1. Introduction
Gamma-aminobutyric acid (GABA) is present in both mammals and invertebrates and is an important inhibitory neurotransmitter [
1]. Among the different types of GABA receptors, the GABA receptor-chloride channel complex is an important target site for insecticides, such as lindane and fipronil [
2]. However, target site mutations have significantly reduced the utility of conventional GABAergic insecticides. Mutation A301S [
3,
4], which was originally described as A302S [
5] and studied in cultured neurons from
Drosophila melanogaster, confers ca. 100-fold and ca. 1000-fold resistance to picrotoxinin and lindane, respectively [
6]. Therefore, development of insecticides that work on novel binding sites of this receptor could help avoid cross resistance problems and contribute to effective control of pest insects.
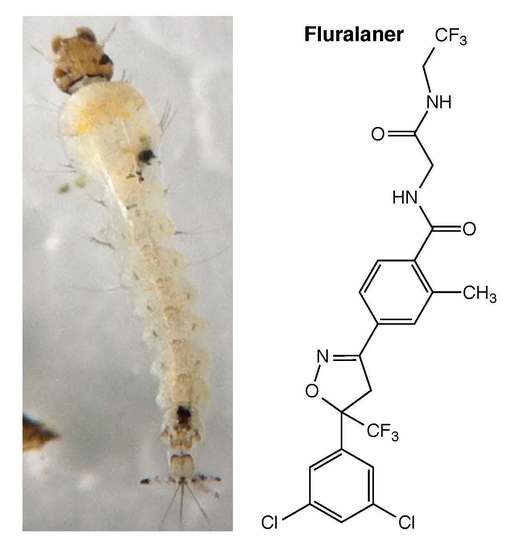
Isoxazolines and
meta-diamides have emerged as second-generation GABAergic compounds in the search for novel insecticides [
7]. Two representative isoxazolines, fluralaner (
Figure 1) and afoxolaner, were developed by scientists from Nissan Chemical Industries in Japan and DuPont in the U.S., respectively [
8,
9]. Both compounds have been approved by the U.S. Food and Drug Administration (FDA) to be commercialized as parasiticides. Fluralaner shows no cross-resistance in both in vivo and in vitro studies against various insects species compared to other classic GABAergic compounds [
10,
11], and demonstrates good selectivity towards mammals vs. invertebrate pests [
10,
12]. According to Gassel et al. [
11], fluralaner has toxicity greater than fipronil against six insect and parasite species, and blocks homo-oligomeric GABA receptors expressed in cell lines with high potency. The only available data on mosquito toxicity of fluralaner is that a low concentration of 1.2 ppt (parts per trillion, 10
−12 g/mL) killed more than 90% of first-instar
Ae. aegypti larvae, which is ca. 16,000-fold more potent than fipronil [
11]. The goal of the present study is to investigate fluralaner toxicity on
Ae. aegypti and
An. gambiae via different exposure routes, and its activity on native GABA receptor responses of CNS preparations of susceptible and resistant
D. melanogaster strains.
2. Materials and Methods
2.1. Chemicals
Samples of dieldrin and
l-aspartic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Fipronil (
Figure 1) was donated by Rhône-Poulenc Ag Co. (now Bayer CropScience, Research Triangle Park, NC, USA), and Triton X-100 was acquired from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Diethyl maleate (DEM) was purchased from Sigma-Aldrich Chemical Co., and piperonyl butoxide (PBO) and
S,S,S-tributyl phosphorotrithioate (DEF) were obtained from Chem Service Inc. (West Chester, PA, USA). Rapeseed-oil methyl ester (RME) was purchased from UCY Energy (Alfter, Germany), and silicon oil was acquired from Dow Corning Co. (Auburn, MI, USA). Ethanol, acetone and dimethyl sulfoxide (DMSO) used as solvents were obtained from Sigma-Aldrich Chemical Co.
