Antioxidant Pre-Treatment Reduces the Toxic Effects of Oxalate on Renal Epithelial Cells in a Cell Culture Model of Urolithiasis
Abstract
:1. Introduction
2. Materials and Methods
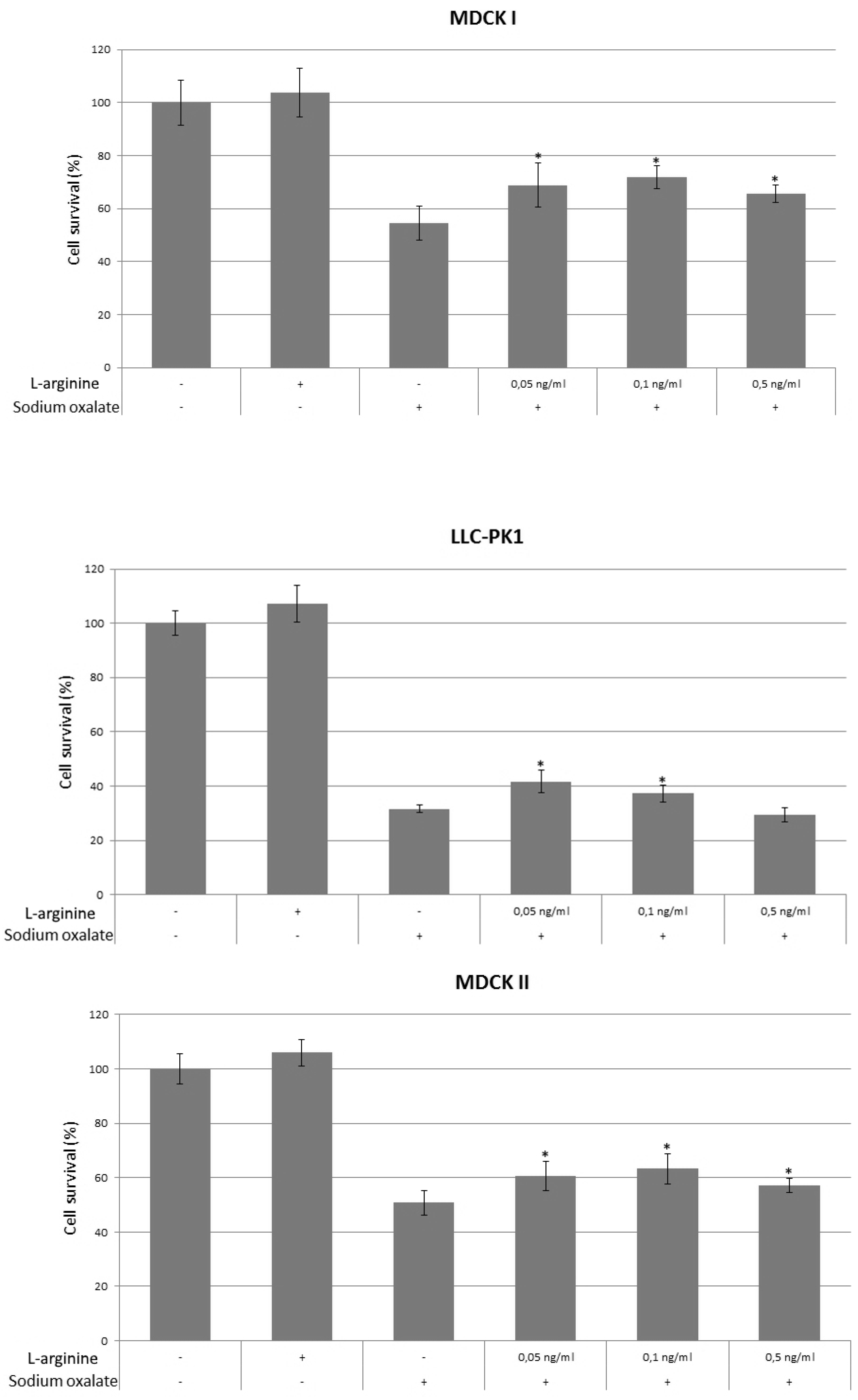
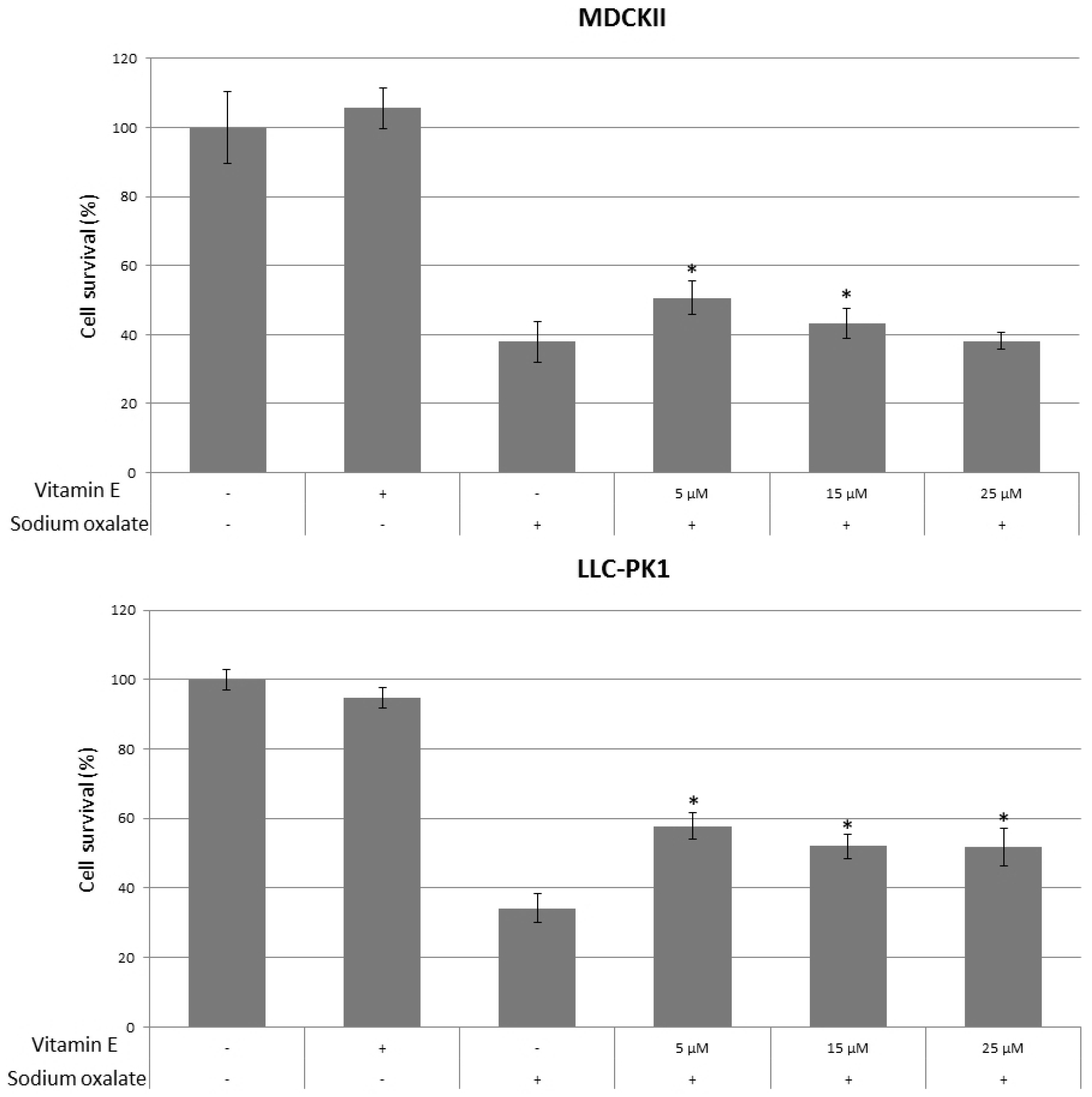
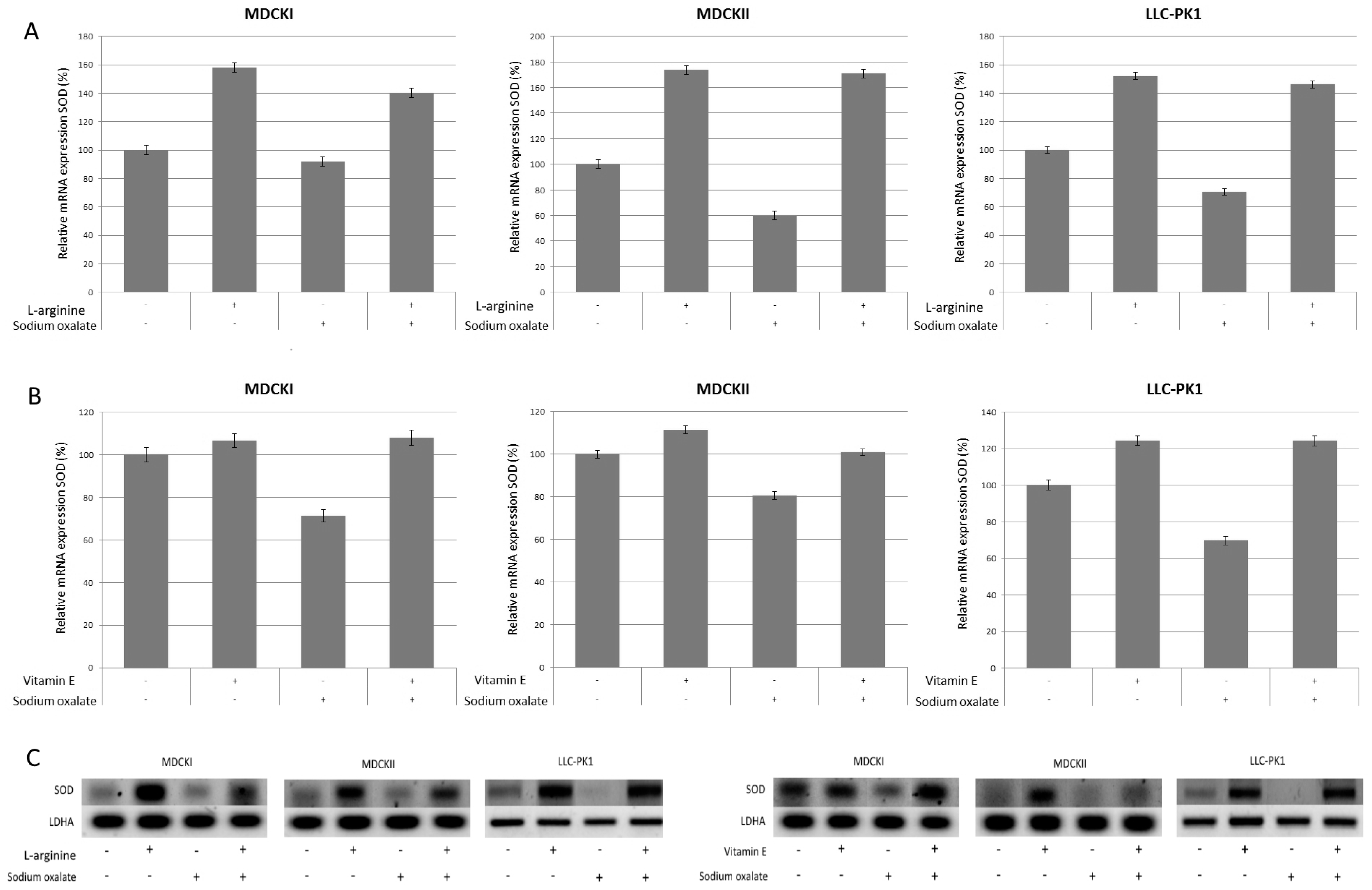
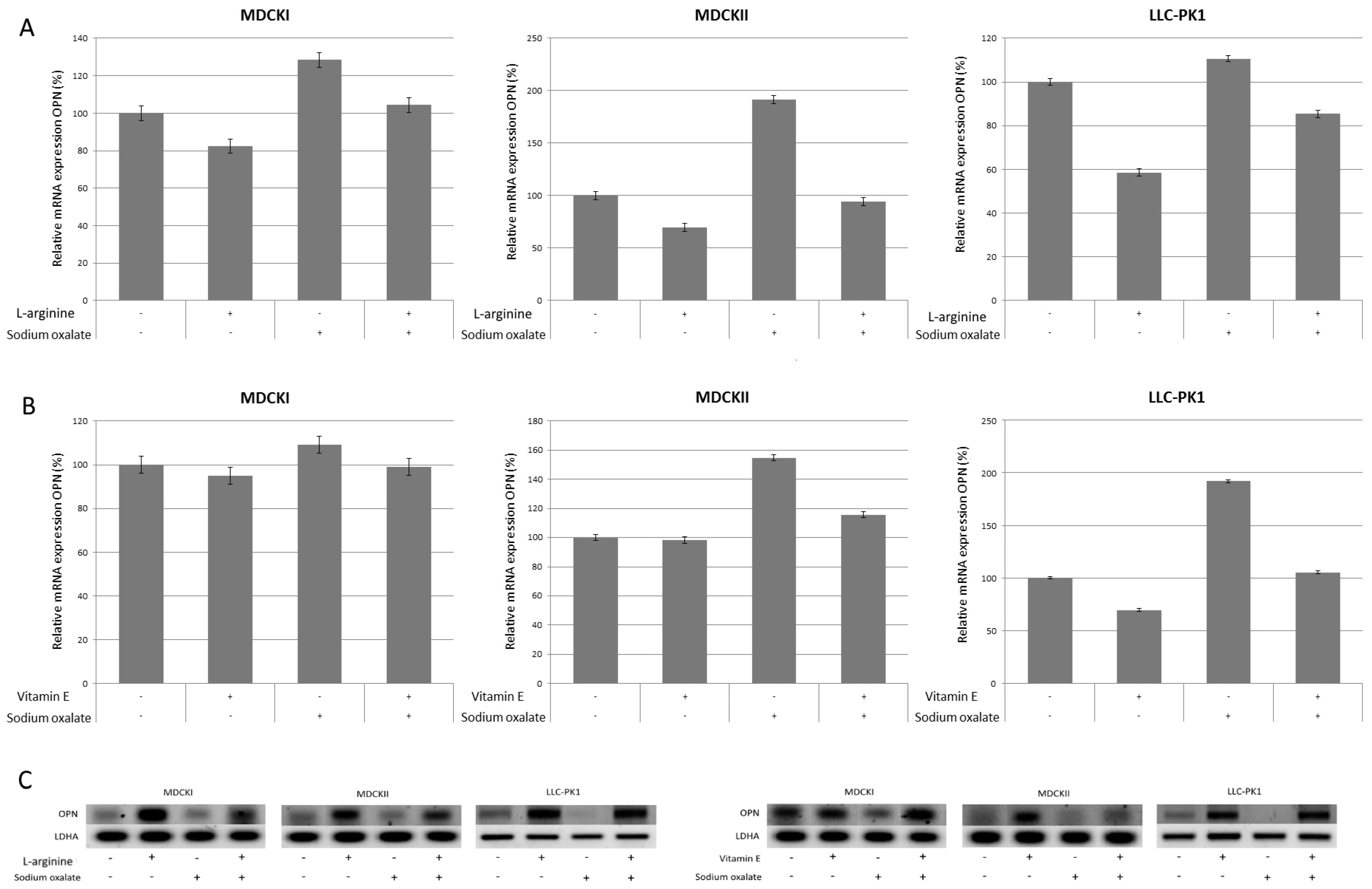
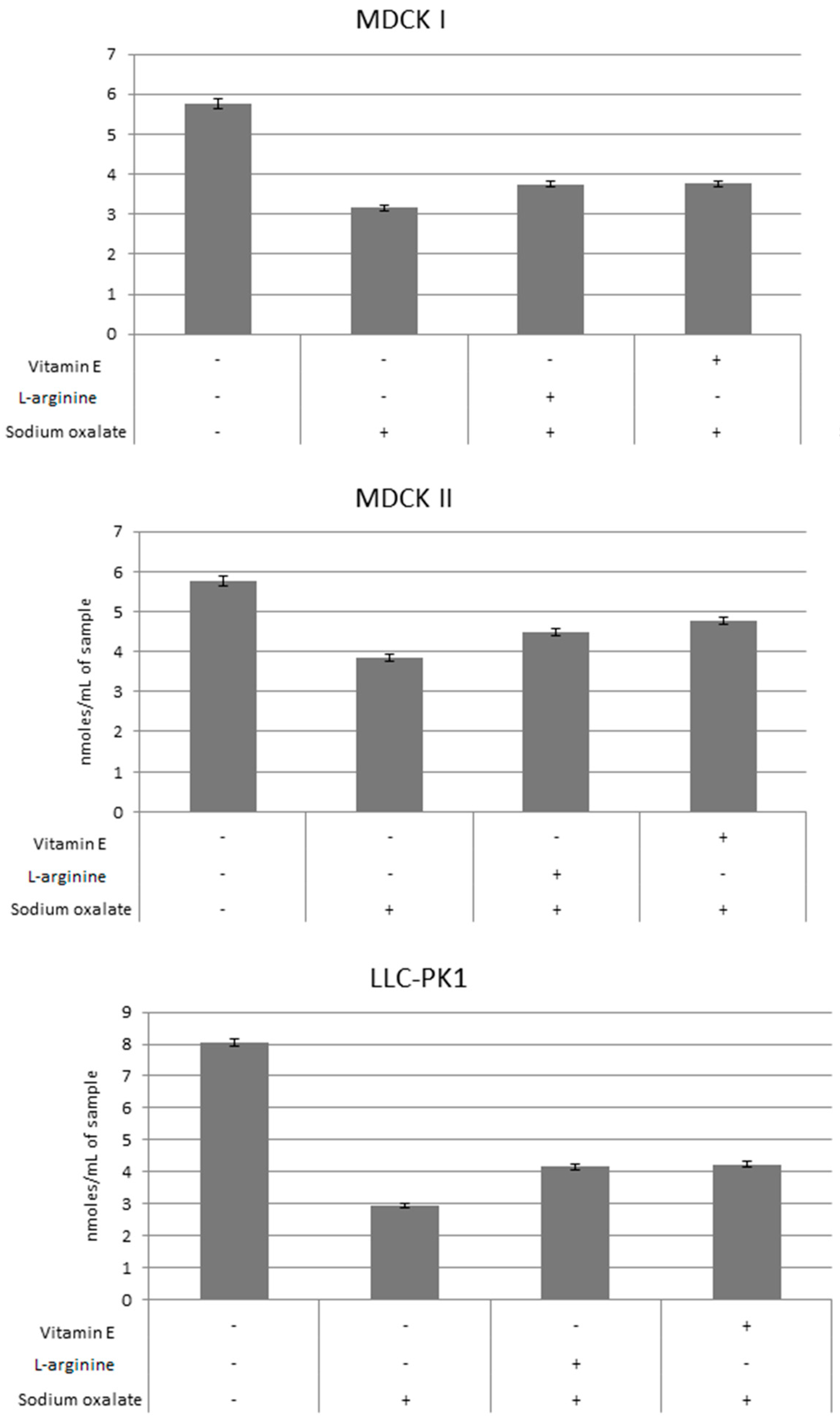
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dardamanis, M. Pathomechanisms of nephrolithiasis. Hippokratia 2013, 17, 100–107. [Google Scholar] [PubMed]
- Worcester, E.M.; Coe, F.L. Clinical practice. Calcium kidney stones. N. Engl. J. Med. 2010, 363, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Obesity, weight gain, and the risk of kidney stones. JAMA 2005, 293, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Brikowski, T.H.; Lotan, Y.; Pearle, M.S. Climate-related increase in the prevalence of urolithiasis in the United States. Proc. Natl. Acad. Sci. USA 2008, 105, 9841–9846. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.F. Facing an uncertain climate. Ann. Intern. Med. 2007, 146, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Fakheri, R.J.; Goldfarb, D.S. Ambient temperature as a contributor to kidney stone formation: Implications of global warming. Kidney Int. 2011, 79, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. Metabolic syndrome, obesity and kidney stones. Arab. J. Urol. 2012, 10, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Evan, A.P.; Worcester, E.M.; Coe, F.L.; Williams, J., Jr.; Lingeman, J.E. Mechanisms of human kidney stone formation. Urolithiasis 2015, 43 (Suppl. 