Significance of Blood Transfusion Units in Determining the Probability of Mortality among Elderly Trauma Patients Based on the Geriatric Trauma Outcome Scoring System: A Cross-Sectional Analysis Based on Trauma Registered Data
Abstract
1. Background
2. Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Statistical Analysis
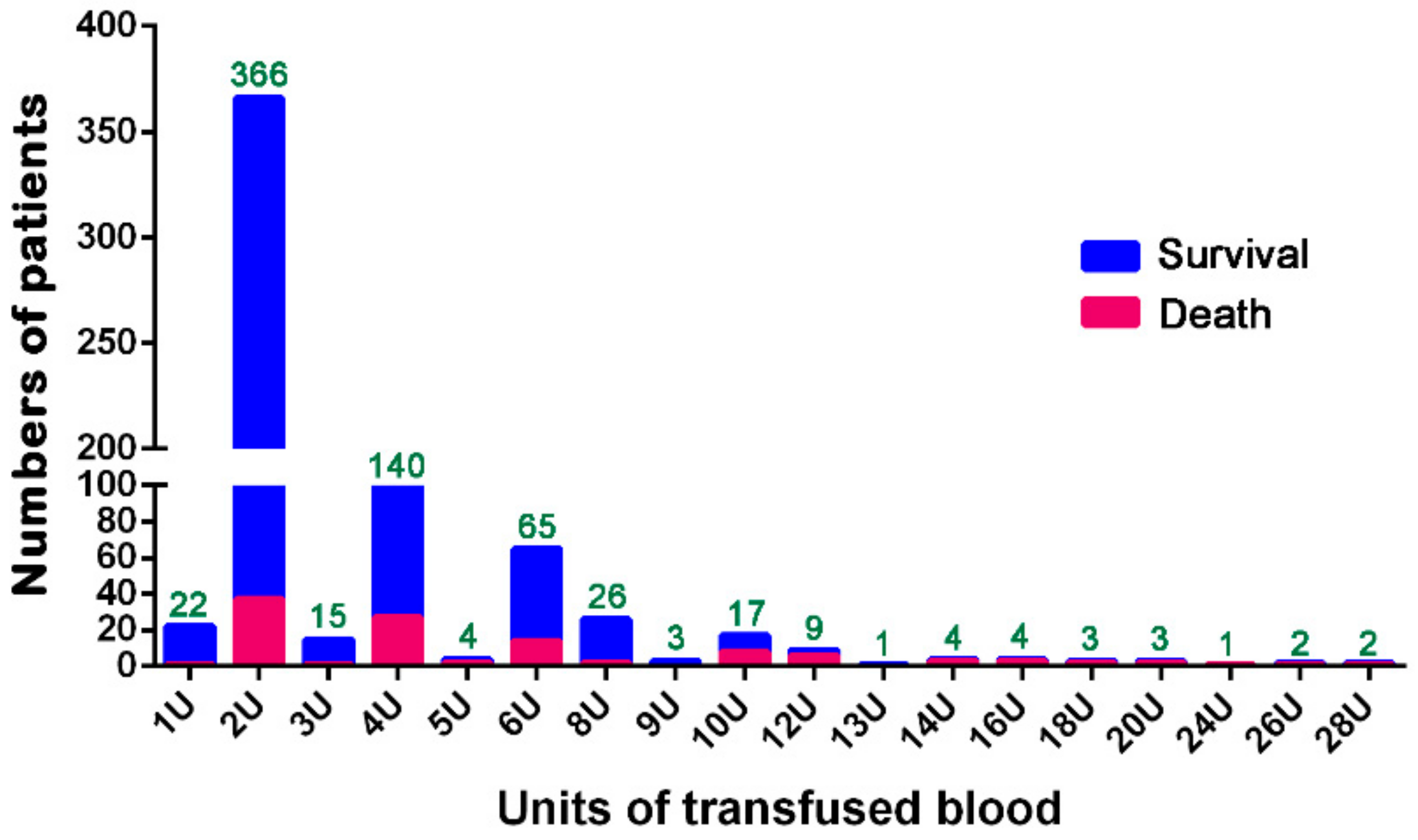
3. Results
3.1. ROC Curve Analysis
3.2. Characteristics and Outcomes of Patients
3.3. Adjusted Mortality Outcomes of the Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keller, J.M.; Sciadini, M.F.; Sinclair, E.; O’Toole, R.V. Geriatric trauma: Demographics, injuries, and mortality. J. Orthop. Trauma 2012, 26, e161–e165. [Google Scholar] [CrossRef] [PubMed]
- Aschkenasy, M.T.; Rothenhaus, T.C. Trauma and falls in the elderly. Emerg. Med. Clin. N. Am. 2006, 24, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, C.A.; Ruchholtz, S.; Kaiser, G.M.; Nast-Kolb, D. Mortality in severely injured elderly trauma patients—When does age become a risk factor? World J. Surg. 2005, 29, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Perdue, P.W.; Watts, D.D.; Kaufmann, C.R.; Trask, A.L. Differences in mortality between elderly and younger adult trauma patients: Geriatric status increases risk of delayed death. J. Trauma 1998, 45, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J. Trauma 1987, 27, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Lefering, R. Trauma scoring systems. Curr. Opin. Crit. Care 2012, 18, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Z.; Wolf, S.E.; Nakonezny, P.A.; Minhajuddin, A.; Rhodes, R.L.; Paulk, M.E.; Phelan, H.A. Estimating geriatric mortality after injury using age, injury severity, and performance of a transfusion: The Geriatric Trauma Outcome Score. J. Palliat. Med. 2015, 18, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.C.; Joseph, B.; Inaba, K.; Nakonezny, P.A.; Bruns, B.R.; Kerby, J.D.; Brasel, K.J.; Wolf, S.E.; Cuschieri, J.; Paulk, M.E.; et al. Multicenter external validation of the Geriatric Trauma Outcome Score: A study by the Prognostic Assessment of Life and Limitations After Trauma in the Elderly (PALLIATE) consortium. J. Trauma Acute Care Surg. 2016, 80, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Madni, T.D.; Ekeh, A.P.; Brakenridge, S.C.; Brasel, K.J.; Joseph, B.; Inaba, K.; Bruns, B.R.; Kerby, J.D.; Cuschieri, J.; Mohler, M.J.; et al. A comparison of prognosis calculators for geriatric trauma: A prognostic assessment of life and limitations after trauma in the elderly consortium study. J. Trauma Acute Care Surg. 2017, 83, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Barea-Mendoza, J.A.; Chico-Fernandez, M.; Sanchez-Casado, M.; Molina-Diaz, I.; Quintana-Diaz, M.; Jimenez-Moragas, J.M.; Perez-Barcena, J.; Llompart-Pou, J.A. Predicting