Fluralaner was extracted and purified from a commercial canine formulation of BRAVECTO™ Chews (for large dogs, 44–88 lbs, each containing 1 g of fluralaner), manufactured by Merck Animal Health. Isolation of the compound was performed with a Teledyne Isco flash chromatography system (Lincoln, NE, USA) using hexanes/ethyl acetate as eluent system. Solvents, hexanes and ethyl acetate were obtained from Acros Organics (Morris Plains, NJ, USA). Melting point was determined on a hot-stage apparatus and is uncorrected. Nuclear magnetic resonance (NMR) analyses were performed at the Nucleic Magnetic Resonance Facility of the University of Florida. NMR spectra were recorded in CDCl3 with tetramethylsilane as the internal standard for 1H (500 MHz) and CDCl3 as the internal standard for 13C (125 MHz).
One half of a Bravecto Chew (3.70 g) was placed in a 250-mL separatory funnel, water (80 mL) was added and the funnel was shaken well to obtain a homogenous suspension. Then, ethyl acetate (80 mL × 3) was used for extraction. Organic phases were combined, washed with aq. Na2CO3 solution, aq. 1 N HCl solution, brine and then dried over anhydrous sodium sulfate. Any remaining solvent was distilled off under reduced pressure and the resultant residue was purified by flash chromatography using hexanes/ethyl acetate nonlinear gradient to obtain 0.48 g of the target product 4-[(5RS)-5-(3,5-dichlorophenyl)-4,5-dihydro-5-(trifluoromethyl)-1,2-oxazol-3-yl]-N-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]-o-toluamide. Product identity and purity (99%) were confirmed with thin layer chromatography (TLC) and NMR analysis. It was a colorless solid after recrystallization from ethanol; m.p. 171.0–172.0 °C; 1H-NMR (CDCl3) δ 7.54–7.41 (m, 6H), 7.29 (t, J = 6.5 Hz, 1H), 6.92 (t, J = 6.9 Hz, 1H), 4.21 (d, J = 5.1 Hz, 2H), 4.10–4.06 (m, 1H), 3.97–3.88 (m, 2H), 3.72–3.68 (m, 1H), 2.44 (s, 3H). 13C-NMR (CDCl3) δ 169.8, 169.4, 155.4, 138.8, 137.4, 137.1, 135.6, 129.8, 129.5, 129.4, 127.7, 123.4 (q, J = 278.9 Hz), 123.7 (q, J = 285.4 Hz), 87.3 (q, J = 30.5 Hz), 43.9, 43.6, 34.9 (q, J = 40.7 Hz), 19.7.
2.2. Insects
Fourth-instar
Ae. aegypti larvae were kindly provided by the Center for Medical, Agricultural & Veterinary Entomology (CMAVE), U.S. Department of Agriculture-Agriculture Research Service, Gainesville, FL, USA. The larvae were fed a mixture of ground liver and yeast, maintained under 75% relative humidity and 28 °C, with a 12 h:12 h dark:light cycle, and reared to adulthood for bioassays. Eggs of
An. gambiae were provided by BEI Resources under the CDC-MR4 program. The emerged larvae were fed with fish flakes (Tetra, Blacksburg, VA, USA), maintained under 75% relative humidity and 28 °C, with a 12 h:12 h dark:light cycle, and reared to adulthood for bioassays. Bioassays were performed on susceptible G3 (MRA-112) strain [
13].
Susceptible (Oregon-R) and cyclodiene-resistant (rdl-1675) strains of D. melanogaster were used in bioassays and electrophysiology experiments. The Oregon-R strain was originally provided by Dr. Doug Knipple from Cornell University, Ithaca, NY, USA, and maintained in culture at the University of Florida since 2009. The rdl-1675 strain was purchased from the Bloomington Drosophila Stock Center at Indiana University, Bloomington, IN, USA. Both strains were reared at 21 °C and provided with artificial media purchased from Carolina Biological Supply, Burlington, NC, USA.
