The Effect of Different Habitat Types and Ontogenetic Stages on the Diet Shift of a Critically Endangered Fish Species, Coreius guichenoti (Sauvage and Dabry de Thiersant, 1874)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Processing
2.3. Data Analysis
3. Results
3.1. Characteristics of Specimens and Sampling Sites
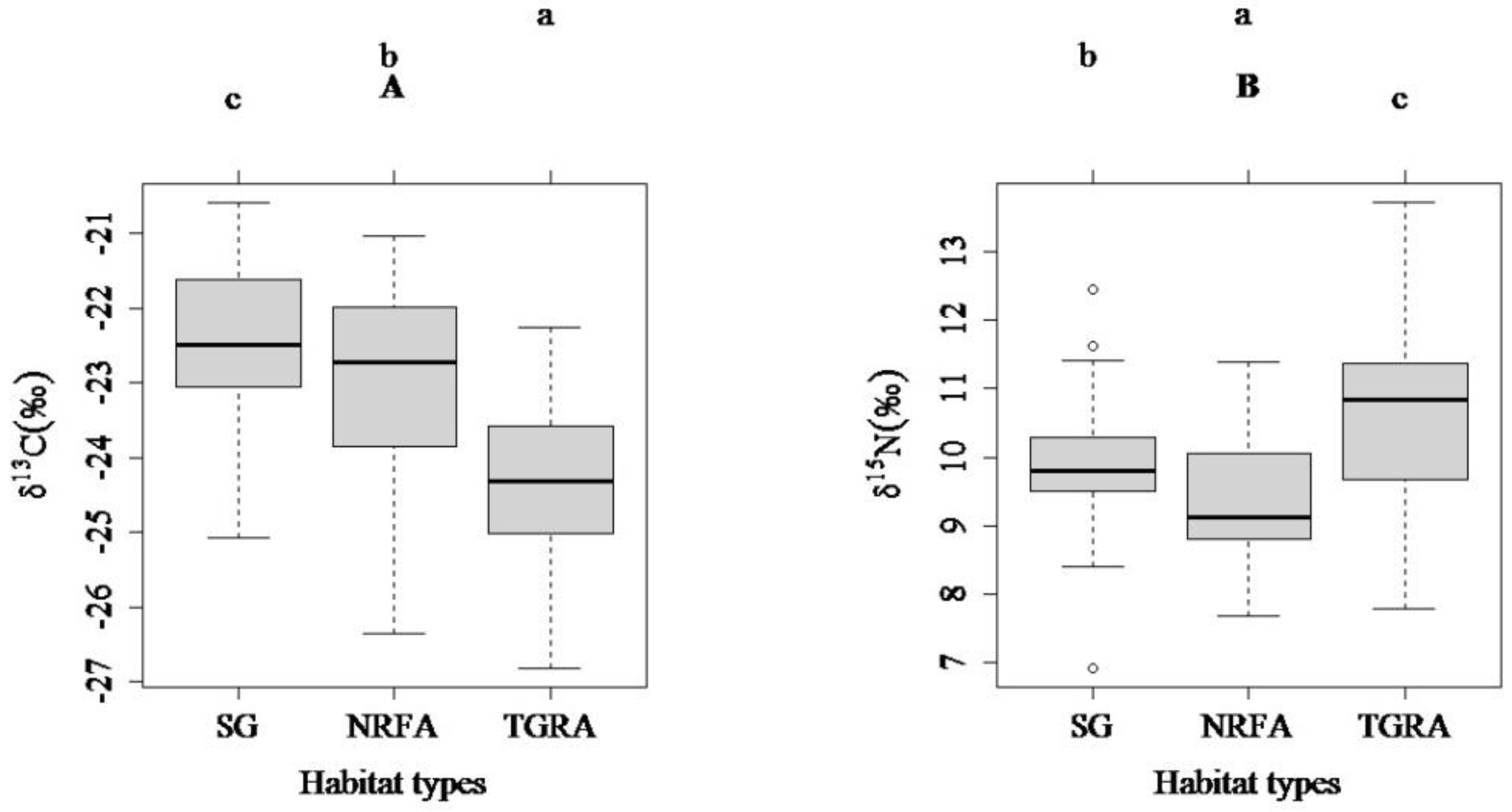
3.2. Comparisons of Carbon and Nitrogen Stable Isotope Ratios among the Three Habitat Types (SG, NRFA and TGRA) and between Two Ontogenetic Stages (FMS and IS)
3.3. Variations in Isotopic Niche Widths among Three Habitat Types (SG, NRFA and TGRA) and between Two Ontogenetic Stages (FMS and IS)
3.4. Differences in Potential Food Sources among the Three Habitat Types (SG, NRFA and TGRA) and between Two Ontogenetic Stages (FMS and IS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenfeld, J.S.; Hatfield, T. Information needs for assessing critical habitat of freshwater fish. Can. J. Fish. Aquat. Sci. 2006, 63, 683–698. [Google Scholar] [CrossRef]
- Heinrichs, J.A.; Bender, D.J.; Gummer, D.L.; Schumaker, N.H. Assessing critical habitat: Evaluating the relative contribution of habitats to population persistence. Biol. Conserv. 2010, 143, 2229–2237. [Google Scholar] [CrossRef]
- Madigan, D.J.; Brooks, E.J.; Bond, M.E.; Gelsleichter, J.; Howey, L.A.; Abercrombie, D.L.; Chapman, D.D. Diet shift and site-fidelity of oceanic whitetip sharks Carcharhinus longimanus along the Great Bahama Bank. Mar. Ecol. Prog. 2015, 529, 185–917. [Google Scholar] [CrossRef]
- Panigada, S.; Donovan, G.P.; Druon, J.N.; Lauriano, G.; Pierantonio, N.; Pirotta, E.; Sciara, G.N. Satellite tagging of Mediterranean fin whales: Working towards the identification of critical habitats and the focusing of mitigation measures. Sci. Rep. 2017, 7, 3365. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.S.; Carl, J.D.; Grønkjær, P.; Støttrup, J.G. Feeding ecology and growth of age 0 year Platichthys flesus (L.) in a vegetated and a bare sand habitat in a nutrient rich fjord. J. Fish Biol. 2005, 66, 531–552. [Google Scholar] [CrossRef]
- Hammar, J. Natural resilience in Arctic charr Salvelinus alpinus: Life history, spatial and dietary alterations along gradients of interspecific interactions. J. Fish Biol. 2014, 85, 81–118. [Google Scholar] [CrossRef] [PubMed]
- Byström, P.; Huss, M.; Persson, L. Ontogenetic constraints and diet shifts in perch (Perca fluviatilis): Mechanisms and consequences for intra-cohort cannibalism. Freshw. Biol. 2012, 57, 847–857. [Google Scholar] [CrossRef]
- Kreitzer, J.D.; Belk, M.C.; Gonzalez, D.B.; Tuckfield, R.C.; Shiozawa, D.K.; Rasmussen, J.E. Ontogenetic diet shift in the June sucker Chasmistes liorus (Cypriniformes, Catostomidae) in the early juvenile stage. Ecol. Freshw. Fish 2010, 19, 433–438. [Google Scholar] [CrossRef]
- Hintz, W.D.; MacVey, N.K.; Asher, A.M.; Porreca, A.P.; Garvey, J.E. Variation in prey selection and foraging success associated with early-life ontogeny and habitat use of American paddlefish (Polyodon spathula). Ecol. Freshw. Fish 2017, 26, 181–189. [Google Scholar] [CrossRef]