1), 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evan, A.; Lingeman, J.; Coe, F.L.; Worcester, E. Randall’s plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006, 69, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Evan, A.P.; Coe, F.L.; Lingeman, J.E.; Shao, Y.; Sommer, A.J.; Bledsoe, S.B.; Anderson, J.C.; Worcester, E.M. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat. Rec. 2007, 290, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, X.; Zhou, W.; Su, Y.; He, S.; Cheng, S.; Lu, J.; Yan, Y.; Pei, X.; Qi, J.; et al. An Explanation of the Underlying Mechanisms for the In Vitro and In Vivo Antiurolithic Activity of Glechoma longituba. Oxid. Med. Cell. Longev. 2016, 2016, 3134919. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Renal tubular damage/dysfunction: Key to the formation of kidney stones. Urol. Res. 2006, 34, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Crystal/cell interaction and nephrolithiasis. Arch. Ital. Urol. Androl. 2011, 83, 1–5. [Google Scholar] [PubMed]
- Khan, S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Fasano, J.M.; Khan, S.R. Intratubular crystallization of calcium oxalate in the presence of membrane vesicles: An in vitro study. Kidney Int. 2001, 59, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Crystal-induced inflammation of the kidneys: Results from human studies, animal models, and tissue-culture studies. Clin. Exp. Nephrol. 