2.3. Larval Mosquito Bioassays
Compounds were dissolved in ethyl alcohol, followed by serial dilution to generate an appropriate number of concentrations. A 5 µL aliquot (or more depending on compound solubility) of this solution was added to 5 mL of tap water containing 10 fourth-instar larvae of each treatment group. Tap water treated with the corresponding amount of ethanol was used as the control group. Larvae were held at room temperature (21 °C) and observed periodically for behavioral effects, such as convulsions and paralysis, for the first 4 h after treatment. Larval mortality was recorded after 24 h, 48 h and 72 h, or until toxicity reached a stable plateau. In these assays, the plateau was defined as the treatment day where toxicity was not significantly different from the value observed 2 days later. Larvae were fed during the experiment with a few grains of ground fish flakes added to the petri dish every day. A Triton X-100 solution of 10 ppm was also tested to see whether it would increase the toxicity of fluralaner.
Paralytic activity of compounds to headless larvae was assessed as described by Islam and Bloomquist [
14], with slight modification. Heads of fourth-instar larvae were detached with forceps and treated as described above, except in physiological saline instead of tap water. The saline was composed of (mM): NaCl (154), KCl (2.7), CaCl
2 (1.4), HEPES (4) at pH 7.2. Mosquito saline treated with 0.5% ethanol and 100 ppm
l-aspartic acid were used as negative and positive controls, respectively. An insect pin was used to probe larvae every hour to observe swimming behavioral responses. Compared to the control group, larvae showing irregular behaviors such as consistent convulsion, twitching or only slight movement were counted as paralyzed. Each concentration was repeated on at least three different batches of mosquitoes. LC
50 (lethal concentration for 50% mortality) and PC
50 (concentration for 50% paralysis) values were calculated as described in the statistics section.
2.4. Adult Mosquito Bioassays
For topical toxicity assays [
15], 5–7 days old adult female mosquitoes (non-blood fed,
n = 10) were chilled on ice for 2 min, during which time 0.2 µL of insecticide solution (in ethanol) was applied to the thorax by a hand-held microdispenser (Hamilton, Reno, NV, USA). A solvent-only treatment was included in each experiment as a negative control. Following treatment, mosquitoes were transferred to paper cups covered with netting. A 10% sugar solution in tap water was supplied via a cotton ball placed on the netting and changed every day. Paper cups were held at room temperature (21 °C), and the mosquitoes were observed for behavioral effects such as convulsion or paralysis and the onset of toxicity for the first 4 h. To test for synergistic effects, synergists were applied topically to the abdomen of mosquitoes 4 h before treatments (PBO, 500 ng per mosquito; DEF, 200 ng per mosquito; or DEM, 1 µg per mosquito) at amounts that generally produced little or no mortality. Mortality was recorded after 24 h, 48 h, and 72 h, or until toxicity reached a stable plateau. Each dose was repeated on at least three different batches of mosquitoes. The LD
50 (lethal dose for 50% mortality) without synergist and LD
50 with synergist were calculated, as described in the statistics section. The synergist ratio was determined by the equation: LD
50 of insecticide alone/LD
50 of insecticide + synergist.
Mosquito injection bioassays [
16] were performed under a microscope with a manual microsyringe pump (World Precision Instruments, Sarasota, FL, USA) and a fine glass pipette (TW100-4, World Precision Instruments) fabricated by a pipette puller (Sutter Instrument, Novato, CA, USA), and broken at the tip (ca. 20 µm). Compound solutions were prepared in mosquito saline (described in headless larvae assays) with 5% ethanol as a vehicle. A 5% ethanol solution in saline was used as control. Non-blood fed female mosquitoes (5–7 days post-emergence,
n = 10) were chilled on ice and injected with 0.2 µL compound solution into the side of their thorax. Treated mosquitoes were then placed into paper cups and maintained as described above for topical assays. Each dose series was repeated on at least three different batches of mosquitoes, with LD
50 values calculated as described in the statistics section.
