Adsorption of Cadmium on Degraded Soils Amended with Maize-Stalk-Derived Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Biochar Samples Preparation
2.2. The Characterization of Soil and Biochar
2.3. Isothermal Adsorption Experiment
2.4. Data Processing
3. The Results and the Discussion
3.1. The Characterization of the Tested Pristine Soils and Biochars
3.2. The Cd Adsorption Isotherms on Degraded Soils
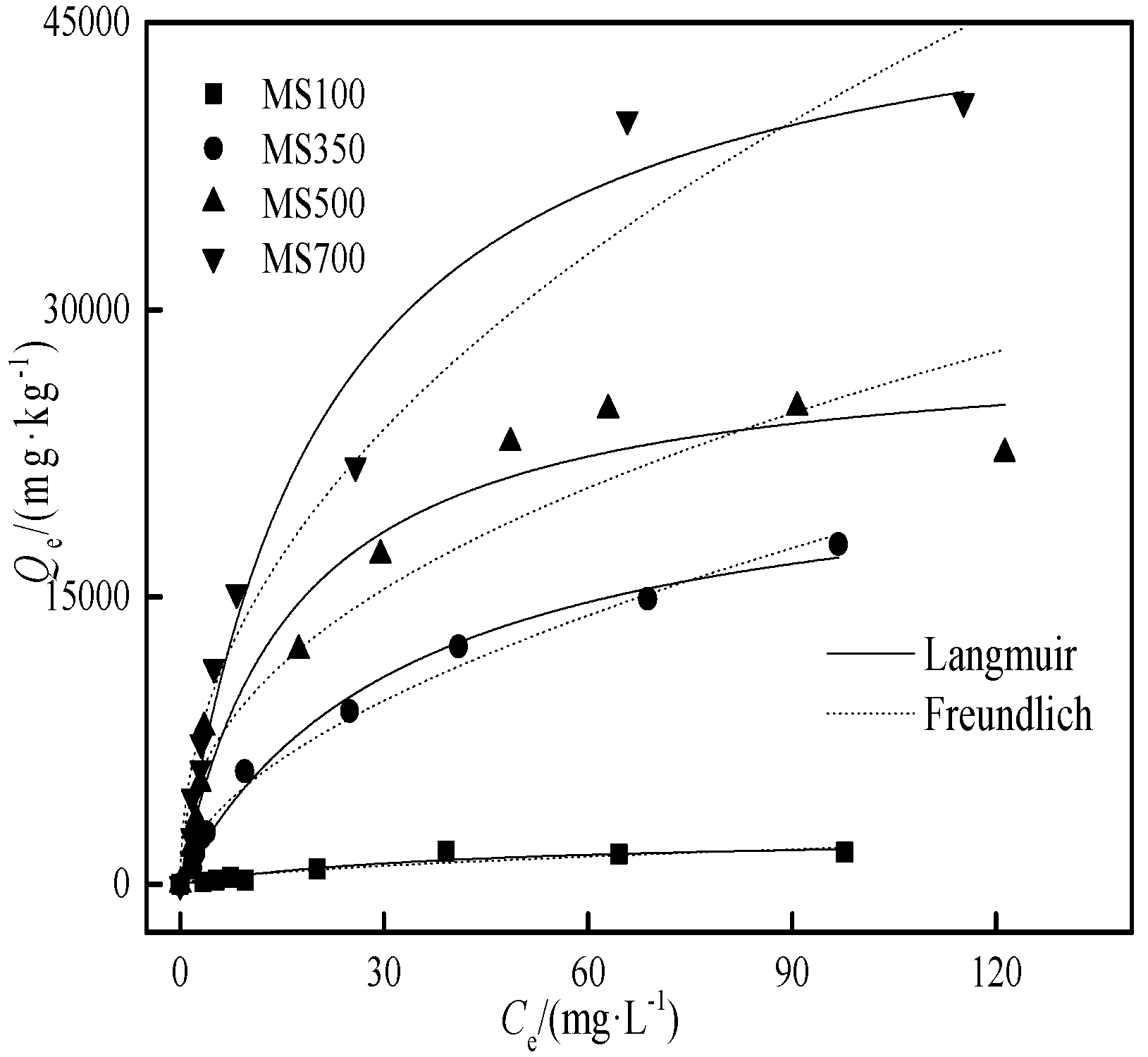
3.3. The Cd Adsorption Isotherms with the Biochars
3.4. The Adsorption Behavior of Cd on the Degraded Soils Amended by Biochars
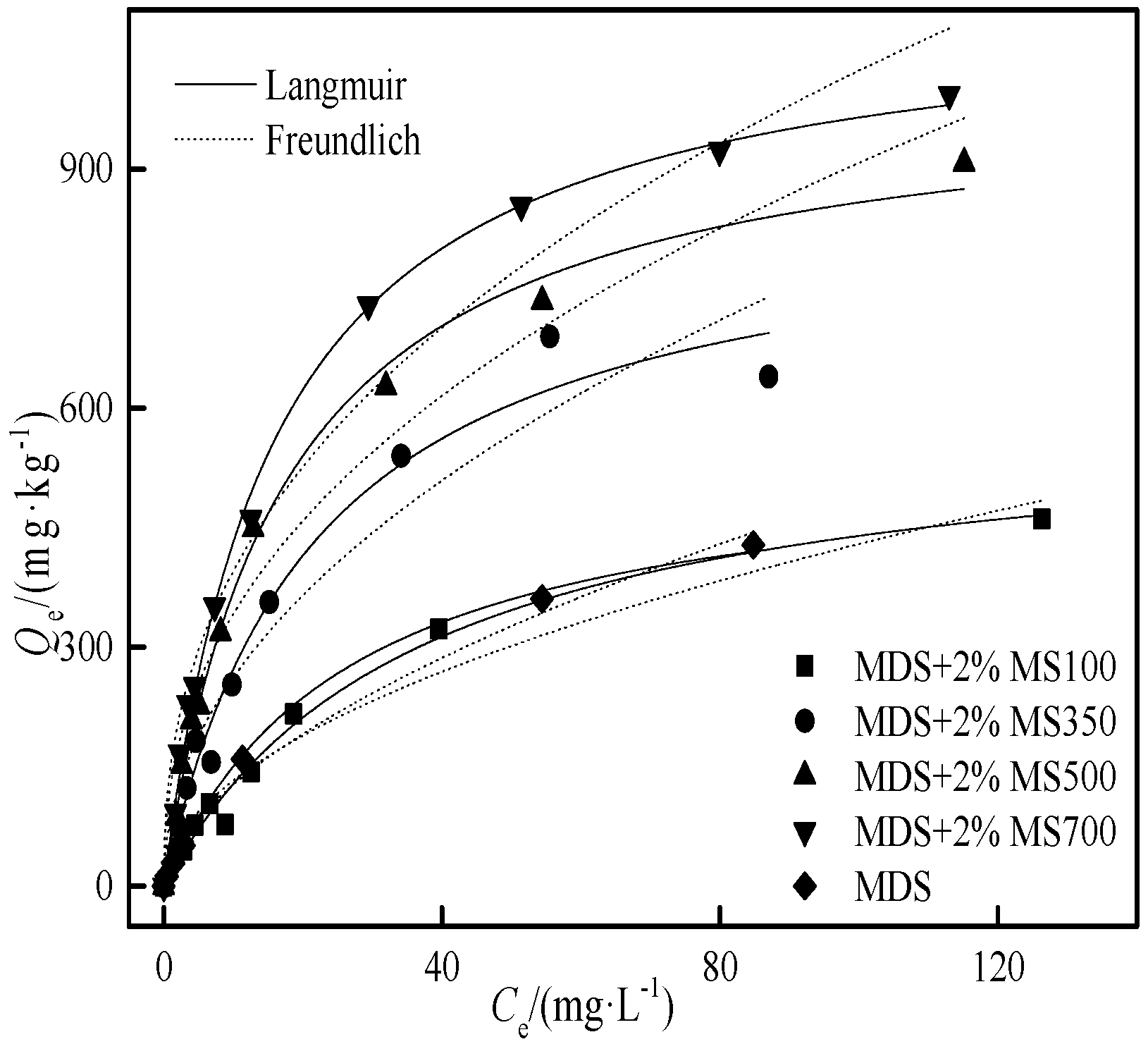
3.4.1. The Effect of Biochar Ratio on Cd Adsorption in Moderately Degraded Soil
3.4.2. The Effect of Biochar Pyrolysis Temperature on Cd Adsorption in Moderately Degraded Soil
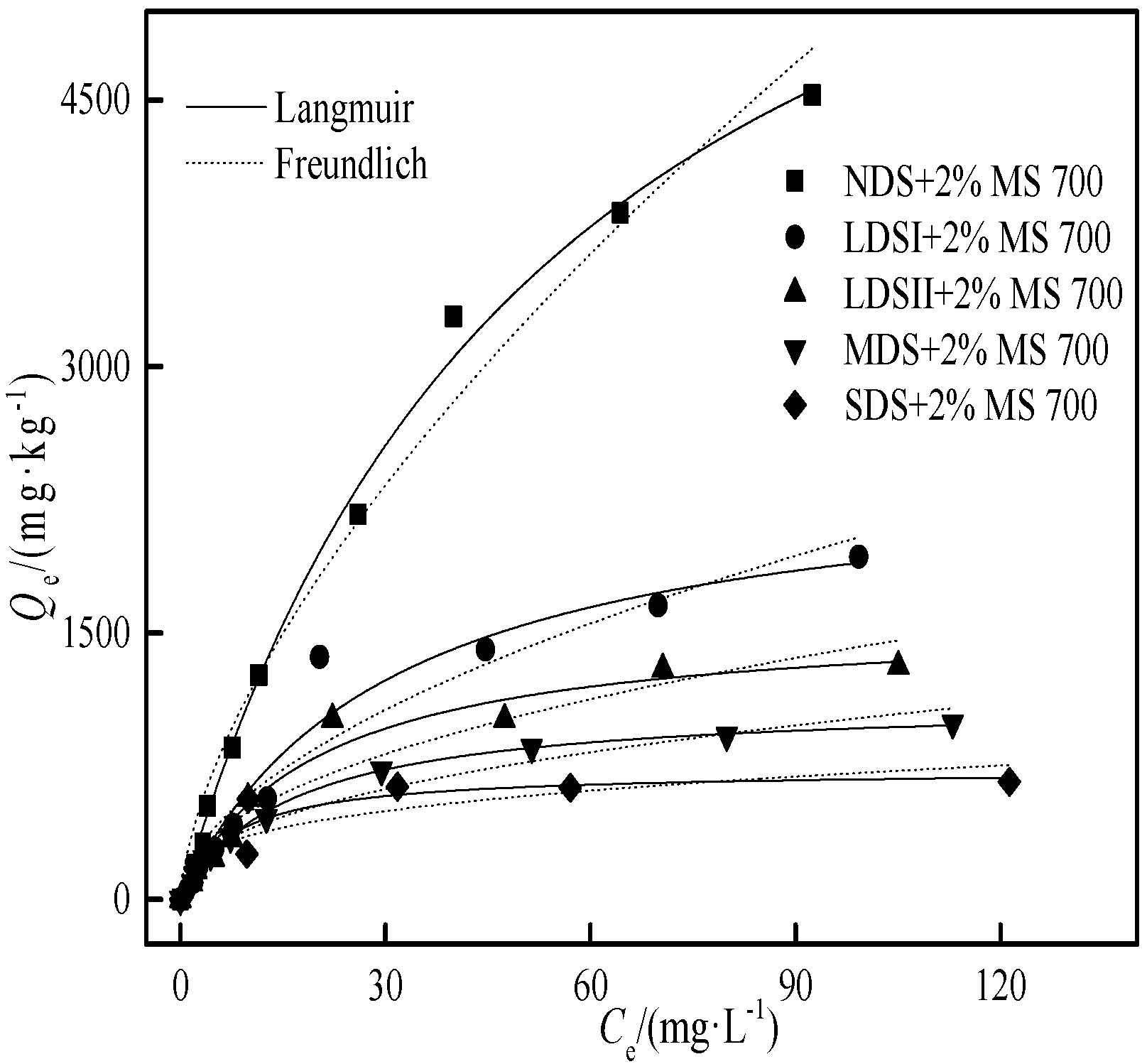
3.4.3. The Cd Adsorption on Different Degraded Soils with 2.0% (wt) MS700
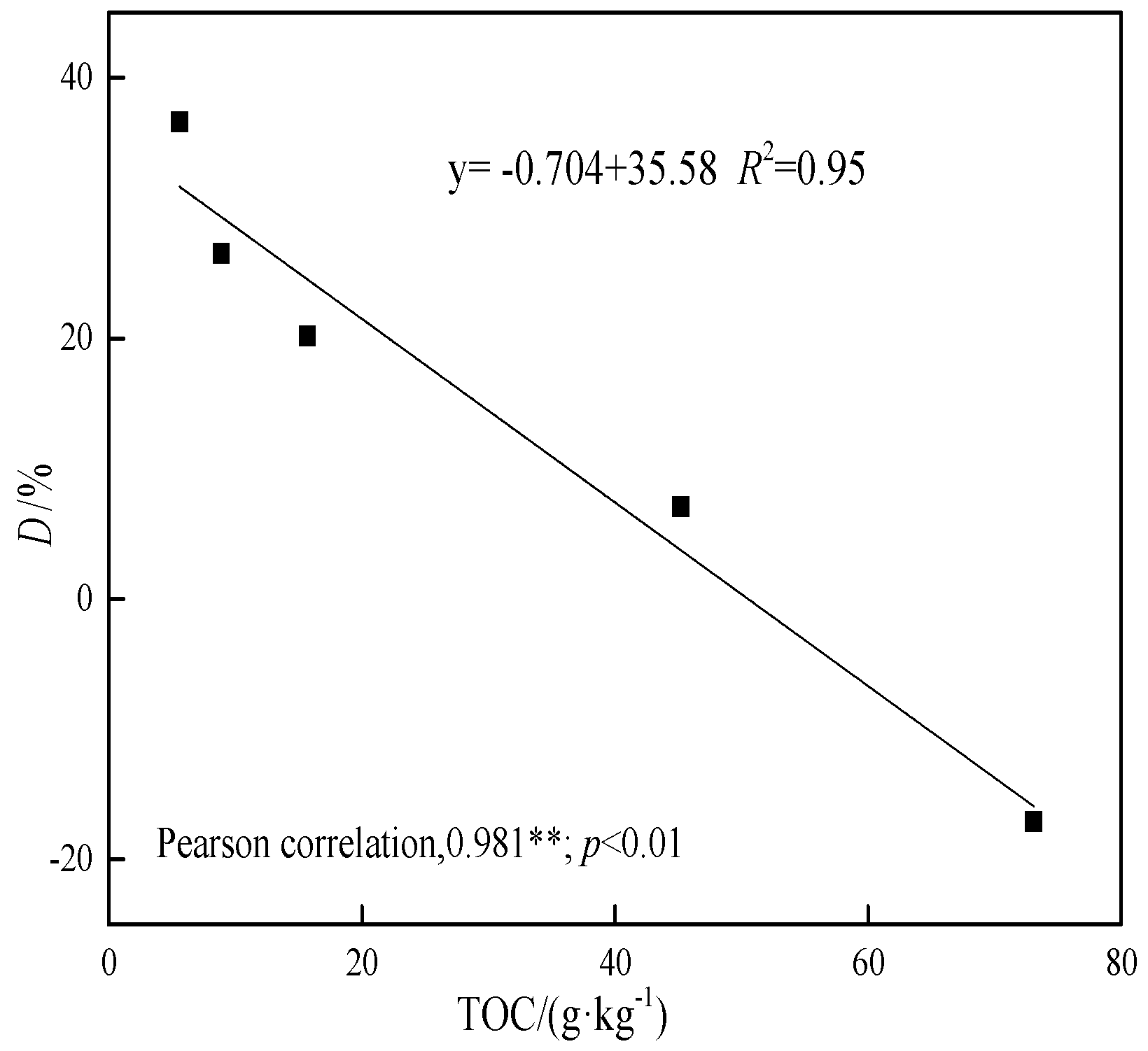
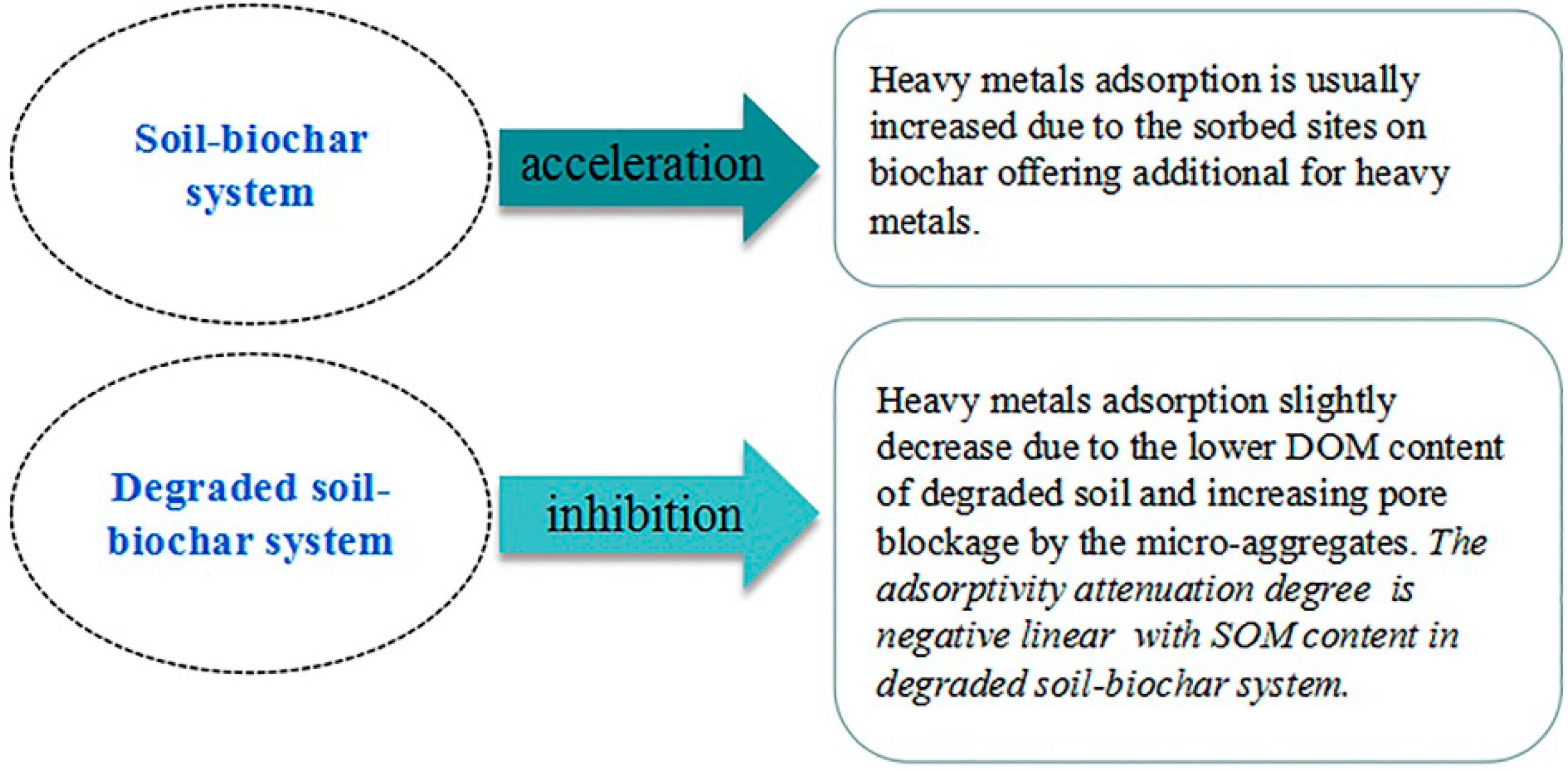
3.5. The Adsorption Inhibition and Acceleration Effect in Soil–Biochar System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in china: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Rötting, T.S.; Mercado, M.; García, M.E.; Quintanilla, J. Environmental