2004, 8, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Umekawa, T.; Tsuji, H.; Uemura, H.; Khan, S.R. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int. 2009, 104, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, J.; Hayashi, T.; Saito, K.; Iida, S.; Matsuoka, K. The Interaction between female sex hormone receptors and osteopontin in a rat hyperoxaluric model. Kurume Med. J. 2010, 57, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, V.; Kalaiselvi, P.; Subashini, B.; Sumitra, K.; Varalakshmi, P. Structural and functional modification of THP on nitration: Comparison with stone formers THP. Nephron Physiol. 2005, 99, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, V.; Kalaiselvi, P.; Sumitra, K.; Srinivasan, S.; Varalakshmi, P. Counteraction of oxalate induced nitrosative stress by supplementation of l-arginine, a potent antilithic agent. Clin. Chim. Acta 2005, 354, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, S.J.; Park, M.N.; Linnes, M.; Han, H.J.; Kim, J.H.; Lieske, J.C.; Kim, S.H. Gallotannin suppresses calcium oxalate crystal binding and oxalate-induced oxidative stress in renal epithelial cells. Biol. Pharm. Bull. 2012, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Yasui, T.; Okada, A.; Tozawa, K.; Hayashi, Y.; Kohri, K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J. Urol. 2005, 173, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.D.; Whitley, P.; Chalmers, A.D. The MDCK variety pack: Choosing the right strain. BMC Cell Biol. 2011, 12, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkoelen, C.F.; van der Boom, B.G.; Kok, D.J.; Houtsmuller, A.B.; Visser, P.; Schröder, F.H.; Romijn, J.C. Cell type-specific acquired protection from crystal adherence by renal tubule cells in culture. Kidney Int. 1999, 55, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Rabito, C.A. Occluding junctions in a renal cell line (LLC-PK1) with characteristics of proximal tubular cells. Am. J. Physiol. 1986, 250, F734–F743. [Google Scholar] [PubMed]
- Sylvester, P.W.; Birkenfeld, H.P.; Hosick, H.L.; Briski, K.P. Fatty acid modulation of epidermal growth factor-induced mouse mammary epithelial cell proliferation in vitro. Exp. Cell Res. 1994, 214, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Yasui, T.; Okada, A.; Tozawa, K.; Hayashi, Y.; Kohri, K. Examination of the anti-oxidative effect in renal tubular cells and apoptosis by oxidative stress. Urol. Res. 2005, 33, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rashed, T.; Menon, M.; Thamilselvan, S. Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: Effect of antioxidants. Am. J. Nephrol. 2004, 24, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Khan, S.R. Herbal medicines in the management of urolithiasis: Alternative or complementary? Planta Med. 2009, 75, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.L.; Shevock, P.N.; Khan, S.R. Cell injury associated calcium oxalate crystalluria. J. Urol. 1990, 144, 1535–1538. [Google Scholar] [PubMed]