survival in geriatric trauma patients: A comparison between the TRISS methodology and the Geriatric Trauma Outcome Score. Cir. Esp. 2018, 96, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ahl, R.; Phelan, H.A.; Dogan, S.; Cao, Y.; Cook, A.C.; Mohseni, S. Predicting in-hospital and 1-year mortality in geriatric trauma patients using Geriatric Trauma Outcome Score. J. Am. Coll. Surg. 2017, 224, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Corwin, H.L. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit. Care Med. 2008, 36, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Watson, S. Massive Transfusion; StatPearls Publishing LLC: Treasure Island, FL, USA, 2018. [Google Scholar]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh-Chabok, S.; Hosseinpour, M.; Kouchakinejad-Eramsadati, L.; Ranjbar, F.; Malekpouri, R.; Razzaghi, A.; Mohtasham-Amiri, Z. Comparison of Revised Trauma Score, Injury Severity Score and Trauma and Injury Severity Score for mortality prediction in elderly trauma patients. Ulus. Travma Acil Cerrahi Derg. 2016, 22, 536–540. [Google Scholar] [PubMed]
- Champion, H.R.; Sacco, W.J.; Copes, W.S.; Gann, D.S.; Gennarelli, T.A.; Flanagan, M.E. A revision of the Trauma Score. J. Trauma 1989, 29, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, H.; Heppner, H.J.; Wohlfart, K.; Turkoglu, A. The geriatric polytrauma: Risk profile and prognostic factors. Ulus. Travma Acil Cerrahi Derg. 2017, 23, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Guerado, E.; Medina, A.; Mata, M.I.; Galvan, J.M.; Bertrand, M.L. Protocols for massive blood transfusion: When and why, and potential complications. Eur. J. Trauma Emerg. Surg. 2016, 42, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Roxby, D. Any changes in recent massive transfusion practices in a tertiary level institution? Transfus. Apher. Sci. 2017, 56, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Cantle, P.M.; Cotton, B.A. Balanced Resuscitation in Trauma Management. Surg. Clin. N. Am. 2017, 97, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Aquilino, A.; Cortese, F.; Scicchitano, P.; Sassara, M.; Mola, E.; Rollo, R.; Caldarola, P.; Giorgino, F.; Pomo, V.; et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc. Health Risk Manag. 2010, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]


| Variables | BT ≥ 2 U n = 665 | BT < 2 U n = 22 | Odds Ratio(95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 77.1 | ±7.5 | 79.2 | ±5.9 | - | 0.199 | |
| Gender, n (%) | 0.665 | ||||||
| Male | 281 | (42.3) | 8 | (36.4) | 1.3 | (0.53–3.09) | |
| Female | 384 | (57.7) | 14 | (63.6) | 0.8 | (0.32–1.89) | |
| Co-morbidities, n (%) | |||||||