For feeding assays [
17], female mosquitoes (5–7 days of age and non-blood fed) were starved for 6 h and chilled on ice for 2 min, then transferred to glass test tubes (
n = 10). Compound solutions were prepared in 10% sugar water with 0.5% ethanol used as vehicle. The control group was assigned 10% sugar water with 0.5% ethanol alone. Cotton balls were treated with 1 mL of prepared compound solution and inserted at the top of the test tubes. Test tubes were maintained at room temperature (21 °C), with the cotton ball changed and solution reapplied every day. To assess synergist effects on feeding assays, synergists (compounds and doses as given above) were topically applied 4 h before treatment. Mortality data was recorded after 24 h, 48 h, and 72 h, or until toxicity reached a stable plateau, with each experiment repeated at least three times. LC
50 values were calculated as described in the statistics section.
A contact filter paper toxicity assay was conducted according to WHO protocol [
18]. Adult female mosquitoes (
n = 10) were 5–7 days of age and non-blood fed at the time of experimentation. Serial dilutions of each insecticide dissolved in ethanol were prepared prior to treatment and 2 mL of each concentration was applied to a 180 cm
2 (12 cm × 15 cm) filter paper (Chromatography Paper, Fisher Scientific Pittsburgh, PA, USA). Papers were left to dry for 24 h prior to use. Mosquitoes were chilled for 2 min on ice, after which they were transferred to a WHO cylindrical plastic holding chamber and held for 1 h to acclimatize. The mosquitoes were then gently transferred into the treatment chamber, which contained the treated paper, and exposed for 1 h in the vertical tube. After the 1-h exposure time, the mosquitoes were transferred to the holding chamber as described above, with 10% sugar solution supplied on the netting and changed every day. Holding chambers were left at room temperature (21 °C) after the treatment, and mortality was recorded after 24 h, 48 h, and 72 h, or until toxicity reached a stable plateau. Each experiment was repeated on at least three different batches of mosquitoes. LC
50 values with and without synergist, as well as synergist ratios were calculated, as described in the statistics section.
Additional surface contact assays were conducted in borosilicate glass test tubes (20 mm × 150 mm) with an inner surface area of ca. 85 cm2 (Fisher Brand 14-961-33). Compounds were dissolved in acetone and 250 µL was added to each tube, with 250 µL of acetone as the control group. The test tube was carefully rotated to allow the acetone to evaporate and to distribute the compound evenly inside the tube. Adult female mosquitoes (n = 10) 5–7 days from emergence (non-blood fed) were chilled on ice for 2 min and transferred into compound-coated tubes. To ensure parallel comparison to WHO paper assay, in another set of experiments mosquitoes were transferred to clean tubes after 1-h exposure to insecticide-coated glass tubes. A cotton ball with 10% sugar water solution was used to seal test tubes and changed every day to ensure food supply for mosquitoes. Test tubes were maintained at room temperature (21 °C) and mortality data was collected after 24 h, 48 h, and 72 h, or until toxicity reached a stable plateau. Each experiment was repeated on at least three different batches of mosquitoes. LC50 values were calculated as described in the statistics section.
2.5. Adult D. melanogaster Bioassays
Adult females (n = 10) from both strains (CS-OR and rdl-1675) that were 5–7 days of age were tested in feeding assays and glass contact assays, and the resistance ratio (LC50 rdl-1675/LC50 CS-OR) was assessed via both routes of exposure. Procedures for both assays were the same as for the mosquito bioassays, except that D. melanogaster was anesthetized by a constant flow of CO2 for several seconds.
