Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options
Abstract
:1. Introduction
2. Epidemiology of Cigarette (Tobacco) Smoking in the United States
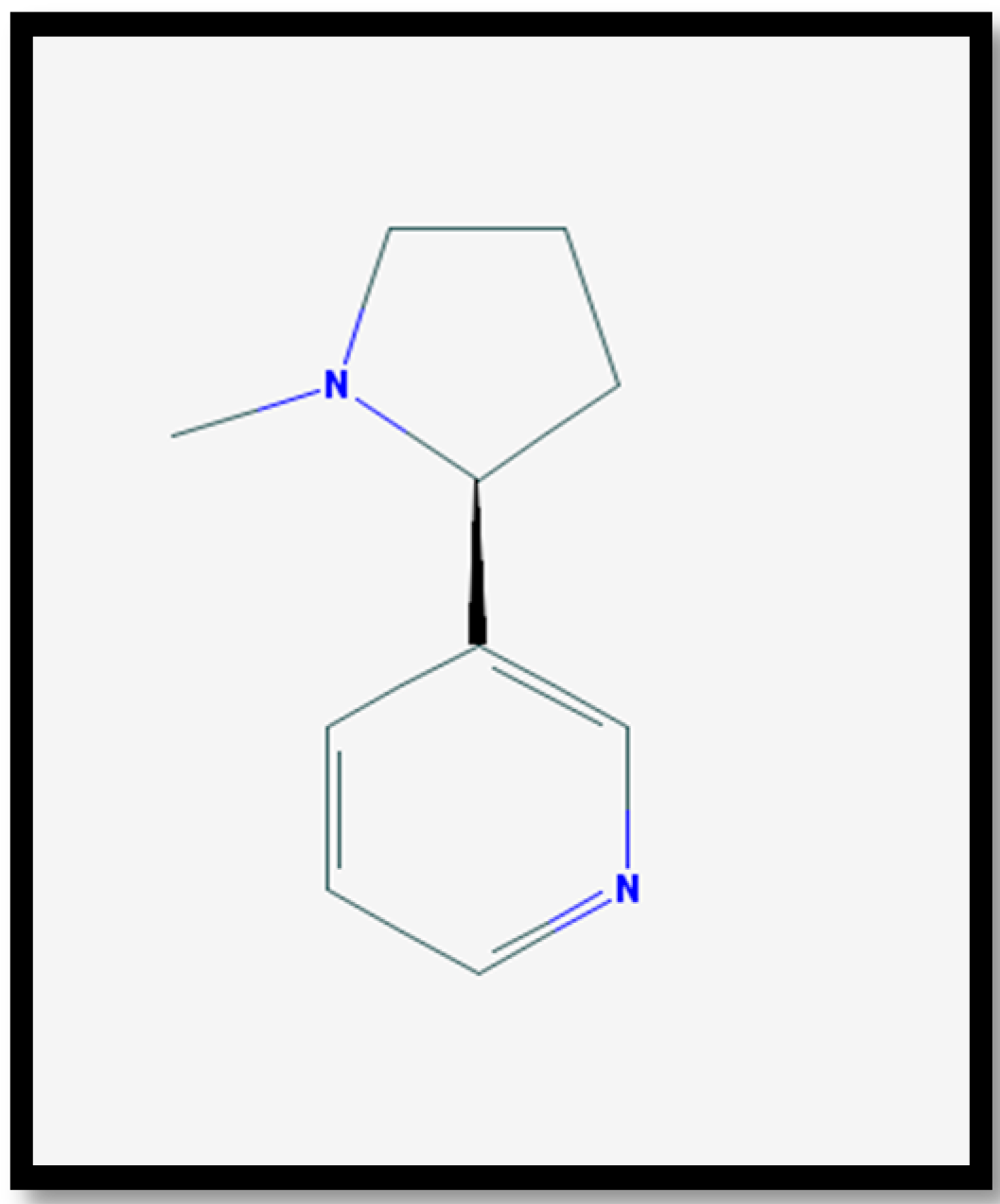
3. Pharmacology of Nicotine
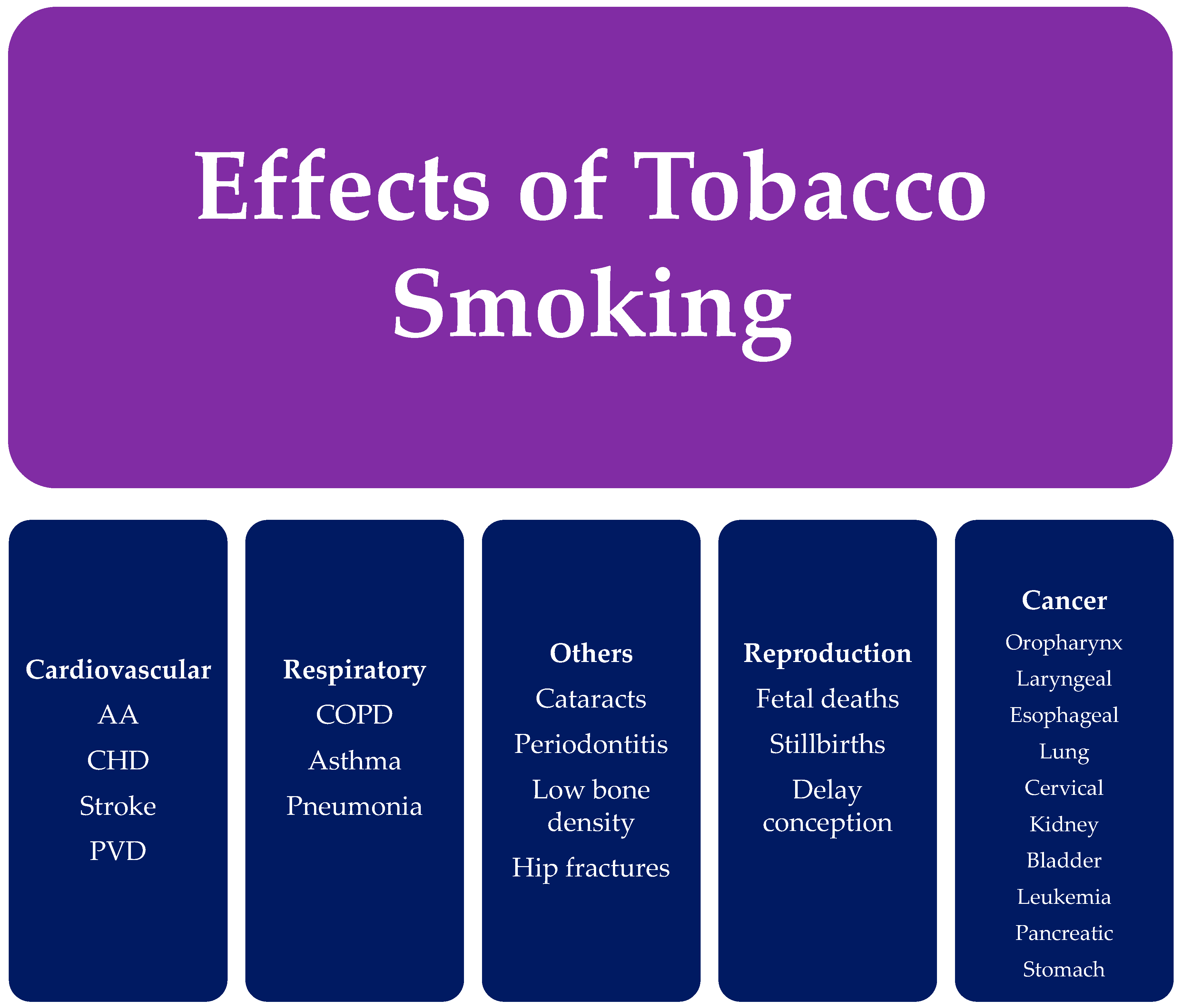
4. Health Effects of Cigarette (Tobacco) Smoking
4.1. Cancer
4.2. Cardiovascular Diseases
4.3. Respiratory Diseases
4.4. Reproductive Effects
4.5. Additional Effects
5. Non-Pharmacologic Treatment of Cigarette (Tobacco) Smoking
6. Pharmacologic Treatment of Cigarette (Tobacco) Smoking
6.1. Nicotine Replacement Therapy (NRT)
6.2. Bupropion Sustained-Release (SR)
6.3. Varenicline
6.4. Clonidine
6.5. Nortriptyline
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, S.S.; Neff, L.; Agaku, I.T.; Cox, S.; Day, H.R.; Holder-Hayes, E.; King, B.A. Tobacco product use among adults—United States, 2013–2014. Morb. Mortal. Wkly. Rep. 2016, 65, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009, 192, 29–60. [Google Scholar]
- Jamal, A.; King, B.A.; Neff, L.J.; Whitmill, J.; Babb, S.D.; Graffunder, C.M. Current cigarette smoking among adults—United States, 2005–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Monitoring Tobacco Use and Prevention Policies, World Health Organization: Geneva, Switzerland, 2017.
