Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Biochars
2.2. Characterization of the Produced Biochars
2.3. Metal Adsorption Experiments
3. Results
3.1. Elemental Compositions and pH of the Ginkgo-Leaf-Derived Biochars
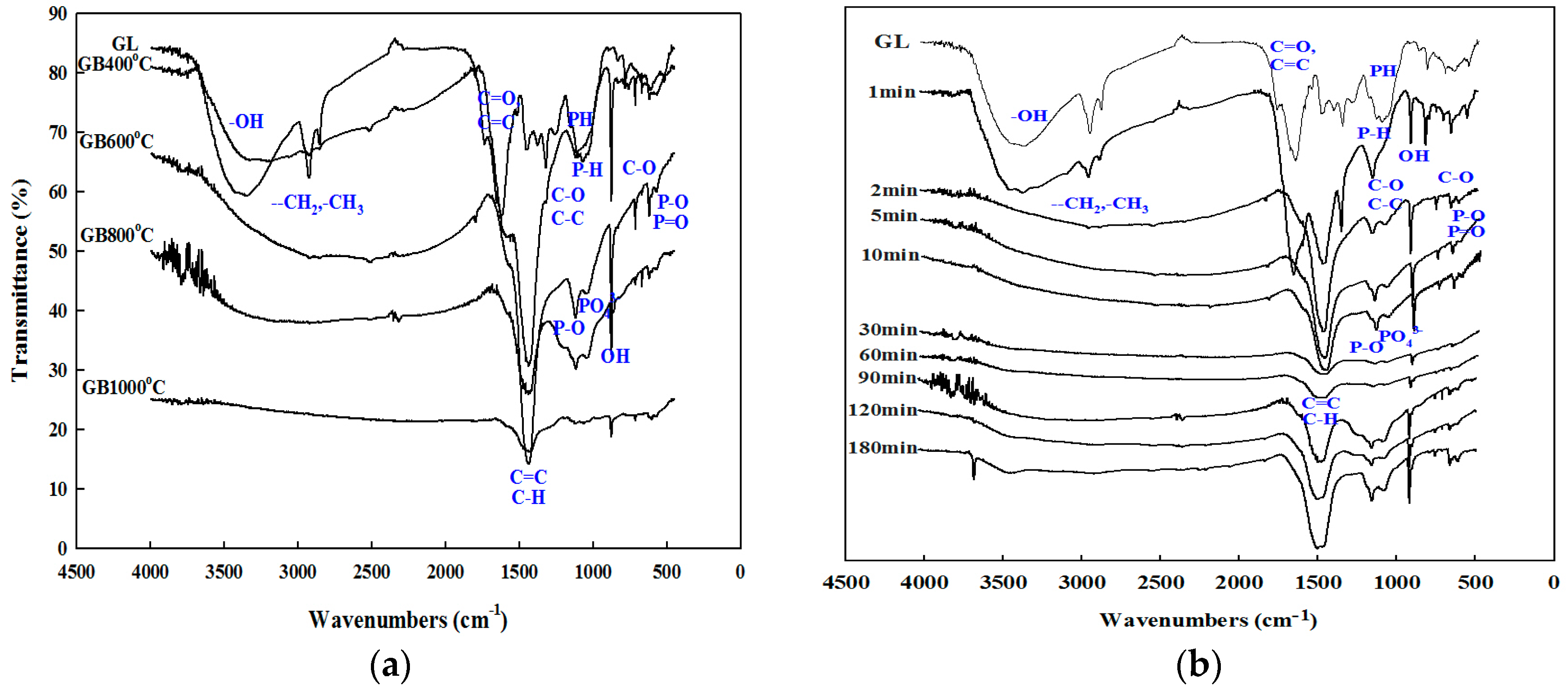
3.2. Functional Groups of the Ginkgo-Leaf-Derived Biochars
3.3. Lead(II) and Cu(II) Adsorption Characteristics of the Biochars
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Dodla, S.K.; Wang, J.J. Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 2016, 142, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, MA, USA, 2010; pp. 47–82. ISBN 978-0-12-381023-6. [Google Scholar]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.; Ok, Y.S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.W.; Seo, D.C.; Kang, S.W.; Lee, S.G.; Seo, Y.J.; Lim, B.J.; Heo, J.S.; Cho, J.S. Adsorption characteristics of heavy metals using sesame waste biochar. Korean J. Soil Sci. Fertil. 2013, 46, 8–15. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.J.; Li, J.Y.; Yuan, J.H.; Xu, R.K. Adsorption of Cu(II) by biochar generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.H.; Ahn, Y.; Chung, J.W. Comparison of heavy metal adsorption by peat moss and peat moss-derived biochar produced under different carbonization conditions. Water Air Soil Pollut. 2015, 226, 9. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Strawn, D.G.; Scheckel, K.G.; Novak, J.M.; Ahmedena, M.; Niandou, M.A.S. Macroscopic and molecular investigations of copper sorption by a steam-activated biochar. J. Environ. Qual. 2012, 41, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Shinogi, Y.; Yoshida, H.; Koizumi, T.; Yamaok, M.; Saito, T. Basic characteristics of low temperature carbon products from waste sludge. Adv. Environ. Res. 2003, 7, 661–665. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Singh, G.; Rana, D.; Matsuura, T.; Ramakrishna, S.; Narbaitz, R.M.; Tabe, S. Removal of disinfection byproducts from water by carbonized electrospun nanofibrous membranes. Sep. Purif. Technol. 2010, 74, 202–212. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, M.; Olesczuk, P. Characterization of biochars produced from residues from biogas production. J. Anal. Appl. Pyrolysis 2015, 115, 157–165. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S. Acid/base-treated activated carbons: Characterization of functional groups and metal adsorptive properties. Langmuir 2004, 20, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.A.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of pyrolysis temperature on physico-chemical properties of corn stover (Zea mays L.) biochar and feasibility for carbon capture and energy balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Krumins, J.; Klavins, M.; Seglins, V. Comparative study of peat composition by using FT-IR spectroscopy. Mater. Sci. Appl. Chem. 2012, 26, 106–114. [Google Scholar]