2.6. Electrophysiological Recording on D. melanogaster Larval CNS
Electrophysiological recordings from
D. melanogaster third-instar larval CNS were performed as described previously [
19], with slight modification. The CNS was dissected in physiological saline containing (mM) NaCl (157), KCl (3), CaCl
2 (2), HEPES (4), at pH 7.2, and either left intact or transected posterior to the cerebral lobes to eliminate the blood-brain barrier (BBB) and facilitate penetration of chemicals into the central synapses. A recording suction electrode was pulled with a pipette puller (Sutter Instrument, Novato, CA, USA) and broken at the tip (ca. 40 µm) by fine forceps before being filled with saline. Several peripheral nerve trunks were drawn into the electrode to record nerve activity descending from the CNS.
Electrical signals were amplified, digitized, and transmitted to the analysis software, wherein spikes were converted to a rate (spikes/s or Hz) by a PowerLab analog to digital converter hardware and LabChart 7 software (ADInstruments, Colorado Springs, CO, USA). To eliminate background noise, spikes were only tallied if they exceeded a fixed threshold, which was set when no peripheral nerves were attached to the suction electrode. After baseline frequency was established, 1 mM GABA was added to the saline bath to inhibit nerve activity, and allowed to incubate for 5 min (
Figure 2).
Experimental compounds (e.g., fluralaner) were then added to the saline bath (1 µL of DMSO solution in a 1 mL bath volume) and mixed by gentle pipetting to assess their ability to reverse the inhibitory effect of GABA. The baseline (pre-treatment) and GABA inhibited nerve firing rates were each averaged over a 3 min period. The drug-induced nerve firing rate was averaged over 3 min periods after treatment (
Figure 2). Fipronil and dieldrin at a concentration of 10 µM were used as positive controls. If, after GABA treatment, the nerve firing rate recovered to at least 25% of the original pretreatment level, the toxicant was considered to have induced a positive response.
Drug effects on larval CNS with intact BBB also were assessed, with experimental compounds added to the bath after establishing the baseline frequency, and each drug concentration was replicated 3–9 times. Fipronil at 10 µM was used as positive control. Recording data obtained within 36 min after addition of experimental compounds were included in the analysis.
2.7. Test of GABAergic Compounds on Mammalian GABAA Receptors
Effects of fluralaner, fipronil, dieldrin, and picrotoxin were tested on GABA α1β3γ2 ion channels expressed in HEK293 cells by ChanTest Corporation, Cleveland, OH, USA. Briefly, HEK293 cells were transfected with cDNA of GABA α1β3γ2 ion channels and maintained in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 with addition of 10% fetal bovine serum, 100 U/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, and 500 μg/mL G418. Extracellular buffer containing (mM): NaCl (137), KCl (4), CaCl2 (1.8), MgCl2 (1), HEPES (10), Glucose (10), at pH 7.4 was loaded into planar patch clamp (PPC) plate wells (11 µL per well). Cell suspension was then pipetted into the intracellular compartment (9 µL per well) of the PPC planar electrode. With whole-cell recording, effects of compounds on transfected HEK293 cells were detected in agonist mode (application of test compounds only) and in antagonist mode (application of GABA with test compound), with solution added 10 µL/s for 2 s. GABA and picrotoxin were used as agonist positive control and antagonist positive control, respectively.
The agonist effect of the test compounds and GABA (positive control) was calculated as: % activation = (ITA/IMax) × 100%, in which ITA was the compound-induced current at various concentrations, and IMax was the mean current induced with 300 μM GABA. Inhibitory effect of the test compounds and picrotoxin on the channel was calculated as: % Inhibition = (ITA/IEC80) × 100%, where ITA was the GABA EC80-induced current in the presence of various concentrations of the test compound and IEC80 was the mean current elicited with GABA EC80. GABA EC80 values were selected based on ChanTest historical data: 60 μM for α1β3γ2 GABA ion channels.