- World Health Organization. WHO Report on the Global Tobacco Epidemic: Raising Taxes on Tobacco, World Health Organization: Geneva, Switzerland, 2015; 52–53.
- Cooperman, N.A.; Bernstein, S.L.; Williams, J.M. Determining smoking cessation related information, motivation, and behavioral skills among opiate dependent smokers in methadone treatment. Subst. Use Misuse 2016, 50, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, L.; Meriwether, M.; Saucedo, C.; Reyes, R.; Cheng, C.; Clark, B.; Tipperman, D.; Schroeder, S.A. From the sidelines to the frontline: How the substance abuse and mental health services administration embraced smoking cessation. Am. J. Public Health 2014, 104, 796–802. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Preventing Tobacco Use among Youth and Young Adults A Report of the Surgeon General Executive Summary; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2012.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014; pp. 1–36.
- Singh, T.; Arrazola, R.A.; Neff, L.J.; Kennedy, S.M.; Holder-Hayes, E.; Jones, C.D. Tobacco use among middle and high school students—United States, 2011–2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 65, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Beard, E.; Kotz, D.; Michie, S.; West, R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: A cross-sectional population study. Addiction 2014, 109, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- D’Ruiz, C.D.; Graff, D.W.; Yan, X.S. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health 2015, 15, 991. [Google Scholar] [CrossRef] [PubMed]
- Callahan-Lyon, P. Electronic cigarettes: Human health effects. Tob. Control 2014. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hann, N.; Wilson, A.; Worrall-Carter, L. Electronic cigarettes: Patterns of use, health effects, use in smoking cessation and regulatory issues. Tob. Induc. Dis. 2014, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. E-Cigarette Use among Youth and Young Adults: A Report of the Surgeon General; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2016.
- Centers for Disease Control and Prevention Office of Smoking and Health. E-Cigarette Information; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2015.
- Protano, C.; Di Milia, L.M.; Orsi, G.B.; Vitali, M. Electronic cigarette: A threat or an opportunity for public health? State of the art and future perspectives. Clin. Ter. 2015, 166, 32–37. [Google Scholar] [PubMed]
- Nicotine|C10H14N2—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/89594#section=Top (accessed on 21 September 2017).
- Burns, D.M. Nicotine addiction. In Harrison’s Principles of Internal Medicine, 19th ed.; Kasper, D., Fauci, A., Hauser, S., Longo, D., Jameson, J.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Doering, P.L.; Li, R.M. Substance-related disorders II: Alcohol, nicotine, and caffeine. In Pharmacotherapy: A Pathophysiologic Approach, 9th ed.; DiPiro, J.T., Talbert, R.L., Yee, G.C., Matzke, G.R., Wells, B.G., Posey, L.M., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2014. [Google Scholar]
- Soghoian, S. Nicotine. In Goldfrank’s Toxicologic Emergencies, 10th ed.; Hoffman, R.S., Howland, M.A., Lewin, N.A., Nelson, L.S., Goldfrank, L.R., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- O’Brien, C.P. Drug addiction. In Goodman and Gilman’s: The Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Nicotine 2D Structure. Available online: https://pubchem.ncbi.nlm.nih.gov/image/imagefly.cgi?cid=89594&width=500&height=500 (accessed on 21 September 2017).
- Benowitz, N.L.; Brunetta, P.G. Smoking Hazards and Cessation, 5th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- United States Department of Health and Human Services. How Tobacco Smoke Causes Disease: A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2010.