- Ramos, J.A.A.; Barletta, M.; Dantas, D.V.; Lima, A.R.A.; Costa, M.F. Trophic niche and habitat shifts of sympatric Gerreidae. J. Fish Biol. 2015, 85, 1446–1469. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.A.; Ebert, T.B. Reproduction, diet and habitat use of leopard sharks, Triakis semifasciata (Girard), in Humboldt Bay, California, USA. Mar. Freshw. Res. 2005, 56, 1089–1098. [Google Scholar] [CrossRef]
- Ward, A.J.W.; Webster, M.M.; Hart, P.J.B. Intraspecific food competition in fishes. Fish Fish. 2006, 7, 231–261. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Fleming, N.E.; Harrod, C.; Newton, J.; Houghton, J.D. Not all jellyfish are equal: Isotopic evidence for inter-and intraspecific variation in jellyfish trophic ecology. PeerJ 2015, 3, e1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhang, M.; Xie, P. Stable isotope changes in freshwater shrimps (Exopalaemon modestus and Macrobrachium nipponensis): Trophic pattern implications. Hydrobiologia 2008, 605, 45–54. [Google Scholar] [CrossRef]
- Pingram, M.A.; Collier, K.J.; Hamilton, D.P.; David, B.O.; Hicks, B.J. Carbon sources supporting large river food webs: A review of ecological theories and evidence from stable isotopes. Freshw. Rev. 2014, 5, 85–103. [Google Scholar] [CrossRef]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.J.; Semmens, B.X.; Schindler, D.E. Including source uncertainty and prior information in the analysis of stable isotope mixing models. Environ. Sci. Technol. 2010, 44, 4645–4650. [Google Scholar] [CrossRef] [PubMed]
- Institute of Hydrobiology (IHB). Fishes in the Yangtze River; Science Press: Beijing, China, 1976. (In Chinese) [Google Scholar]
- Li, L.; Wei, Q.W.; Guo, W.; Lin, D.Q.; Wu, J.M. Interspecies diet relationship of Coreius from Yibin reach of Yangtze River, China. Chin. J. Appl. Ecol. 2015, 26, 1877–1882. (In Chinese) [Google Scholar]
- Jiang, Z.G.; Jiang, J.P.; Wang, Y.Z.; Zhang, E.; Zhang, Y.Y.; Li, L.L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.M.; et al. Red list of China’s vertebrates. Biodivers. Sci. 2016, 24, 500–551. (In Chinese) [Google Scholar]
- Chen, D.Q.; Chang, J.B.; Gu, H.B. Impacts of Jinsha River first stage project on ecology and environment of nature reserve and its countermeasures. J. Yangtze River Sci. Res. Inst. 2005, 22, 21–24. (In Chinese) [Google Scholar]
- Yang, Z.; Tang, H.Y.; Zhu, D.; Liu, H.G.; Wan, L.; Tao, J.P.; Chang, J.B. Spatiotemporal patterns of fish community structures in the Three Gorges Reservoir and its upstream during the 175-m-deep impoundment. Acta Ecol. Sin. 2015, 15, 5064–5075. (In Chinese) [Google Scholar]
- Huang, X.; Deng, Z.L. Study on the feeding habits of Coreius guichenoti (Sauvage et Dabry) below the Gezhouba Dam, Yichang. Freshw. Fish. 1990, 6, 11–14. (In Chinese) [Google Scholar]