Inhibitory concentration-response data were fitted to an equation of the form: % Inhibition = % VC + {(% PC − % VC)/[1 + ([Test]/IC50)N]}, where [Test] was the concentration of the compound, IC50 was the concentration of the compound producing 50% inhibition, N was the Hill coefficient, % VC was the mean current at the passive control (picrotoxin EC50), % VC was the mean current at the vehicle control (GABA EC50) and % inhibition was the percentage of ion channel current inhibited at each concentration of the test compound. Nonlinear least squares fits were solved with the XLfit add-in for Excel software (Microsoft, Redmond, WA, USA).
2.8. Statistical Analysis
Control mortality was corrected by Abbott’s formula and LC50, LD50, and PC50 values were calculated by SAS software (Proc Probit, SAS 9.4, SAS Institute Inc., Cary, NC, USA). EC50 and IC50 values, and concentration-response curves of drugs in Drosophila larval CNS recordings were obtained by nonlinear regression to a four-parameter logistic equation in GraphPad Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA). The two-tailed Student’s t-test, one-way ANOVA with Bonferroni or Tukey’s post-test used to analyze results between treatment group means, with a p < 0.05 considered to be statistically significant.
4. Discussion
Gassel et al. [
11] performed toxicity comparisons of fluralaner with other commercialized compounds, including fipronil. On
Ctenocephalides felis (cat flea),
Ae. aegypti,
Lucilia cuprina Meigen (sheep blowfly), and
Stomoxys calcitrans Linnaeus (stable fly), fluralaner outperformed dieldrin and imidacloprid, as well as deltamethrin, except on
S. calcitrans. Ozoe et al. [
10] also reported that fipronil outperformed fluralaner on
C. felis by a factor of five in dry film contact assays. For mosquitoes, Gassel et al. [
11] reported a single finding that fluralaner (48 h LC
90 value = 1.2 ppt) was ca. 16,000-fold more potent than fipronil on
Ae. aegypti first-instar larvae (48 h LC
90 value = 20 ppb). In the present study, fluralaner gave a 48 h LC
90 value of 2.2 ppb on fourth-instar
Ae. aegypti larvae, differing from the data on first-instar larvae by over 1800-fold. However, fipronil showed a 48-h LC
90 value of 11 ppb, similar to toxicity observed by Gassel et al. [
11] for first instars (20 ppb for >90% mortality). In addition, on fourth-instar
Ae. aegypti larvae, fipronil was 10- to 13-fold less potent than fluralaner. These data suggest that fluralaner might be an excellent larvicide, although the life stage of the mosquito larvae has a large impact on chemical sensitivity. On adult
Ae. aegypti, fipronil had higher toxicity than fluralaner in topical (7- to 21-fold), feeding (ca. 100-fold), and glass contact assays (8-fold at 48 h). These findings, plus the greater speed of action, suggest fipronil would be a better overall adult mosquitocide, at least in the absence of resistance.
Bioassays and CNS recordings provide some insight into the slow toxicity of fluralaner. It took 3 days for fluralaner toxicity to plateau, and its toxicity was not enhanced much by injection (about two-fold compared to topical application). In contrast, the LD
50 value by injection can be reduced by more than 10-fold compared to topical treatments for carbamates such as propoxur [
16], another compound that must reach central synapses to exert its effects. Cuticle thickness is known to have a significant and positive correlation with the time to knock down by permethrin, suggesting that thicker cuticle led to a slower rate of insecticide penetration [
20]. For larval assays of
Ae. aegypti, the concentration leading to 50% paralysis on intact larvae was 10-fold higher than that for headless larvae. Thus, the cuticle of fourth-instar
Ae. aegypti proved to be a more important factor influencing fluralaner toxicity than in adults. The large size (molecular weight = 556) and high lipophilicity (log
p = 5.0) could influence fluralaner penetration of barriers and contribute to its slowly developing toxicity. These barriers would include the blood-brain-barrier, and although there was no significant difference between speed of nerve discharge block in severed vs. intact