- Rakel, R.E.; Houston, T. Nicotine Addiction. In Textbook of Family Medicine; Rakel, R.E., Rakel, D.P., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2011; pp. 1105–1122. [Google Scholar]
- Global Initiative for Chronic Obsructive Lung Disease (GOLD) 2017. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obsructive Lung Disease. 2017. Available online: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (accessed on 10 January 2017).
- Harris, K.K.; Zopey, M.; Friedman, T.C. Metabolic effects of smoking cessation. Nat. Rev. Endocrinol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.C.; Jaén, C.R.; Baker, T.B.; Bailey, W.C.; Benowitz, N.L.; Curry, S.J.; Dorfman, S.F.; Froelicher, E.S.; Goldstein, M.G.; Healton, C.G.; et al. Treating Tobacco Use and Dependence: 2008 Update; Clinical Practice Guideline; U.S. Department of Health and Human Services: Rockville, MD, USA, 2008.
- Fiore, M.C.; Baker, T.B. Treating smokers in the health care setting. N. Engl. J. Med. 2011, 365, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Niaura, R. Nonpharmacologic therapy for smoking cessation: Characteristics and efficacy of current approaches. Am. J. Med. 2008, 121, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Twyman, L.; Bonevski, B.; Paul, C.; Bryant, J. Perceived barriers to smoking cessation in selected vulnerable groups: A systematic review of the qualitative and quantitative literature. BMJ Open 2014, 4, e006414. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Rampes, H.; Liu, J.P.; Stead, L.F.; Campbell, J. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Hajek, P.; Stead, L.F. Aversive smoking for smoking cessation. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Barnes, J.; Dong, C.Y.; McRobbie, H.; Walker, N.; Mehta, M.; Stead, L.F. Hypnotherapy for smoking cessation. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Stead, L.F.; Lancaster, T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Nides, M. Update on pharmacologic options for smoking cessation treatment. Am. J. Med. 2008, 121, S20–S31. [Google Scholar] [CrossRef] [PubMed]
- Hays, J.T.; McFadden, D.D.; Ebbert, J.O. Pharmacologic agents for tobacco dependence treatment: 2011 update. Curr. Atheroscler. Rep. 2012, 14, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.J.; Filion, K.B.; Yavin, D.; Belisle, P.; Mottillo, S.; Joseph, L.; Gervais, A.; O’Loughlin, J.; Paradis, G.; Rinfret, S.; et al. Pharmacotherapies for smoking cessation: A meta-analysis of randomized controlled trials. CMAJ 2008, 179, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Stead, L.; Perera, R.; Bullen, C.; Mant, D.; Cahill, K.; Lancaster, T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Hajek, P.; West, R.; Foulds, J.; Nilsson, F.; Burrows, S.; Meadow, A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch. Intern. Med. 1999, 159, 2033–2038. [Google Scholar] [CrossRef] [PubMed]
- Hays, J.T.; Ebbert, J.O. Adverse effects and tolerability of medications for the treatment of tobacco use and dependence. Drugs 2010, 70, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Benowitz, N.L. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001, 24, 277–322. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Stead, L.; Cahill, K.; Lancaster, T. Antidepressants for smoking cessation. Cochrane Libr. 2014. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Information for Healthcare Professionals: Varenicline (Marketed as Chantix) and Bupropion (Marketed as Zyban, Wellbutrin, and GEnerics). Available online: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm (accessed on 5 July 2017).
- GlaxoSmithKline. Zyban (Bupropion Hydrochloride) [Prescribing Information], Research Triangle Park: Durham, NC, USA, 2017.
- Tonstad, S.; Farsang, C.; Klaene, G.; Lewis, K.; Manolis, A.; Perruchoud, A.P.; Silagy, C.; van Spiegel, P.I.; Astbury, C.; Hider, A.; et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: A multicentre, randomised study. Eur. Heart J. 2003, 24, 946–955. [Google Scholar] [CrossRef]
- Cahill, K.; Lindson-Hawley, N.; Thomas, K.; Fanshawe, T.; Lancaster, T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: Chantix (Varenicline) Drug Label Now Contains Updated Efficacy and Safety Information. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm264436.htm (accessed on 5 July 2017).
- U.S. Food and Drug Administration. FDA Drug Safety Communication: Chantix (Varenicline) May Increase the Risk of Certain Cardiovascular Adverse Events in Patients with Cardiovascular Disease. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm259161.htm (accessed on 5 July 2017).