- Liu, F.; Dan, S.G.; Wang, J.W.; Cao, W.X. Feeding habits of Coreius guichenoti (Sauvage et Dabry) in the upper Yangtze River. Acta Hydrobiol. Sin. 2012, 36, 1081–1086. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Z.; Tang, H.Y.; Tao, J.P.; Zhao, N. The effect of cascaded huge dams on the downstream movement of Coreius guichenoti (Sauvage & Dabry de Thiersant, 1874) in the upper Yangtze River. Environ. Biol. Fishes 2017, 100, 1507–1516. [Google Scholar]
- Ge, Z.S.; Liu, Q.Y.; Xu, Q.M.; Li, H.Z.; Xue, G.F.; Mei, Y.T.; Xu, Y.H. The geomorphic evolution and characteristics of the riverbed in the lower reaches of Jinshajiang River. Quat. Sci. 2006, 26, 421–428. (In Chinese) [Google Scholar]
- Duan, X.B.; Tian, H.W.; Gao, T.H.; Liu, S.P.; Wang, K.; Chen, D.Q. Resources status of ichthyoplankton in the upper Yangtze River before the storage of Jinsha River first stage project. Resour. Environ. Yangtze Basin 2015, 24, 1358–1365. (In Chinese) [Google Scholar]
- Wang, J.W. Reproduction biology of Gobiocypris Rarus. Acta Hydrobiol. Sin. 1992, 16, 165–174. (In Chinese) [Google Scholar]
- Dulčić, J.; Pallaoro, A.; Matić-Skoko, S.; Dragičević, B.; Tutman, P.; Grgičević, R.; Kraljević, M. Age, growth and mortality of common two-banded seabream, Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817), in the eastern Adriatic Sea (Croatian coast). J. Appl. Ichthyol. 2011, 27, 1254–1258. [Google Scholar] [CrossRef]
- Svensson, E.; Freitas, V.; Schouten, S.; Middelburg, J.J.; van der Veer, H.W.; Damsté, J.S.S. Comparison of the stable carbon and nitrogen isotopic values of gill and white muscle tissue of fish. J. Exp. Mar. Biol. Ecol. 2014, 457, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.M.; Xiong, J.; Qiu, J.W.; Wu, J.M.; Wang, J.W.; Xie, Z.C. Structure of macroinvertebrate communities in relation to environmental variables in a subtropical Asian river system. Int. Rev. Hydrobiol. 2010, 95, 42–57. [Google Scholar] [CrossRef]
- Vaslet, A.; Phillips, D.L.; France, C.; Feller, I.C.; Baldwin, C.C. The relative importance of mangroves and seagrass beds as feeding areas for resident and transient fishes among different mangrove habitats in Florida and Belize: Evidence from dietary and stable-isotope analyses. J. Exp. Mar. Biol. Ecol. 2012, 434, 81–93. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Schmidt, S.N.; Krueger, C.C.; Vander Zanden, M.J.; Eshenroder, R.L. Ontogenetic niche shifts and resource partitioning of lake trout morphotypes. Can. J. Fish. Aquat. Sci. 2009, 66, 1007–1018. [Google Scholar] [CrossRef]
- Messina, S.; Battino, D.; Croci, D.; Mamoli, D.; Ratti, S.; Perucca, E. Phenobarbital pharmacokinetics in old age: A case-matched evaluation based on therapeutic drug monitoring data. Epilepsia 2005, 46, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER-stable isotope Bayesian ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual, Version 3.0. 2005. Available online: http://github.com/briansttock/MixSIAR/ (accessed on 10 October 2018).