| DM | 191 | (28.7) | 9 | (40.9) | 0.6 | (0.25–1.38) | 0.235 |
| HTN | 376 | (56.5) | 15 | (68.2) | 0.6 | (0.24–1.51) | 0.382 |
| CAD | 69 | (10.4) | 3 | (13.6) | 0.7 | (0.21–2.54) | 0.719 |
| CHF | 14 | (2.1) | 2 | (9.1) | 0.2 | (0.05–1.01) | 0.090 |
| CVA | 60 | (9.0) | 1 | (4.5) | 2.1 | (0.28–15.76) | 0.712 |
| ESRD | 19 | (2.9) | 3 | (13.6) | 0.2 | (0.05–0.68) | 0.030 |
| ISS (median, IQR) | 9 | (9–20) | 9 | (9–13.8) | - | 0.020 | |
| <16, n (%) | 410 | (61.7) | 17 | (77.3) | 0.5 | (0.17–1.30) | 0.181 |
| 16–24, n (%) | 113 | (17.0) | 3 | (13.6) | 1.3 | (0.38–4.46) | 0.783 |
| >24, n (%) | 142 | (21.4) | 2 | (9.1) | 2.7 | (0.63–11.75) | 0.194 |
| GTOS | 135.8 | ±21.9 | 131.0 | ±13.4 | - | 0.115 | |
| Mortality, n (%) | 110 | (16.5) | 1 | (4.5) | 4.2 | (0.55–31.27) | 0.154 |
| Variables | BT ≥ 3 U n = 299 | BT < 3 U n = 388 | Odds Ratio (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 76.8 | ±7.6 | 77.5 | ±7.4 | - | 0.235 | |
| Gender, n (%) | 0.019 | ||||||
| Male | 141 | (42.7) | 148 | (38.1) | 1.4 | (1.07–1.97) | |
| Female | 158 | (52.8) | 240 | (61.9) | 0.7 | (0.51–0.94) | |
| Co-morbidities, n (%) | |||||||
| DM | 75 | (25.1) | 125 | (32.2) | 0.7 | (0.50–0.99) | 0.043 |
| HTN | 151 | (50.5) | 240 | (61.9) | 0.6 | (0.46–0.85) | 0.003 |
| CAD | 33 | (11.0) | 39 | (10.1) | 1.1 | (0.68–1.81) | 0.707 |
| CHF | 5 | (1.7) | 11 | (2.8) | 0.6 | (0.20–1.70) | 0.446 |
| CVA | 21 | (7.0) | 40 | (10.3) | 0.7 | (0.38–1.14) | 0.139 |
| ESRD | 5 | (1.7) | 17 | (4.4) | 0.4 | (0.14–1.02) | 0.050 |
| ISS (median, IQR) | 16 | (9–25) | 9 | (9–16) | - | <0.001 | |
| <16, n (%) | 145 | (48.5) | 282 | (72.7) | 0.4 | (0.26–0.49 | <0.001 |
| 16–24, n (%) | 57 | (19.1) | 59 | (15.2) | 1.3 | (0.88–1.96) | 0.184 |
| >24, n (%) | 97 | (32.4) | 47 | (12.1) | 3.5 | (2.36–5.14) | <0.001 |
| GTOS | 142.1 | ±23.7 | 130.8 | ±18.7 | - | <0.001 | |
| Mortality, n (%) | 73 | (24.4) | 38 | (9.8) | 3.0 | (1.94–4.56) | <0.001 |
| Variables | BT ≥ 4 U n = 284 | BT < 4 U n = 403 | Odds Ratio (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 76.6 | ±7.5 | 77.5 | ±7.5 | - | 0.118 | |
| Gender, n (%) | 0.010 | ||||||
| Male | 136 | (47.9) | 153 | (38.0) | 1.5 | (1.10–2.04) | |
| Female | 148 | (52.1) | 250 | (62.0) | 0.7 | (0.49–0.91) | |
| Co-morbidities, n (%) | |||||||
| DM | 69 | (24.3) | 131 | (32.5) | 0.7 | (0.47–0.94) | 0.021 |
| HTN | 141 | (49.6) | 250 | (62.0) | 0.6 | (0.44–0.82) | 0.001 |
| CAD | 31 | (10.9) | 41 | (10.2) | 1.1 | (0.66–1.77) | 0.801 |
| CHF | 5 | (1.8) | 11 | (2.7) | 0.6 | (0.22–1.86) | 0.454 |
| CVA | 21 | (7.4) | 40 | (9.9) | 0.7 | (0.42–1.26) | 0.278 |
| ESRD | 5 | (1.8) | 17 | (4.2) | 0.4 | (0.15–1.12) | 0.081 |
| ISS (median, IQR) | 16 | (9–25) | 9 | (9–16) | - | <0.001 | |
