossRef]
- Richter, C.; Gogvadze, V.; Laffranchi, R.; Schlapbach, R.; Schweizer, M.; Suter, M.; Walter, P.; Yaffee, M. Oxidants in mitochondria: From physiology to diseases. Biochim. Biophys. Acta 1995, 1271, 67–74. [Google Scholar] [CrossRef]
- Hug, H.; Strand, S.; Grambihler, A.; Galle, J.; Hack, V.; Stremmel, W.; Krammer, P.H.; Galle, P.R. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 1997, 272, 28191–28193. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.P. Advances in the interpretation and prediction of CYP2E1 metabolism from a biochemical perspective. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.H.; Martin, H.C.; Caro, A.A.; Pearce, A.R.; Miller, G.P. Subcellular localization of rat CYP2E1 impacts metabolic efficiency toward common substrates. Toxicology 2015, 338, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Wu, X.; Boor, P.J.; Ansari, G.A.S. Oxidative modification of proteins and lipids in aniline-induced splenic toxicity. Toxicol. Sci. 1999, 48, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Wu, X.; Ansari, G.A.S.; Boor, P.J. Malondialdehyde-protein adducts in spleens of aniline-treated rats: Immunohistochemical detection and localization. J. Toxicol. Environ. Health Part A 2003, 66, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Devadas, S.; Hinshaw, J.A.; Zaritskaya, L.; Williams, M.S. Fas-stimulated generation of reactive oxygen species or exogenous oxidative stress sensitize cells to Fas-mediated apoptosis. Free Radic. Biol. Med. 2003, 35, 648–661. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. Induction of superoxide dismutase isozymes in rabbit lung due to aniline exposure. J. Appl. Toxicol. 1987, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Upaganlawar, A.B.; Upasani, C. Protective effects of dioscorea alata L. in aniline exposure-induced spleen toxicity in rats: A biochemical study. Toxicol. Int. 2014, 21, 294–299. [Google Scholar] [PubMed]
- Polyak, K.; Xia, Y.; Zweier, J.L.; Kinzler, K.W.; Vogelstein, B. A model for p53-induced apoptosis. Nature 1997, 389, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar] [PubMed]