- Deng, H.T. Studies on Fish Food Web Structures and Energy flow of Daning River in the Three Gorges Reservoir Areas. Ph.D. Thesis, Southwest University, Chongqing, China, 2015. (In Chinese). [Google Scholar]
- McClain-Counts, J.P.; Demopoulos, A.W.; Ross, S.W. Trophic structure of mesopelagic fishes in the Gulf of Mexico revealed by gut content and stable isotope analyses. Mar. Ecol. 2007, 38, e12449. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing. 2014. Available online: http://www.R-project.org/ (accessed on 10 October 2018).
- Kim, S.L.; Tinker, M.T.; Estes, J.A.; Koch, P.L. Ontogenetic and among-individual variation in foraging strategies of northeast pacific white sharks based on stable isotope analysis. PLoS ONE 2012, 7, e45068. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, N.; Fujimoto, Y.; Shimada, T.; Shikano, S.; Kikuchi, E. Ontogenetic dietary shifts of largemouth bass do not increase trophic position in a shallow eutrophic lake in Japan. Int. J. Limnol. 2016, 52, 355–364. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Faulk, C.K. Batch spawning facilitates transfer of an essential nutrient from diet to eggs in a marine fish. Biol. Lett. 2013, 9, 20130593. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.B.; Tang, H.Y.; Chen, S.; Yang, Z.; Dong, F.Y. Effects of the first phase of Jinsha River hydropower project on fish recruitment: Early life history stages of Coreius guichenoti in the Upper Yangtze River. J. Hydroecol. 2015, 36, 6–10. (In Chinese) [Google Scholar]
- Yang, Z.; Wan, L.; Tao, J.P.; Cai, Y.P.; Zhang, Y.Y.; Qiao, Y. Age and growth of Coreius guichenoti in the mainstream of the Yangtze River. J. Hydroecol. 2011, 32, 46–52. (In Chinese) [Google Scholar]
- Little, B.; Furness, R.W. Long-distance molt migration by British Goosanders Mergus merganser. Ringing Migr. 1985, 6, 77–82. [Google Scholar] [CrossRef]
- De Magalhães Lopes, J.; Alves, C.B.M.; Peressin, A.; Pompeu, P.S. Influence of rainfall, hydrological fluctuations, and lunar phase on spawning migration timing of the Neotropical fish Prochilodus costatus. Hydrobiologia 2018, 818, 145–161. [Google Scholar] [CrossRef]
- Harris, J.; Hightower, J. Movement patterns of American shad transported upstream of dams on the Roanoke River, North Carolina and Virginia. N. Am. J. Fish. Manag. 2011, 31, 240–256. [Google Scholar] [CrossRef]
- Tao, J.; Yang, Z.; Cai, Y.; Wang, X.; Chang, J. Spatiotemporal response of pelagic fish aggregations in their spawning grounds of middle Yangtze to the flood process optimized by the Three Gorges Reservoir operation. Ecol. Eng. 2017, 103, 86–94. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montana, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.O.; Booth, D.J.; Lee, R.W.; Simpson, S.J.; Pile, A.J. Ontogenetic diet shifts in the reef fish Pseudanthias rubrizonatus from isolated populations on the north-west shelf of Australia. Mar. Ecol. Prog. 2010, 419, 211–222. [Google Scholar] [CrossRef]