distribution and health impacts of As and Pb in crops and soils near Vinto smelter, Oruro, Bolivia. Int. J. Environ. Sci. Technol. 2014, 11, 935–948. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Puga, A.P.; Coscione, A.R.; Beesley, L.; Abreu, C.A.; Camargo, O.A. Sorption and desorption of cadmium and zinc in two tropical soils amended with sugarcane-straw-derived biochar. J. Soils Sediment 2016, 16, 226–234. [Google Scholar] [CrossRef]
- Matei, N.; Popescu, A.; Radu, G.L.; Artem, V.; Pavalache, G. Cadmium and lead occurrence in soil and grape from murfatlar vineyard. Ovidius Univ. Ann. Chem. 2015, 26, 37–40. [Google Scholar] [CrossRef]
- Naushad, M.; ALothman, Z.A.; Islan, M. Adsorption of cadmium ion using a new composite cation-exchanger; polyaniline sn(iv) silicate: Kinetics, thermodynamic and isotherm; studies. Int. J. Environ. Sci. Technol. 2013, 10, 567–578. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Alothman, Z.A.; Shar, M.A.; Alhokbany, N.S.; Alshehri, S.M. Synthesis, characterization and application of curcumin formaldehyde resin for the removal of cd 2+, from wastewater: Kinetics, isotherms and thermodynamic studies. J. Ind. Eng. Chem. 2015, 29, 78–86. [Google Scholar] [CrossRef]