- Collins, A.R.; Ma, A.G.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995, 336, 69–77. [Google Scholar] [CrossRef]
- Ahamed, M.; Siddiqui, M.A.; Akhtar, M.J.; Ahmad, I.; Pant, A.B.; Alhadlaq, H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef] [PubMed]

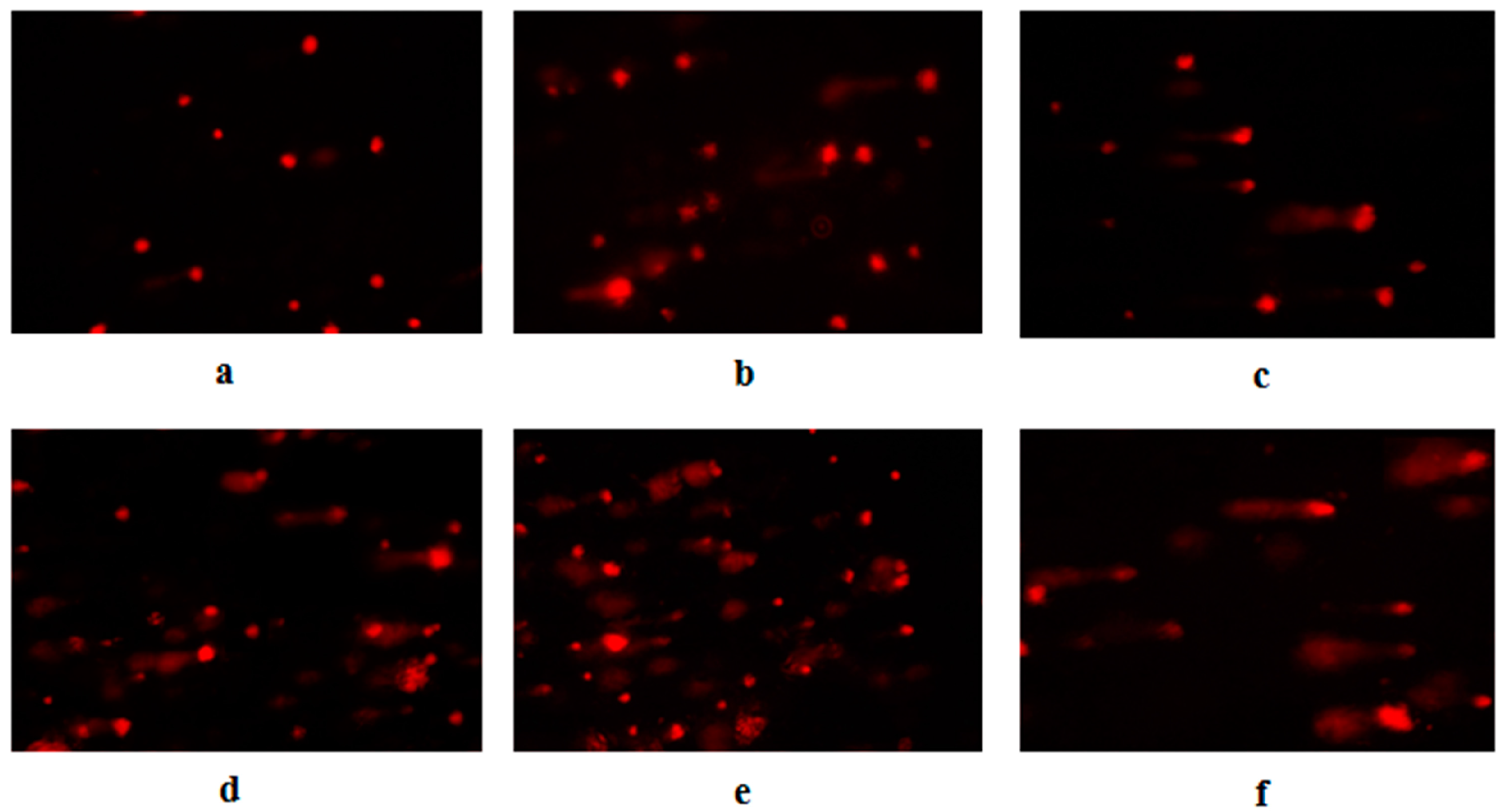
| Number | Group | Amount of Cells | Amount of Comet Cells | Percent of Comet Cells (%) | Length of Comet Tail (μm) |
|---|---|---|---|---|---|
| 1 | 0 μg/mL | 600 | 23 | 3.83 ± 1.17 | 12.02 ± 1.79 |
| 2 | Positive control | 600 | 583 | 97.17 ± 1.83 | 43.58 ± 6.77 |
| 3 | 2.50 μg/mL | 600 | 64 | 10.67 ± 4.59 * | 16.54 ± 3.27 * |
| 4 | 5.00 μg/mL | 600 | 127 | 21.17 ± 2.64 * | 28.67 ± 5.01 * |
| 5 | 10.00 μg/mL | 600 | 214 | 35.67 ± 4.89 * | 41.24 ± 6.12 * |
| 6 | 10.00 μg/mL+ | 600 | 142 | 23.67 ± 3.67 *,Δ | 30.46 ± 5.15 *,Δ |
| 20 mM NAC |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gao, H.; Na, X.-L.; Dong, S.-Y.; Dong, H.-W.; Yu, J.; Jia, L.; Wu, Y.-H. Aniline Induces Oxidative Stress and Apoptosis of Primary Cultured Hepatocytes. Int. J. Environ. Res. Public Health 2016, 13, 1188. https://doi.org/10.3390/ijerph13121188
Wang Y, Gao H, Na X-L, Dong S-Y, Dong H-W, Yu J, Jia L, Wu Y-H. Aniline Induces Oxidative Stress and Apoptosis of Primary Cultured Hepatocytes. International Journal of Environmental Research and Public Health. 2016; 13(12):1188. https://doi.org/10.3390/ijerph13121188
Chicago/Turabian StyleWang, Yue, Hong Gao, Xiao-Lin Na, Shu-Ying Dong, Hong-Wei Dong, Jia Yu, Li Jia, and Yong-Hui Wu. 2016. "Aniline Induces Oxidative Stress and Apoptosis of Primary Cultured Hepatocytes" International Journal of Environmental Research and Public Health 13, no. 12: 1188. https://doi.org/10.3390/ijerph13121188
APA StyleWang, Y., Gao, H., Na, X.-L., Dong, S.-Y., Dong, H.-W., Yu, J., Jia, L., & Wu, Y.-H. (2016). Aniline Induces Oxidative Stress and Apoptosis of Primary Cultured Hepatocytes. International Journal of Environmental Research and Public Health, 13(12), 1188. https://doi.org/10.3390/ijerph13121188