- Cao, X.D.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wily & Sons Ltd.: Chichester, UK, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Kennedy, L.J.; Vijaya, J.J.; Sekaran, G. Effect of two-stage process on the preparation and characterization of porous carbon composite from rice husk by phosphoric acid activation. Ind. Eng. Chem. Res. 2004, 43, 1832–1838. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta Part A 2016, 168, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, J.C.; Morera, M.T.; Mazkiaran, C.; Garrido, J.J. Competitive sorption of heavy metal by soils. Isotherms and fractional factorial experiments. Environ. Pollut. 1998, 101, 275–284. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Matsufuru, H.; Takaoka, M.; Tanida, H.; Sato, T. Impacts of chemical amendments and plant growth on lead speciation and enzyme activities in a shooting range soil: An X-ray absorption fine structure investigation. J. Environ. Qual. 2009, 38, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.A.; Zhang, P.; Hesterberg, D.; Chou, J.; Sayers, D.E. Formation of chloropyromorphite in a lead-contaminated soil smended with hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.; Marculescu, C.; Badea, A.; Dinca, C.; Apostol, T. Effect of Pyrolysis Conditions on Bio-Char Production from Biomass. In Proceedings of the 3rd WSEAS International Conference on Renewable Energy Sources, Tenerife, Canary Islands, Spain, 1–3 July 2009; pp. 239–241. [Google Scholar]
- Park, J.H.; Lee, S.J.; Lee, M.E.; Chung, J.W. Comparison of heavy metal immobilization in contaminated soils amended with peat moss and peat moss-derived biochar. Environ. Sci. Process. Impacts 2016, 18, 514–520. [Google Scholar] [CrossRef] [PubMed]
| Sample | Carbonization Temperature (°C) | Elemental Composition (%) | Atomic Ratio | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Ash | O/C | H/C | (O + N)/C | |||
| GL | 45.14 | 5.54 | 1.21 | 0.73 | 33.99 | 13.40 | 0.565 | 1.473 | 0.588 | 4.60 | |
| GB | 400 | 51.15 | 2.07 | 1.37 | 1.02 | 16.38 | 28.02 | 0.240 | 0.486 | 0.263 | 10.19 |
| 600 | 48.14 | 3.81 | 1.29 | 0.87 | 17.31 | 32.63 | 0.270 | 0.950 | 0.293 | 10.72 | |
| 800 | 42.48 | 0.47 | 1.65 | 1.85 | 17.96 | 35.60 | 0.317 | 0.133 | 0.350 | 12.43 | |