- Ministry of environmental protection; Land and resources bureauNational. Soil pollution survey communique. China Environ. Prot. Ind. 2014, 36, 10–11. [Google Scholar]
- Zhu, W.; Du, W.; Shen, X.; Zhang, H.; Ding, Y. Comparative adsorption of Pb2+ and Cd2+ by cow manure and its vermicompost. Environ. Pollut. 2017, 227, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chen, B.M.; Liu, L. Soil Heavy Metal Pollution of Cultivated Land in China. Res. Soil Water Conserv. 2013, 20, 293–298. [Google Scholar]
- Khan, M.A.; Ding, X.; Khan, S.; Brusseau, M.L.; Khan, A.; Nawab, J. The influence of various organic amendments on the bioavailability and plant uptake of cadmium present in mine-degraded soil. Sci. Total Environ. 2018, 636, 810. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.F.; Li, M.X.; Sun, T. The Status and Trend Analysis of Desertification and Sandification. For. Resour. Manag. 2016, 1, 1–5. [Google Scholar]
- Khanamani, A.; Fathizad, H.; Karimi, H.; Shojaei, S. Assessing desertification by using soil indices. Arab. J. Geosci. 2017, 10, 287. [Google Scholar] [CrossRef]
- Gad, A.; Abdelsamie, A.G. Study on desertification of irrigated arable lands in egypt. ii-salinization. Egypt. J. Soil Sci. 2000, 40, 373–384. [Google Scholar]
- Li, J.Y.; Liang, C.H.; Du, L.Y. Effects of Organic Matter on Mercury Adsorption-Desorption in Brown Soil. Bull. Soil Water Conserv. 2014, 34, 32–35. [Google Scholar]
- Song, B.; Zeng, W.Q. Effects of Organic Matter on the Remediation of Cadmium-Contaminated Soil-A Review. Chin. J. Soil Sci. 2015, 46, 1018–1024. [Google Scholar]
- Wu, T.; Li, X.P.; Cai, Y.; Ai, Y.W.; Sun, X.M.; Yu, H.T. Geochemical behavior and risk of heavy metals in different size lead-polluted soil particles. China Environ. Sci. 2017, 37, 4212–4221. [Google Scholar]
- Clemente, R.; Pardo, T.; Madejón, P.; Madejón, E.; Bernal, M.P. Food byproducts as amendments in trace elements contaminated soils. Food Res. Int. 2015, 73, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.W.; Zhang, X.H.; Zhang, J.F.; et al. Biochar soil amendment as a solution to prevent cd-tainted rice from china: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Gao, F.X.; Li, M.K.; Luo, X.P. Effects of Biochar-Derived Sewage Sludge on Heavy Metal Adsorption and Immobilization in