D. melanogaster larval CNS, the blood brain barrier in adult mosquitoes may have a different permeability to fluralaner.
At present, there is no published information on the metabolism of fluralaner in insects. A decrease in toxicity was observed with DEM in adult sugar feeding assays (
Table 5), but the overall effect was small, as was the potentiation of toxicity by PBO. Both findings argue against significant glutathione-
S-transferase and P450 monooxygenase metabolism. DEF is a potent carboxylesterase inhibitor [
21] and in this study had the most significant positive synergist ratios in feeding assays with
Ae. aegypti adults. However, little synergism was noted in topical applications of fluralaner, suggesting that the factor responsible resides in the alimentary system. No ester linkage is present in the fluralaner molecule, but it does have two adjacent amide groups. Carboxylamidases have been identified in different insect species, including
Lepidoptera,
Orthoptera, and
Dictyoptera, which can use
p-nitroacetanilide as a model substrate, and were found most abundantly in the midgut [
22,
23]. The only purified carboxylamidase studied from insects was insensitive to DEF [
22]; however, a carboxylamidase might be present in adult
Ae. aegypti that metabolizes fluralaner in a DEF-sensitive fashion. Alternatively, DEF might have other mechanisms for potentiating the oral toxicity of fluralaner. All these possibilities await further investigation.
Fluralaner showed no target site cross resistance with other GABA receptor-directed compounds. Ozoe et al. [
10] reported fluralaner to be equally effective in topical assays on dieldrin-resistant and susceptible strains of houseflies, with LD
50 values of 1.01 ng/mg and 0.85 ng/mg, respectively. These values were similar on a per mg insect weight basis to the topical toxicity found for fluralaner on
Ae. aegypti adults (1.3 ng/mg at 24 h). Additionally, Asahi et al. [
24] showed that fluralaner had similar potent toxicity on fipronil-resistant and susceptible strains of
Laodelphax striatellus Fallén, whereas fipronil showed a resistance ratio of about 1700. These findings are in agreement with results of both feeding and glass contact assays on dieldrin-resistant and susceptible strains of
D. melanogaster reported here, where fluralaner showed similar activity at all recorded time points.
On homo-oligomeric RDL (
resistant to
die
ldrin) GABA receptors, fluralaner showed no cross-resistance to classical GABA non-competitive antagonists. Gassel et al. [
11] reported fluralaner to be a potent blocker of dieldrin-resistant
D. melanogaster and
C. felis RDL recombinant, homomultimeric receptors in cell-based fluorescence dye assays, with IC
50 values as low as 2.8 nM and 1.7 nM, respectively. In two-electrode voltage clamp (TEVC) recordings of oocytes expressing
M. domestica RDL receptors, fluralaner had similar IC
50 values of 2.8 nM on
rdl-type receptors with A299S mutation and 5.3 nM on wild-type receptors [
10]. Fluralaner also had an IC
50 value of 12 nM on fipronil-resistant RDL receptors from a plant-feeding mite,
Tetranychus urticae Koch, in TEVC experiments, whereas 30 µM of fipronil only blocked 27% of GABA-induced current [
24]. In the present study, fluralaner was tested on native
D. melanogaster GABA receptors, in situ, instead of heterologously expressed RDL receptors. In line with data on expressed RDL receptors, fluralaner showed similar potent activity on native dieldrin-resistant and -susceptible GABA receptors of
D. melanogaster larval CNS, with EC
50 values of 0.29 µM and 0.34 µM, respectively. The lower potency in CNS preparations can be attributed to the need for penetration into the neuropile, as well as any differences between native and recombinant homomultimeric receptors. These results further document that fluralaner has potent activity on insect strains that are resistant to classical GABAergic compounds.
Gassel et al. [
11] also reported 18-fold resistance to fipronil in
D. melanogaster homo-oligomeric RDL GABA receptors (Ser isoform). In the present study, no resistance to fipronil was observed in RDL larval CNS preparations, at least when tested at 10 µM (
Figure 8). We would note that this concentration is over 10-fold greater than the IC
50 for blocking the Ala isoform in homo-oligomeric receptors, reported to be 663 nM [
11]. So, the resistance may be largely circumvented at this concentration.