- Varela, J.L.; Rodríguez-Marín, E.; Medina, A. Estimating diets of prespawning Atlantic bluefin tuna from stomach content and stable isotope analyses. J. Sea Res. 2013, 76, 187–192. [Google Scholar] [CrossRef]
- Hayden, B.; Massa-Gallucci, A.; Harrod, C.; O’grady, M.; Caffrey, J.; Kelly-Quinn, M. Trophic flexibility by roach Rutilus rutilus in novel habitats facilitates rapid growth and invasion success. J. Fish Biol. 2014, 84, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Munroe, S.E.; Heupel, M.R.; Fisk, A.T.; Simpfendorfer, C.A. Geographic and temporal variation in the trophic ecology of a small-bodied shark: Evidence of resilience to environmental change. Can. J. Fish. Aquat. Sci. 2014, 72, 343–351. [Google Scholar] [CrossRef]
- Willson, J.D.; Winne, C.T.; Pilgrim, M.A.; Romanek, C.S.; Gibbons, J.W. Seasonal variation in terrestrial resource subsidies influences trophic niche width and overlap in two aquatic snake species: A stable isotope approach. Oikos 2010, 119, 1161–1171. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Castello, L.; Murphy, B.R.; Xie, S. Potential effects of dam cascade on fish: Lessons from the Yangtze River. Rev. Fish Biol. Fish. 2015, 25, 569–585. [Google Scholar] [CrossRef]



| Biological Parameter | SG | NRFA | TGRA | ||||
|---|---|---|---|---|---|---|---|
| Immature | Fully Mature | Immature | Fully Mature | Immature | Fully Mature | ||
| Total length (mm) | Mean ± SD | 233 ± 60 a | 368 ± 42 b | 289 ± 63 | none | 286 ± 47 | none |
| Range | 95–343 | 304–409 | 131–396 | none | 194–346 | none | |
| Standard length (mm) | Mean ± SD | 194 ± 52 a | 307 ± 35 b | 240 ± 53 | none | 238 ± 41 | none |
| Range | 75–292 | 260–405 | 107–331 | none | 157–291 | none | |
| Body weight (g) | Mean ± SD | 128.9 ± 92 a | 524.8 ± 236.7 b | 262.1 ± 175.4 | none | 233.2 ± 114.0 | none |
| Range | 5.5–418.6 | 235.1–1359.9 | 19.4–866.4 | none | 52.7–411.7 | none | |
| Age (years) | Mean ± SD | 1.79 ± 0.64 a | 2.46 ± 1.11 b | 2.47 ± 0.79 | none | 2.40 ± 0.72 | none |
| Range | 1–3 | 3–6 | 1–4 | none | 1–3 | none | |
| N | 43 | 25 | 55 | 0 | 52 | 0 | |
| Parameter | SG | NRFA | TGRA | p |
|---|---|---|---|---|
| Dissolved oxygen (DO, mg/L) | 9.19 ± 0.20 | 7.64 ± 0.32 | 8.04 ± 0.51 | 0.013 |
| pH | 8.13 ± 0.08 | 8.38 ± 0.04 | 8.48 ± 0.02 | 0.001 |
| Total phosphorus (TP, mg/L) | 0.08 ± 0.02 | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.018 |
| Total nitrogen (TN, mg/L) | 2.36 ± 0.91 | 2.32 ± 0.18 | 2.55 ± 0.27 | 0.933 |
| Chemical oxygen demand (CODMn, mg/L) | 2.83 ± 1.33 | 2.68 ± 0.18 | 2.57 ± 0.23 | 0.946 |
| Biochemical oxygen demand (BOD5, mg/L) | 1.11 ± 0.56 | 1.95 ± 0.55 | 1.50 ± 0.70 | 0.436 |
| Velocity of water flow (Velocity, m/s) | 2.29 ± 0.11 | 1.87 ± 0.20 | 0.59 ± 0.13 | <0.001 |
| Water temperature (T, °C) | 20.17 ± 0.62 | 22.60 ± 0.08 | 23.37 ± 0.62 | 0.002 |
| CPB (%) | 54.61 ± 8.56 | 34.90 ± 5.33 | 7.72 ± 2.56 | <0.001 |
| PPG (%) | 28.46 ± 10.96 | 36.63 ±6.26 | 12.28 ± 6.25 | 0.002 |
| SPS (%) | 16.93 ± 4.35 | 28.48 ± 3.08 | 80.01 ± 6.81 | <0.001 |