| <16, n (%) | 132 | (46.5) | 295 | (73.2) | 0.3 | (0.23–0.44) | <0.001 |
| 16–24, n (%) | 55 | (19.4) | 61 | (15.1) | 1.3 | (0.90–2.01) | 0.149 |
| >24, n (%) | 97 | (34.2) | 47 | (11.7) | 3.9 | (2.66–5.81) | <0.001 |
| GTOS | 142.9 | ±23.9 | 130.6 | ±18.5 | - | <0.001 | |
| Mortality, n (%) | 72 | (25.4) | 39 | (9.7) | 3.2 | (2.07–4.85) | <0.001 |
| Variables | BT ≥ 6 U n = 140 | BT < 6 U n = 574 | Odds Ratio (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 75.7 | ±6.9 | 77.6 | ±7.6 | - | 0.008 | |
| Gender, n (%) | 0.001 | ||||||
| Male | 77 | (55.0) | 212 | (38.8) | 1.9 | (1.33–2.81) | |
| Female | 63 | (45.0) | 335 | (61.2) | 0.5 | (0.36–0.75) | |
| Co-morbidities, n (%) | |||||||
| DM | 33 | (23.6) | 167 | (30.5) | 0.7 | (0.46–1.08) | 0.118 |
| HTN | 65 | (46.4) | 326 | (59.6) | 0.6 | (0.40–0.85) | 0.006 |
| CAD | 20 | (14.3) | 52 | (9.5) | 1.6 | (0.91–2.76) | 0.121 |
| CHF | 3 | (2.1) | 13 | (2.4) | 0.9 | (0.25–3.20) | 1.000 |
| CVA | 5 | (3.6) | 56 | (10.2) | 0.3 | (0.13–0.83) | 0.019 |
| ESRD | 4 | (2.9) | 18 | (3.3) | 0.9 | (0.29–2.60) | 1.000 |
| ISS (median, IQR) | 18.5 | (9–27) | 9 | (9–16) | - | <0.001 | |
| <16, n (%) | 51 | (36.4) | 376 | (68.7) | 0.3 | (0.18–00.38) | <0.001 |
| 16-24, n (%) | 31 | (22.1) | 85 | (15.5) | 1.5 | (0.98–2.45) | 0.076 |
| >24, n (%) | 58 | (41.4) | 86 | (15.7) | 3.8 | (2.52–5.70) | <0.001 |
| GTOS | 147.2 | ±25.2 | 132.7 | ±19.7 | - | <0.001 | |
| Mortality, n (%) | 43 | (30.7) | 68 | (12.4) | 3.1 | (2.01–4.85) | <0.001 |
| Variables | BT ≥ 8 U n = 75 | BT < 8 U n = 612 | Odds Ratio (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 75.0 | ±6.7 | 77.4 | ±7.5 | - | 0.008 | |
| Gender, n (%) | 0.025 | ||||||
| Male | 41 | (54.7) | 248 | (40.5) | 1.8 | (1.09–2.87) | |
| Female | 34 | (45.3) | 364 | (59.5) | 0.6 | (0.35–0.92) | |
| Co-morbidities, n (%) | |||||||
| DM | 17 | (22.7) | 183 | (29.9) | 0.7 | (0.39–1.21) | 0.226 |
| HTN | 29 | (38.7) | 362 | (59.2) | 0.4 | (0.27–0.71) | 0.001 |
| CAD | 11 | (14.7) | 61 | (10.0) | 1.6 | (0.78–3.10) | 0.229 |
| CHF | 2 | (2.7) | 14 | (2.3) | 1.2 | (0.26–5.25) | 1.000 |
| CVA | 2 | (2.7) | 59 | (9.6) | 0.3 | (0.07–1.07) | 0.050 |
| ESRD | 0 | (0.0) | 22 | (3.6) | - | 0.156 | 1.000 |
| ISS (median, IQR) | 21 | (10–29) | 9 | (9–17) | - | <0.001 | |
| <16, n (%) | 25 | (33.3) | 402 | (65.7) | 0.3 | (0.16–0.43) | <0.001 |
| 16–24, n (%) | 15 | (20.0) | 101 | (16.5) | 1.3 | (0.69–2.32) | 0.513 |
| >24, n (%) | 35 | (46.7) | 109 | (17.8) | 4.0 | (2.45–6.65) | <0.001 |
| GTOS | 150.4 | ±27.1 | 133.9 | ±20.3 | - | <0.001 | |
| Mortality, n (%) | 29 | (38.7) | 82 | (13.4) | 4.1 | (2.42–6.85) | <0.001 |
| Variables | BT ≥ 10 U n = 46 | BT < 10 U n = 641 | Odds Ratio (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 75.0 | ±6.3 | 77.3 | ±7.5 | - | 0.045 | |