- Khan, S.R. Heterogeneous nucleation of calcium oxalate crystals in mammalian urine. Scanning Microsc. 1995, 9, 597–614, discussion 614–616. [Google Scholar] [PubMed]
- Patel, A.B.; Robertson, W.G.; Choong, S.; Hothersall, J.S. Heat-shock protein 25 ameliorates calcium oxalate crystal-mediated oxidative stress in renal epithelial cells. BJU Int. 2006, 98, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lu, F.J.; Yeh, R.H.; Li, Z.L.; Chen, C.H. Synergistic antioxidant activity of resveratrol with genistein in high-glucose treated Madin-Darby canine kidney epithelial cells. Biomed. Rep. 2016, 4, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Kobayashi, S.; Yoshikawa, Y.; Watanabe, K.; Orino, K. Effects of oxidative stress caused by tert-butylhydroquinone on cytotoxicity in MDCK cells. J. Vet. Med. Sci. 2012, 74, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, E.B.; Pessoa, B.S.; Biswas, S.K.; Lopes de Faria, J.B. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am. J. Nephrol. 2009, 29, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, P.N.; Dey, S.; Sahoo, M.; Choudhary, S.S.; Mahajan, S. Alteration in Oxidative/nitrosative imbalance, histochemical expression of osteopontin and antiurolithiatic efficacy of Xanthium strumarium (L.) in ethylene glycol induced urolithiasis. Biomed. Pharmacother. 