- Pfizer Inc. Chantix (Varenicline) [Prescribing Information], Pfizer Inc.: New York, NY, USA, 2016.
- Gourlay, S.; Stead, L.; Benowitz, N. Clonidine for smoking cessation. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Hughes, J.R.; Stead, L.F.; Lancaster, T. Nortriptyline for smoking cessation: A review. Nicotine Tob. Res. 2005, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Truven Health Analytics. Red Book Online® System (Electronic Version), Truven Health Analytics: Greenwood Village, CO, USA, 2016.
- U.S. Department of Health and Human Services. Healthy People 2020: Tobacco Use. Available online: https://www.healthypeople.gov/2020/topics-objectives/topic/tobacco-use (accessed on 5 July 2017).
- Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2014; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014.
| Cause of Death | Total |
|---|---|
| Smoking-related cancers | 6,587,000 |
| Cardiovascular and metabolic diseases | 7,787,000 |
| Pulmonary diseases | 3,804,000 |
| Conditions related to pregnancy and birth | 108,000 |
| Residential fires | 86,000 |
| Lung cancers caused by exposure to secondhand smoke | 263,000 |
| Coronary heart disease caused by exposure to secondhand smoke | 2,194,000 |
| Total | 20,830,000 |
| Stages of Change | Description |
|---|---|
| Pre-contemplation | The patient is not yet ready to quit at this time or within six months |
| Contemplation | The patient is considering quitting at some point in the future, but has not yet taken any action towards quitting. |
| Preparation | Patient is planning to quit in the next 30 days |
| Action | Patient is in the process of quitting or has quit within the last six months. |
| Maintenance | The patient has quit smoking for at least three months. |
| Intervention | Description |
|---|---|
| Ask | Implement a system to ensure that all patients are asked their tobacco use status at every visit. |
| Advise | Urge every tobacco user to quit. Advice should be clear, strong, and personalized Clear—“I think it is important for you to quit smoking now and I can help you.” Strong—“As your clinician, I need you to know that quitting smoking is the most important thing you can do to protect your health now and in the future.” Personalized—“Continuing to smoke makes your asthma worse, and quitting may dramatically improve your health.” |
| Assess | Assess every tobacco user’s willingness to quit smoking. If the patient is willing to make a quit attempt, provide assistance. If the patient will participate in an intensive treatment, deliver or refer treatment. If the patient clearly states he or she is unwilling to quit provide motivational intervention. If the patient is a member of a special population (e.g., adolescent, pregnant smoker), provide information specific to that population. |
| Assist | Provide aid for the patient to quit. This includes:
|
| Arrange | Schedule follow-up contact, either in person or by telephone. Follow-up contact should occur soon after the quit date, preferably during the first week. A second follow-up contact is recommended within the first month. Schedule further follow-up contacts as indicated. |
| Intervention | Description |
|---|---|
| Relevance | Motivational information to a patient is more effective if it is personally relevant to a patient |
| Risk | The acute and long-term risks of smoking should be stressed. It is most effective if smoking can be tied to the patient’s current health or illnesses and the health of others |
| Rewards | Encourage the patient to identify potential benefits of smoking (e.g., Improved health, saving money, etc.) |
| Roadblocks | Ask the patient to identify barriers or impediments to quitting and provide treatment that address these barriers |
| Repetition | Repeat the motivational intervention each time an unmotivated smoker visits the clinic |
| Studies | Intervention | Description | Efficacy |
|---|---|---|---|
| Clinical Approaches | |||
| Niaura [31] | Self-help programs | Printed or electronic materials given to patients to increase motivation and provide advice to quit. | Generic materials: OR, 1.24 (95% confidence interval (CI): 1.07–1.45); Tailored materials: OR, 1.42 (1.26–1.61) (based on 11 trials with ≥6-month follow-up) |
