Volatile Organic Compounds in Anatomical Pathology Wards: Comparative and Qualitative Assessment of Indoor Airborne Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. VOC Sampling
2.3. VOC Analyses
2.4. Statistical Methods
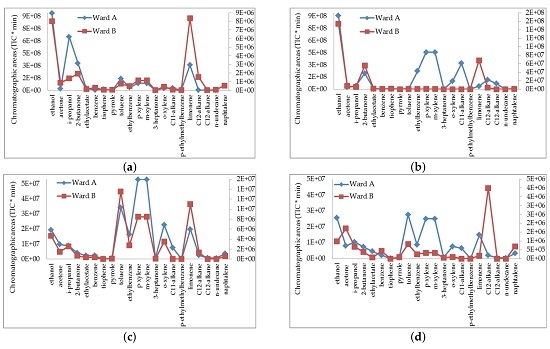
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Bessonneau, V.; Mosqueron, L.; le Berrubé, A.; Mukensturm, G.I.; Buffet-Bataillon, S.; Gangneux, J.P.; Thomas, O. VOC Contamination in Hospital, from Stationary Sampling of a Large Panel of Compounds, in View of Healthcare Workers and Patients Exposure Assessment. PLoS ONE 2013, 8, e55535. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Human: Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol; World Health Organization (WHO): Lyon, France, 2006; Volume 88, pp. 37–325. [Google Scholar]
- World Health Organization Regional Office for Europe Copenhagen. Air Quality Guidelines for Europe, 2nd ed.; European Series No. 91; WHO: Copenhagen, Denmark, 2000. [Google Scholar]
- Orsière, T.; Sari-Minodier, I.; Iarmarcovai, G.; Botta, A. Genotoxic risk assessment of pathology and anatomy laboratory workers exposed to formaldehyde by use of personal air sampling and analysis of DNA damage in peripheral lymphocytes. Mutat. Res. 2006, 605, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Pala, M.; Ugolini, D.; Ceppi, M.; Rizzo, F.; Maiorana, L.; Bolognesi, C.; Schilirò, T.; Gilli, G.; Bigatti, P.; Bono, R.; et al. Occupational exposure to formaldehyde and biological monitoring of Research Institute workers. Cancer Detect. Prev. 2008, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, J.G.; Van Ryan, K.; Galarpe, R. Determination of formaldehyde in air in selected hospital histopathology laboratories in Cagayan de Oro, Philippines. J. Chem. Health Saf. 2015, 22, 10–14. [Google Scholar] [CrossRef]
- Ladeira, C.; Viegas, S.; Carolino, E.; Prista, J.; Gomes, M.C.; Brito, M. Genotoxicity biomarkers in occupational exposure to formaldehyde—The case of histopathology laboratories. Mutat. Res. 2011, 721, 15–20. [Google Scholar] [CrossRef] [PubMed]
- LeBouf, R.F.; Virji, M.A.; Saito, R.; Henneberger, P.K.; Simcox, N.; Stefaniak, A.B. Exposure to volatile organic compounds in healthcare settings. Occup. Environ. Med. 2014, 71, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-C.; Wu, P.-C.; Tseng, C.H.; Su, H.J. Indoor air quality varies with ventilation types and working areas in hospitals. Build. Environ. 2015, 85, 190–195. [Google Scholar] [CrossRef]
- Arndt, T.; Schröfel, S.; Güssregen, B.; Stemmerich, K. Inhalation but not transdermal resorption of hand sanitizer ethanol causes positive ethyl glucuronide findings in urine. Forensic Sci. Int. 2014, 237, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Church, A.S.; Witting, M.D. Laboratory testing in ethanol, methanol, ethylene glycol, and isopropanol toxicities. J. Emerg. Med. 1997, 15, 687–692. [Google Scholar] [CrossRef]
- Kalantari, N.; Bayanib, M.; Ghaffaric, T. Deparaffinization of formalin-fixed paraffin-embedded tissue blocks using hot water instead of xylene. Anal. Biochem. 2016, 507, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ruan, H.; Qi, X.; Guo, X.; Zheng, J.; Liu, C.; Fang, Y.; Huang, M.; Xu, M.; Shen, W. Increased apoptosis and abnormal visual behavior by histone modifications with exposure to para-xylene in developing Xenopus. Neuroscience 2016, 331, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Clausen, P.A.; Larsen, K.; Hammer, M.; Larsen, S.T.; Nielsen, G.D. Acute airway effects of ozone-initiated d-limonene chemistry: Importance of gaseous products. Toxicol. Lett. 2008, 181, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Acebo, I.; Llorca, J.; Ortiz-Revuelta, C.; Angulo, B.; Gómez-Álvarez, S.; Dierssen-Sotos, T. Sick building syndrome in a general hospital and the risks for pregnant workers. Int. J. Gynecol. Obstet. 