2016, 84, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Boonla, C.; Hunapathed, C.; Bovornpadungkitti, S.; Poonpirome, K.; Tungsanga, K.; Sampatanukul, P.; Tosukhowong, P. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int. 2008, 101, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, Q.; Sun, Y.; Diao, L.; Qin, Z.; Wang, W.; Lu, J.; Fu, S.; Ma, B.; Yue, Z. Potential Mechanisms Responsible for the Antinephrolithic Effects of an Aqueous Extract of Fructus Aurantii. Evid. Based Complement. Altern. Med. 2015, 2015, 491409. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Tohru, U.; Hirotsugu, U.; Masanori, I.; Yuji, H.; Takashi, K. Urinary concentration of osteopontin and association with urinary supersaturation and crystal formation. Int. J. Urol. 2007, 14, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Sarica, K.; Kafkasli, A.; Narter, F.; Ozturk, O.; Yazici, O.; Hamarat, B.; Sahin, C.; Eryildirim, B. Hyperoxaluria-induced tubular ischemia: The effects of verapamil on the antioxidant capacity of the affected kidneys. Urolithiasis 2016, 44, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Ma, M.C.; Chen, J.; Chen, C.F. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J. Urol. 2002, 167, 2584–2593. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kizivat, T.; Smolić, M.; Marić, I.; Tolušić Levak, M.; Smolić, R.; Bilić Čurčić, I.; Kuna, L.; Mihaljević, I.; Včev, A.; Tucak-Zorić, S. Antioxidant Pre-Treatment Reduces the Toxic Effects of Oxalate on Renal Epithelial Cells in a Cell Culture Model of Urolithiasis. Int. J. Environ. Res. Public Health 2017, 14, 109. https://doi.org/10.3390/ijerph14010109
Kizivat T, Smolić M, Marić I, Tolušić Levak M, Smolić R, Bilić Čurčić I, Kuna L, Mihaljević I, Včev A, Tucak-Zorić S. Antioxidant Pre-Treatment Reduces the Toxic Effects of Oxalate on Renal Epithelial Cells in a Cell Culture Model of Urolithiasis. International Journal of Environmental Research and Public Health. 2017; 14(1):109. https://doi.org/10.3390/ijerph14010109
Chicago/Turabian StyleKizivat, Tomislav, Martina Smolić, Ivana Marić, Maja Tolušić Levak, Robert Smolić, Ines Bilić Čurčić, Lucija Kuna, Ivan Mihaljević, Aleksandar Včev, and Sandra Tucak-Zorić. 2017. "Antioxidant Pre-Treatment Reduces the Toxic Effects of Oxalate on Renal Epithelial Cells in a Cell Culture Model of Urolithiasis" International Journal of Environmental Research and Public Health 14, no. 1: 109. https://doi.org/10.3390/ijerph14010109
APA StyleKizivat, T., Smolić, M., Marić, I., Tolušić Levak, M., Smolić, R., Bilić Čurčić, I., Kuna, L., Mihaljević, I., Včev, A., & Tucak-Zorić, S. (2017). Antioxidant Pre-Treatment Reduces the Toxic Effects of Oxalate on Renal Epithelial Cells in a Cell Culture Model of Urolithiasis. International Journal of Environmental Research and Public Health, 14(1), 109. https://doi.org/10.3390/ijerph14010109