| Gender, n (%) | 0.442 | ||||||
| Male | 22 | (47.8) | 267 | (41.7) | 1.3 | (0.71–2.34) | |
| Female | 24 | (52.2) | 374 | (58.3) | 0.8 | (0.43–1.42) | |
| Co-morbidities, n (%) | |||||||
| DM | 11 | (23.9) | 189 | (29.5) | 0.8 | (0.37–1.51) | 0.503 |
| HTN | 14 | (30.4) | 377 | (58.8) | 0.3 | (0.16–0.59) | <0.001 |
| CAD | 8 | (17.4) | 64 | (10.0) | 1.9 | (0.85–4.25) | 0.131 |
| CHF | 0 | (0.0) | 16 | (2.5) | - | 0.411 | 1.000 |
| CVA | 1 | (2.2) | 60 | (9.4) | 0.2 | (0.03–1.59) | 0.111 |
| ESRD | 0 | (0.0) | 22 | (3.4) | - | 0.390 | 1.000 |
| ISS (median, IQR) | 25 | (16–32) | 9 | (9–17) | - | <0.001 | |
| <16, n (%) | 10 | (21.7) | 417 | (65.1) | 0.1 | (0.07–0.31) | <0.001 |
| 16–24, n (%) | 8 | (17.4) | 108 | (16.8) | 1.0 | (0.47–2.29) | 1.000 |
| >24, n (%) | 28 | (60.9) | 116 | (18.1) | 7.0 | (3.77–13.16) | <0.001 |
| GTOS | 158.2 | ±26.4 | 134.1 | ±20.5 | - | <0.001 | |
| Mortality, n (%) | 27 | (58.7) | 84 | (13.1) | 9.4 | (5.02–17.70) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-C.; Rau, C.-S.; Kuo, P.-J.; Liu, H.-T.; Hsu, S.-Y.; Hsieh, C.-H. Significance of Blood Transfusion Units in Determining the Probability of Mortality among Elderly Trauma Patients Based on the Geriatric Trauma Outcome Scoring System: A Cross-Sectional Analysis Based on Trauma Registered Data. Int. J. Environ. Res. Public Health 2018, 15, 2285. https://doi.org/10.3390/ijerph15102285
Wu S-C, Rau C-S, Kuo P-J, Liu H-T, Hsu S-Y, Hsieh C-H. Significance of Blood Transfusion Units in Determining the Probability of Mortality among Elderly Trauma Patients Based on the Geriatric Trauma Outcome Scoring System: A Cross-Sectional Analysis Based on Trauma Registered Data. International Journal of Environmental Research and Public Health. 2018; 15(10):2285. https://doi.org/10.3390/ijerph15102285
Chicago/Turabian StyleWu, Shao-Chun, Cheng-Shyuan Rau, Pao-Jen Kuo, Hang-Tsung Liu, Shiun-Yuan Hsu, and Ching-Hua Hsieh. 2018. "Significance of Blood Transfusion Units in Determining the Probability of Mortality among Elderly Trauma Patients Based on the Geriatric Trauma Outcome Scoring System: A Cross-Sectional Analysis Based on Trauma Registered Data" International Journal of Environmental Research and Public Health 15, no. 10: 2285. https://doi.org/10.3390/ijerph15102285
APA StyleWu, S.-C., Rau, C.-S., Kuo, P.-J., Liu, H.-T., Hsu, S.-Y., & Hsieh, C.-H. (2018). Significance of Blood Transfusion Units in Determining the Probability of Mortality among Elderly Trauma Patients Based on the Geriatric Trauma Outcome Scoring System: A Cross-Sectional Analysis Based on Trauma Registered Data. International Journal of Environmental Research and Public Health, 15(10), 2285. https://doi.org/10.3390/ijerph15102285