| Telephone counseling | Quit hotlines or using a counselor to call patients. | OR, 1.56 (95% CI: 1.38–1.77) (based on 27 trials with ≥6-month follow-up) | |
| Cognitive-behavioral therapy Individual | Individual or group sessions that focus on addressing and changing thinking and behavior in smokers. | OR, 1.56 (95% CI: 1.32–1.84) (based on 21 trials with ≥6-mo follow-up) | |
| Cognitive-behavioral therapy Group | OR, 2.17 (95% CI: 1.37–3.45) (based on 55 trials with ≥6-month follow-up) | ||
| Healthcare provider interventions | Advice given to patients from clinicians during routine contact. | Meta-analysis of 37 studies with a mean sample size of 507 each, physician advice had the greatest impact on increasing cessation (p = 0.002) | |
| Exercise programs | Exercise based interventions | OR, 2.36 (95% CI: 0.97–5.70) (based on 1 trial with 12-month follow-up) | |
| Public Health Approaches | |||
| Niaura [31] | Community-level interventions | Include various approaches such as distribution of “quit kits”, support groups, smoke-free areas, and others. | COMMIT trial demonstrated modestly higher odds of quitting only in light smokers (less than 25 cigarettes/day) in an intervention community compared with a control community (OR, 1.17; p < 0.05) |
| Workplace interventions | Include various approaches such as seminars, online interventions, and others. | Meta-analysis of 19 studies demonstrated significantly improved odds of abstinence at 6 and 12 months, but not thereafter | |
| Multimedia interventions | Use differing multimedia such as internet, videos, to aid in cessation. | Large scale campaign in NY that used education, referrals, school-based programs, and poster contests resulted in an absolute decrease in smoking prevalence of 10% over the 5-year study period | |
| Public health policy [ 31] | Include smoking bans | Ban of all public smoking in Italy resulted in a 2.3% decrease in smoking prevalence <1 year. later | |
| Alternative Approaches | |||
| White, et al. [33] | Acupuncture | Involves penetration of the skin with needles to stimulate certain points on the body. | Meta-analysis of 33 randomized trials found no differences in long-term abstinence rates for acupuncture |
| Hajek, et al. [34] | Aversive therapy | Increasing the amount of smoking over time with the goal of inducing a sense of displeasure. | Meta-analysis of 25 randomized trials found insufficient evidence to support a clear dose-response relationship between aversive therapy and smoking cessation |
| Barnes, et al. [35] | Hypnosis | Creates unconscious change in patients undergoing hypnosis in the form of new thoughts or attitudes. | Systematic review of 11 randomized trials found insufficient data to support the use of hypnotherapy for smoking cessation |
| Generic Name | Brand Name(s) | Mechanism of Action | Common Adverse Effects | Dose |
|---|---|---|---|---|
| Nicotine gum | Nicorette, Equate, Top Care, others | Partially replace the nicotine formally obtained from tobacco, which aids smoking cessation by reducing the severity of withdrawal symptoms and cravings | Jaw pain, mouth, soreness, dyspepsia, hiccups | The 2-mg gum is for patients smoking less than 25 cigarettes/day; the 4 mg gum for patients smoking 25 or more cigarettes/day. Use at least 1 piece every 1 to 2 h for the 1st 6 weeks; the gum should be used for up to 12 weeks with no more than 24 pieces to be used per day |
| Nicotine lozenge | Sunmark, Top Care, others | See above | Mouth and throat, hiccups | 2 mg lozenge for patients who smoke their 1st cigarette more than 30 min after waking, and the 4 mg lozenge for patients who smoke their 1st cigarette within 30 min of waking. Generally, smokers should use at least 9 lozenges/day in the first 6 weeks; the lozenge should be used for up to 12 weeks, with no more than 20 lozenges/day |
| Nicotine patch | Nicoderm CQ, Equate, others | See above | Mild skin irritation at placement site | For those who smoke more than 10 cigarettes/day: 21 mg patch for 6–8 weeks, decrease 14 mg for 2–4 weeks, then 7 mg for 2–4 weeks. For less than 10 cigarettes/day: 14 mg for 6 weeks, decrease to 7 mg for 2–4 weeks. |