| Food Sources | δ13C | δ15N | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SG | NRFA | TGRA | p | SG | NRFA | TGRA | p | ||
| Mollusks | Mean | −20.03 | −23.41 | −23.24 | 0.001 | 9.78 | 9.72 | 6.78 | 0.012 |
| SD | 0.52 | 0.21 | 0.17 | 0.41 | 0.14 | 0.24 | |||
| Macrocrustaceans | Mean | −24.81 | −24.06 | −25.11 | <0.001 | 10.42 | 6.24 | 5.21 | 0.007 |
| SD | 0.33 | 0.48 | 0.47 | 0.37 | 0.32 | 0.32 | |||
| Aquatic insect larvae | Mean | −25.24 | −24.08 | −23.18 | <0.001 | 8.78 | 7.12 | 6.12 | <0.001 |
| SD | 0.22 | 0.21 | 0.53 | 0.19 | 0.54 | 0.45 | |||
| POM | Mean | −24.78 | −22.13 | −21.42 | 0.002 | 6.79 | 4.78 | 3.11 | <0.001 |
| SD | 0.82 | 0.19 | 0.09 | 0.53 | 0.47 | 0.08 | |||
| Potential Food Sources | SG | NRFA | TGRA | |||
|---|---|---|---|---|---|---|
| 50% | Range | 50% | Range | 50% | Range | |
| Aquatic insect larvae | 0.5 | 0.1–3.2 | 1.6 | 0.1–8.7 | 0.7 | 0.1–5.5 |
| Macrocrustaceans | 3.4 | 0.9–8.1 | 56.4 | 16.8–82.8 | 49.4 | 35.6–65.0 |
| Mollusks | 3.7 | 0.4–13.3 | 6.1 | 2.7–11.0 | 47.4 | 31.9–61.4 |
| POM | 91.0 | 81.2–97.3 | 34.4 | 8.9–73.9 | 1.4 | 0.5–3.4 |
| Potential Food Sources | FMS | IS | ||
|---|---|---|---|---|
| 50% | Range | 50% | Range | |
| Aquatic insect larvae | 5.2 | 0.4–17.4 | 1.0 | 0.1–4.1 |
| Macrocrustaceans | 2.7 | 0.2–10.2 | 0.6 | 0.1–3.0 |
| Mollusks | 4.3 | 0.5-–13.1 | 3.0 | 0.1–13.4 |
| POM | 85.1 | 74.4–93.7 | 94.2 | 84.3–98.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Chen, X.; Zhao, N.; Tang, H.; Tao, J.; Zhang, P.; Shi, F.; Wan, C. The Effect of Different Habitat Types and Ontogenetic Stages on the Diet Shift of a Critically Endangered Fish Species, Coreius guichenoti (Sauvage and Dabry de Thiersant, 1874). Int. J. Environ. Res. Public Health 2018, 15, 2240. https://doi.org/10.3390/ijerph15102240
Yang Z, Chen X, Zhao N, Tang H, Tao J, Zhang P, Shi F, Wan C. The Effect of Different Habitat Types and Ontogenetic Stages on the Diet Shift of a Critically Endangered Fish Species, Coreius guichenoti (Sauvage and Dabry de Thiersant, 1874). International Journal of Environmental Research and Public Health. 2018; 15(10):2240. https://doi.org/10.3390/ijerph15102240
Chicago/Turabian StyleYang, Zhi, Xiaojuan Chen, Na Zhao, Huiyuan Tang, Jiangping Tao, Peng Zhang, Fang Shi, and Chengyan Wan. 2018. "The Effect of Different Habitat Types and Ontogenetic Stages on the Diet Shift of a Critically Endangered Fish Species, Coreius guichenoti (Sauvage and Dabry de Thiersant, 1874)" International Journal of Environmental Research and Public Health 15, no. 10: 2240. https://doi.org/10.3390/ijerph15102240
APA StyleYang, Z., Chen, X., Zhao, N., Tang, H., Tao, J., Zhang, P., Shi, F., & Wan, C. (2018). The Effect of Different Habitat Types and Ontogenetic Stages on the Diet Shift of a Critically Endangered Fish Species, Coreius guichenoti (Sauvage and Dabry de Thiersant, 1874). International Journal of Environmental Research and Public Health, 15(10), 2240. https://doi.org/10.3390/ijerph15102240