Good selectivity of fluralaner between mammals and invertebrates also has been demonstrated in both in vivo and in vitro studies. Currently commercialized as a parasiticide, fluralaner was reported to be safe to dogs at or above recommended treatments [
25,
26]. In radioligand binding studies on rat brain membranes, 10 μM of fluralaner showed around 40% inhibition of radiolabeled 4-ethynyl-4-
N-propylbicycloorthobenzoate (EBOB) binding, which is more than 2000-fold less sensitive than its binding to housefly GABA receptors [
10,
12]. Additionally, fluralaner had low activities on either recombinant β
3 homopentamers or α
1β
2γ
2 heteropentamers [
10,
12]. In the present study, mammalian GABA
A α
1β
3γ
2 receptors were tested against fluralaner and fipronil. In line with previous reports, the IC
50 value of fluralaner was higher than 30 μM. Moreover, the IC
50 value of fipronil was 4.9 μM, suggesting fluralaner has a lower toxicity to mammals than fipronil.
The mode of action and toxicology of isoxazolines such as fluralaner are similar to another new insecticide class, the
meta-diamides, which also work on invertebrate GABA receptors. According to Nakao et al. [
27],
meta-diamide
7 has potent activity on three mutant GABA receptors that are resistant to GABA receptor-directed non-competitive antagonists. Additionally, mutations G336M in M3, I277F and L281C in M1 reduce the activity of fluralaner on RDL GABA receptors, while having only a small impact on the activity of fipronil. Further molecular modeling suggests that
meta-diamide binds to T9’ to S15’ region in M2, close to the avermectin target site and different from the classical convulsant site [
28]. Homology modeling also suggests that fluralaner might share the same binding site as
meta-diamide
7 [
28]. According to most recent Mode of Action Classification Scheme from the Insecticide Resistance Action Committee (IRAC) [
2], GABAergic insecticides have been categorized into Groups 2A (cyclodiene and organochlorines, i.e., chlordane), 2B (phenylpyrazoles, i.e., fipronil), while glutamate-gated chloride channel activators, avermectins and milbemycins, are in Group 6. As of this writing, isoxazolines and
meta-diamides have not received a category from IRAC, but these compounds will probably be assigned to a new category because of their novel actions on RDL GABA receptors.
5. Conclusions
In this study, the toxicity of fluralaner against Ae. aegypti, An. gambiae, and D. melanogaster was assessed in various exposure routes. Compared to fipronil, fluralaner had a more slowly developing toxicity, and generally a 7- to 100-fold lower potency in adult bioassays. These findings suggest that fipronil would be a better overall mosquitocide than fluralaner in the absence of resistance. The data also imply that the moderate potency, low contact toxicity, and slow action of fluralaner might preclude its use as a mosquitocide for vector control, despite its favorable mammalian selectivity and lack of cross resistance in rdl carrying insects.
For larval assays on Ae. aegypti, the concentration leading to 50% paralysis of intact larvae was 10-fold higher than that on headless larvae, so the cuticle seems to be an important factor influencing fluralaner toxicity. Compared to fluralaner, fipronil was 10- to 13-fold less potent in larval assays, which differed from the results seen in adult mosquito bioassays.
In synergism assays, DEF was found to increase fluralaner toxicity by 3.8–8 fold in feeding assays on each day of exposure, with other synergists less active. This finding suggests that carboxylamidases might be involved in the metabolism of fluralaner in mosquitoes.
This study provides additional evidence for selectivity and lack of cross resistance of fluralaner. It was tested on mammalian GABAA α1β3γ2 receptors, which gave an IC50 value larger than 30 µM. Additionally, feeding and glass contact assays of fluralaner against susceptible and rdl strains of D. melanogaster showed similar activity, consistent with the equal sensitivity of larval CNS recordings towards fluralaner in both strains.