| Nicotine inhaler | Nicotrol | See above | Mouth and throat irritation, cough | A dose from consists 1 inhalation. Recommended dosage is 6–16 cartridges/day. Recommended duration of therapy is up to 6 months. |
| Nicotine nasal spray | Nicotrol NS | See above | Runny nose, throat and nasal irritation, cough | Spray 1–2 doses/h, increasing as needed for symptom relief. Minimum recommended treatment is 8 doses/day, with a maximum of 40 doses/day (5 doses/h). Each bottle contains approximately 100 doses. Recommended duration of therapy is 3–6 months |
| Bupropion SR | Zyban, Wellbutrin SR | Inhibitor of dopamine and norepinephrine reuptake, but its mechanism of action in smoking cessation is not well understood | Insomnia, dry mouth, headache, tremors, nausea, anxiety | Begin treatment 1–2 weeks. quit date. Begin with a dose of 150 mg every morning for 3 days, then 150 mg twice daily. Dosage should not exceed 300 mg/day. Dosing at 150 mg twice daily should continue for 7–12 weeks. For long-term therapy, consider use for up to 6 months post-quit |
| Varenicline | Chantix | Partial agonist specific for the neuronal nicotinic acetylcholine receptor subtype α4β2. | Nausea, insomnia, abnormal dreaming, headache | Start 1 week before the quit date at 0.5 mg once daily for 3 days, then 0.5 mg twice daily for 4 days, then 1 mg twice daily for 3 months approved for up to 6 months. Note: Patient should be instructed to quit smoking on day 8, when dosage is increased to 1 mg twice daily |
| Drug | Some Available Formulations | Usual Adult Maintenance a Dosage (Max Dose) | Cost b |
|---|---|---|---|
| Nicotinic Receptor Agonists | |||
| Nicotine polacrilex gum-eneric | 2, 4 mg/piece | 48 mg/day, 96 mg/day | $151.79–$205.99 |
| Nicorette Gum (GSK) | $173.91–$195.64 | ||
| Nicotine polacrilex lozenge-generic | 2, 4 mg/lozenge | 40 mg/day, 80 mg/day | $224.07 c |
| Nicorette Gum (GSK) | $232.43 c | ||
| Nicotine transdermal patch-generic | 7, 14, 21 mg/24 h patches | 1 patch/day d | $49.78 c,e |
| Nicoderm CQ (GSK) | $52.32 c,e | ||
| Nicotine nasal spray—Nicotrol NS (Pharmacia & Upjohn) | 200 sprays/10 mL bottle (0.5 mg/spray) | 1 dose (2 sprays) 40mg/day (max 5 doses/h) f | $333.69 g |
| Nicotine oral inhaler—Nicotrol (Pharmacia & Upjohn) | 10 mg cartridges | 16 cartridges/day | $317.80 h |
| Dopaminergic-Noradrenergic Reuptake Inhibitors | |||
| Bupropion SR-generic | 100, 150, 200 mg SR tabs j | 150 mg bid k | $27.00 |
| Wellbutrin SR (GSK) i | $377.00 | ||
| Zyban | $235.97 | ||
| Nicotinic Receptor Partial Agonist | |||
| Varenicline—Chantix (Pfizer) | 0.5, 1 mg tabs | 1 mg bid l | $337.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onor, I.O.; Stirling, D.L.; Williams, S.R.; Bediako, D.; Borghol, A.; Harris, M.B.; Darensburg, T.B.; Clay, S.D.; Okpechi, S.C.; Sarpong, D.F. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int. J. Environ. Res. Public Health 2017, 14, 1147. https://doi.org/10.3390/ijerph14101147
Onor IO, Stirling DL, Williams SR, Bediako D, Borghol A, Harris MB, Darensburg TB, Clay SD, Okpechi SC, Sarpong DF. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. International Journal of Environmental Research and Public Health. 2017; 14(10):1147. https://doi.org/10.3390/ijerph14101147
Chicago/Turabian StyleOnor, IfeanyiChukwu O., Daniel L. Stirling, Shandrika R. Williams, Daniel Bediako, Amne Borghol, Martha B. Harris, Tiernisha B. Darensburg, Sharde D. Clay, Samuel C. Okpechi, and Daniel F. Sarpong. 2017. "Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options" International Journal of Environmental Research and Public Health 14, no. 10: 1147. https://doi.org/10.3390/ijerph14101147
APA StyleOnor, I. O., Stirling, D. L., Williams, S. R., Bediako, D., Borghol, A., Harris, M. B., Darensburg, T. B., Clay, S. D., Okpechi, S. C., & Sarpong, D. F. (2017). Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. International Journal of Environmental Research and Public Health, 14(10), 1147. https://doi.org/10.3390/ijerph14101147