| 1000 | 43.34 | 0.33 | 0.78 | 2.19 | 6.81 | 46.56 | 0.118 | 0.091 | 0.133 | 12.90 | |
| Elemental Composition (%) | Carbonization Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | 30 | 60 | 90 | 120 | 180 | |
| C | 51.19 | 44.55 | 41.94 | 40.33 | 48.30 | 45.84 | 40.51 | 39.31 | 23.32 |
| H | 1.09 | 1.061 | 0.92 | 0.85 | 1.30 | 1.31 | 1.13 | 1.27 | 1.19 |
| N | 1.32 | 1.08 | 1.05 | 1.09 | 1.28 | 1.25 | 1.55 | 1.26 | 1.26 |
| S | 1.08 | 1.01 | 1.04 | 1.17 | 1.64 | 1.70 | 1.77 | 1.79 | 0.59 |
| O | 14.02 | 17.05 | 18.54 | 19.37 | 11.93 | 14.21 | 19.44 | 16.09 | 19.33 |
| Ash | 31.31 | 35.26 | 36.52 | 37.18 | 35.55 | 35.69 | 35.6 | 40.29 | 54.31 |
| Carbonization Temperature (°C) | Biochar Yield (%) | Equilibrium Adsorption Capacity (mg/g) | |
|---|---|---|---|
| Pb(II) | Cu(II) | ||
| Control (GL) | - | 24.81 ± 0.19 d | 11.71 ± 0.35 c |
| 400 | 34.63 ± 2.28 a | 51.13 ± 0.42 b | 14.28 ± 0.32 b |
| 600 | 29.47 ± 1.21 b | 56.53 ± 0.00 a | 9.88 ± 0.11 d |
| 800 | 26.90 ± 0.89 b | 57.55 ± 0.02 a | 18.22 ± 1.00 a |
| 1000 | 20.62 ± 3.88 c | 42.89 ± 2.76 c | 11.03 ± 0.72 c,d |
| Carbonization Time (min) | Biochar Yield (%) | Equilibrium Adsorption Capacity (mg/g) | |
|---|---|---|---|
| Pb(II) | Cu(II) | ||
| Control (GL) | - | 39.17 ± 0.74 f | 18.50 ± 0.71 b,c |
| 1 | 44.50 ± 0.37 a | 67.71 ± 1.30 d | 17.89 ± 1.09 c |
| 2 | 35.54 ± 0.46 b | 63.06 ± 3.53 e | 10.58 ± 1.52 e |
| 5 | 31.38 ± 0.65 c | 67.51 ± 0.61 d | 15.46 ± 1.99 d |
| 10 | 28.77 ± 0.44 d,e | 74.1 ± 0.31 c | 19.37 ± 0.57 b,c |
| 30 | 27.47 ± 0.12 e | 60.03 ± 1.89 e | 14.41 ± 0.75 d |
| 60 | 30.31 ± 0.16 c,d | 73.22 ± 1.86 c | 17.83 ± 0.18 c |
| 90 | 26.90 ± 0.89 e | 93.22 ± 1.63 a | 22.58 ± 0.20 a |
| 120 | 26.90 ± 0.31 e | 88.28 ± 0.26 b | 21.25 ± 0.64 b |
| 180 | 19.30 ± 2.96 f | 85.37 ± 0.52 b | 20.02 ± 0.28 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. Int. J. Environ. Res. Public Health 2017, 14, 1528. https://doi.org/10.3390/ijerph14121528
Lee M-E, Park JH, Chung JW. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. International Journal of Environmental Research and Public Health. 2017; 14(12):1528. https://doi.org/10.3390/ijerph14121528
Chicago/Turabian StyleLee, Myoung-Eun, Jin Hee Park, and Jae Woo Chung. 2017. "Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times" International Journal of Environmental Research and Public Health 14, no. 12: 1528. https://doi.org/10.3390/ijerph14121528
APA StyleLee, M.-E., Park, J. H., & Chung, J. W. (2017). Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. International Journal of Environmental Research and Public Health, 14(12), 1528. https://doi.org/10.3390/ijerph14121528