Soils. Int. J. Environ. Res. Public Health 2017, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Li, Y.; Pei, G.; Qiao, X.; Zhu, Y.; Li, H. Remediation of cadmium contaminated water and soil using vinegar residue biochar. Environ. Sci. Pollut. R. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (pahs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246. [Google Scholar] [CrossRef] [PubMed]
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Liu, J.J.; Yang, X.; Lu, K.P.; Zhang, X.K.; Huang, H.G.; Wang, H.L. Effect of bamboo and rice straw biochars on the transformation and bioavailability of heavy metals in soil. Acta Sci. Circumstantiae 2015, 35, 3679–3687. [Google Scholar] [CrossRef]
- Qi, F.; Kuppusamy, S.; Naidu, R.; Bolan, N.S.; Ok, Y.S.; Lamb, D.; Li, Y.B.; Yu, L.B.; Semple, K.T.; Wang, H.L. Pyrogenic carbon and its role in contaminant immobilization in soils. Crit. Rev. Environ. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Zhou, F.S.; Wang, H.; Fang, S.E.; Zhang, W.H.; Qiu, R.L. Pb(II), Cr(VI) and atrazine sorption behavior on sludge-derived biochar: Role of humic acids. Environ. Sci. Pollut. Res. 2015, 22, 16031–16039. [Google Scholar] [CrossRef] [PubMed]