2011, 113, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Lin, J.M.; Chen, Y.Y.; Chen, Y.C. Building-related symptoms among office employees associated with indoor carbon dioxide and total volatile organic compounds. Int. J. Environ. Res. Public Health 2015, 12, 5833–5845. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Won, Y.L.; Park, D.J.; Kim, Y.S.; Jin, E.S.; Lee, S.K. Combined Toxic Effects of Polar and Nonpolar Chemicals on Human Hepatocytes (HepG2) Cells by Quantitative Property—Activity Relationship Modeling. Toxicol. Res. 2016, 32, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, M.T.; Cipolla, M.; Stella, A.; Ceppi, M.; Bruzzone, M.; Izzotti, A.; Valerio, F. Indoor pollution and burning practices in wood stove management. J. Air Waste Manag. Assoc. 2014, 64, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 4th ed.; Blackwell Science Inc.: Malden, MA, USA, 2002; pp. 187–207. [Google Scholar]
- Kandyala, R.; Raghavendra, S.P.; Rajasekharan, S.T. Xylene: An overview of its health hazards and preventive measures. J. Oral. Maxillofac. Pathol. 2010, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Buesa, R.J.; Peshkov, M.V. Histology without xylene. Ann. Diagn. Pathol. 2009, 13, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.T.N.; Min, P.A. BTEX pollution caused by motorcycles in the megacity of HoChiMinh. J. Environ. Sci. 2013, 25, 348–356. [Google Scholar] [CrossRef]
- Buzyuska, A.J.; Krata, A.; Stranger, M.; Locateli Godoi, A.F.; Kontozova-Deutsch, V.; Bencs, L.; Naveau, I.; Roekens, E.; Van Grieken, R. Atmospheric BTEX-concentrations in an area with intensive street traffic. Atmos. Environ. 2009, 43, 311–318. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Karakitsios, S.S.; Gotti, A.; Liakos, I.L.; Katsoviannis, A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, T.; Zenkner, F.F.; Ellwanger, J.H.; Prá, D.; Rieger, A. DNA damage and cytotoxicity in pathology laboratory technicians exposed to organic solvents. An. Acad. Bras. Cienc. 2016, 88, 227–236. [Google Scholar] [CrossRef] [PubMed]



| VOC | Retention Time (min) | VOC | Retention Time (min) |
|---|---|---|---|
| Ethanol | 4.96 | m-xylene * | 28.27 |
| Acetone | 5.58 | 3-heptanone | 28.79 |
| i-propanol | 5.93 | o-xylene | 29.76 |
| 2-butanone | 9.69 | C11-alkane | 34.94 |
| ethylacetate | 11.01 | p-ethylmethylbenzene | 37.61 |
| benzene | 14.08 | Limonene | 38.61 |
| thiophene | 14.42 | C12-alkane | 38.35 |
| pyrrole | 19.74 | C12-alkane | 38.93 |
| toluene | 21.30 | n-undecane | 41.02 |
| ethylbenzene | 27.71 | naphthalene | 43.55 |
| p-xylene * | 28.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipolla, M.; Izzotti, A.; Ansaldi, F.; Durando, P.; Piccardo, M.T. Volatile Organic Compounds in Anatomical Pathology Wards: Comparative and Qualitative Assessment of Indoor Airborne Pollution. Int. J. Environ. Res. Public Health 2017, 14, 609. https://doi.org/10.3390/ijerph14060609
Cipolla M, Izzotti A, Ansaldi F, Durando P, Piccardo MT. Volatile Organic Compounds in Anatomical Pathology Wards: Comparative and Qualitative Assessment of Indoor Airborne Pollution. International Journal of Environmental Research and Public Health. 2017; 14(6):609. https://doi.org/10.3390/ijerph14060609
Chicago/Turabian StyleCipolla, Massimo, Alberto Izzotti, Filippo Ansaldi, Paolo Durando, and Maria Teresa Piccardo. 2017. "Volatile Organic Compounds in Anatomical Pathology Wards: Comparative and Qualitative Assessment of Indoor Airborne Pollution" International Journal of Environmental Research and Public Health 14, no. 6: 609. https://doi.org/10.3390/ijerph14060609
APA StyleCipolla, M., Izzotti, A., Ansaldi, F., Durando, P., & Piccardo, M. T. (2017). Volatile Organic Compounds in Anatomical Pathology Wards: Comparative and Qualitative Assessment of Indoor Airborne Pollution. International Journal of Environmental Research and Public Health, 14(6), 609. https://doi.org/10.3390/ijerph14060609