- You, Q.G.; Xue, X.; Peng, F.; Dong, S.Y. Alpine Meadow Degradation Effect on Soil Thermal and Hydraulic Properties and Its Environmental Impacts. J. Desert Res. 2015, 35, 1183–1192. [Google Scholar]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Wan, T.; Tu, W.G.; Xi, H.; Li, Y.D.; Tang, X.F.; Yang, Y.C. Study on Vegetation and Soil Characteristics of Desertification Grassland in Northwest Sichuan. Acta Agrestia Sin. 2013, 21, 650–657. [Google Scholar]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Alqadami, A.A. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2015, 330, 1351–1360. [Google Scholar] [CrossRef]
- Yang, H.M.; Li, Y.G.; Wu, C.X.; Jiang, R.T.; Li, F.C.; Zhao, L.; Wei, L. Sorption of bisphenol A on degraded soils in Northwest Sichuan. China Environ. Sci. 2018, 38, 1424–1432. [Google Scholar] [CrossRef]
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Schmidt, M.W.I. Characterisation and evaluation of reference materials for black carbon analysis using elemental composition, colour, BET surface area and C NMR spectroscopy. Appl. Geochem. 2008, 23, 2113–2122. [Google Scholar] [CrossRef]
- Yu, J.T.; Dehkhoda, A.M.; Ellis, N. Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels 2011, 25, 337–344. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel metal–organic framework (mof) based composite material for the sequestration of u(vi) and th(iv) metal ions from aqueous environment. Acs Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.A.M.; Matos, G.S.B.D.; Fernandes, A.R.; Barros, K.R.M.; Amarante, C.B.D. Adsorption of cadmium and copper in representative soils of eastern amazonia, brazil. Semin. Cienc. Agrar. 2016, 37, 3005–3016. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z. Cadmium adsorption in soil influenced by dissolved organic matter derived from rice straw and sediment. Ying Yong Sheng Tai Xue Bao 2002, 13, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Zhu, X.F.; Qian, W.; Zhao, M.H.; Xu, R.K.; Yu, Y.C. Adsorption of Cd(II) by two variable-charge soils in the presence of pectin. Environ. Sci. Pollut. Res. 2016, 23, 12976–12982. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ren, J.; Tao, L.; Li, H.; Hao, J.X. Effect of sludge biochars obtained at different pyrolysis temperatures on the adsorption of Cd(II) by loess in northwestern china. Polish J. Environ. Stud. 2017, 26. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, Y.; Sun, K.; Gao, B.; Wang, Z.; Jin, J.; Zhang, Z.Y.; Wang, S.F.; Yan, Y.; Liu, X.T. Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties. Chemosphere 2014, 111, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yan, J.; Li, L.; Quan, G.; Ding, C.; Chen, T.M.; Yin, C.T.; Gao, J.F.; Hussain, Q. Does biochar alter the speciation of cd and pb in aqueous solution? Bioresources 2014, 10, 88–104. [Google Scholar] [CrossRef]
- Li, D.; Hockaday, W.C.; Masiello, C.A.; Alvarez, P.J.J. Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol. Biochem. 2013, 43, 1732–1737. [Google Scholar] [CrossRef]
- Wang, T.T.; Li, Y.S.; Jiang, A.C.; Lu, M.X.; Liu, X.J.; Yu, X.Y. Suppression of Chlorantraniliprole Sorption on Biochar in Soil-Biochar Systems. Bull. Environ. Contam. Toxicol. 2015, 95, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; He, A.F.; Jiang, J.; Feng, J.Y.; Sheng, G.D. Quantitative relationships between the adsorptivity of carbonaceous materials in soil for Pb(II) and soil organic matter content. Sci. Total Environ. 2016, 572, 369–378. [Google Scholar] [CrossRef] [PubMed]
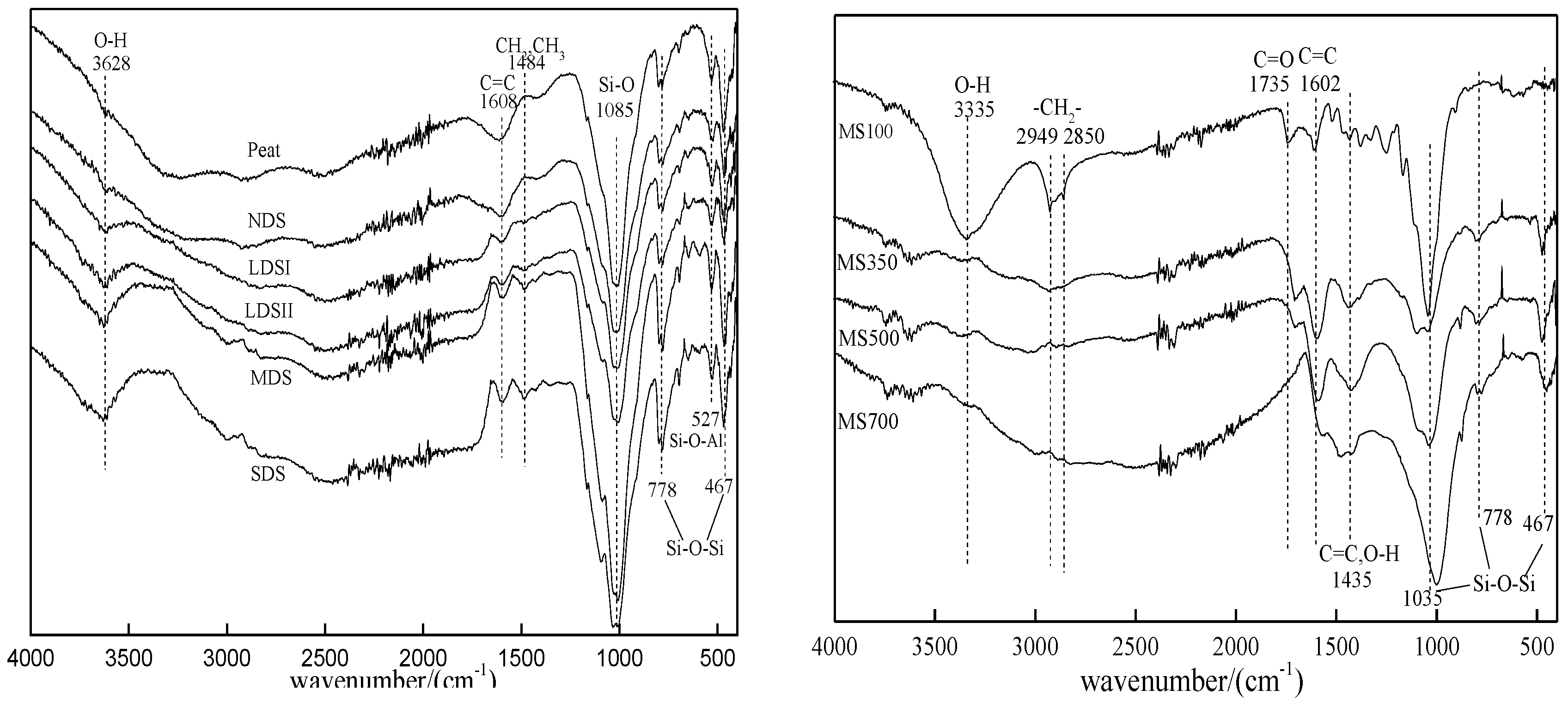
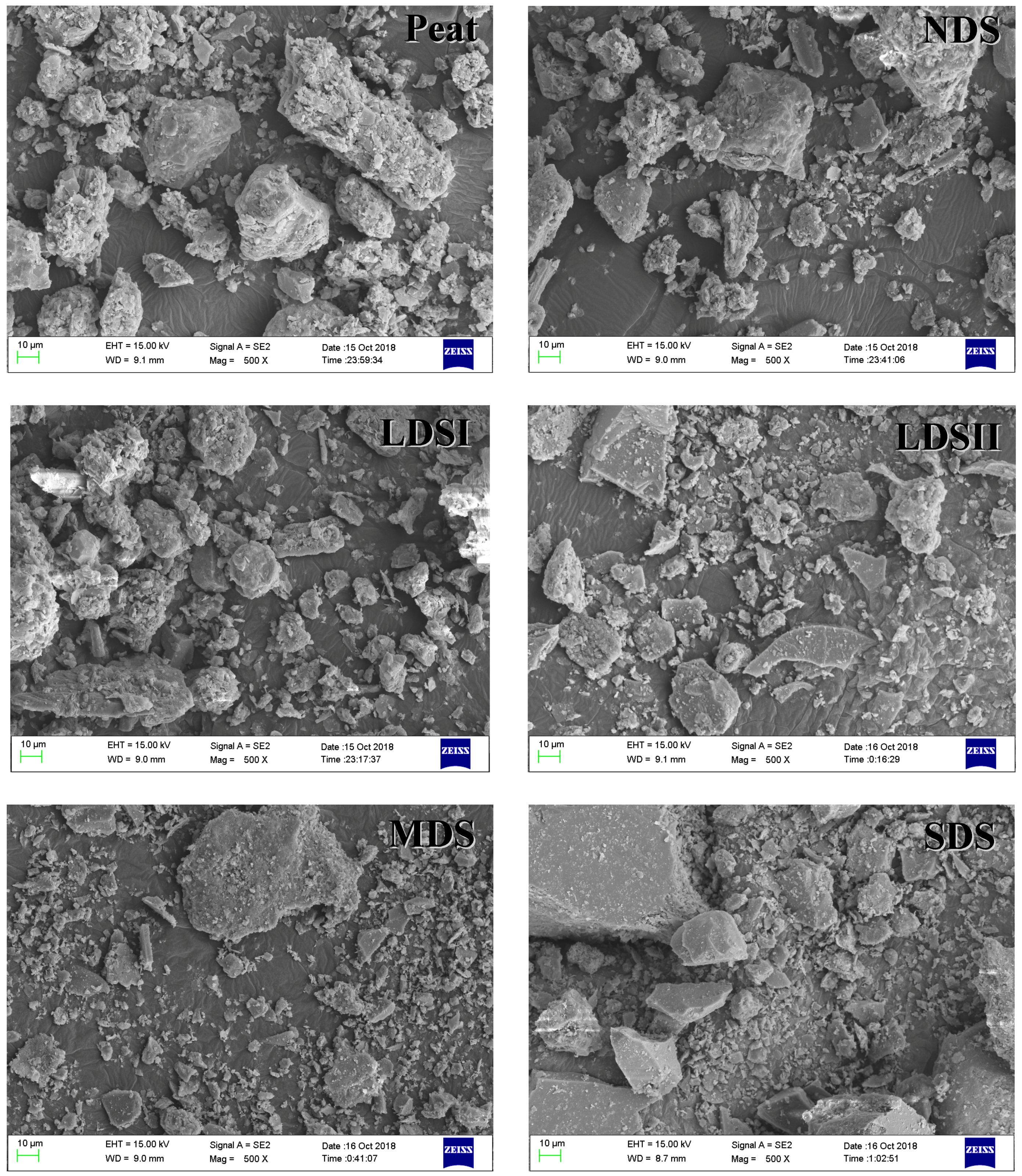
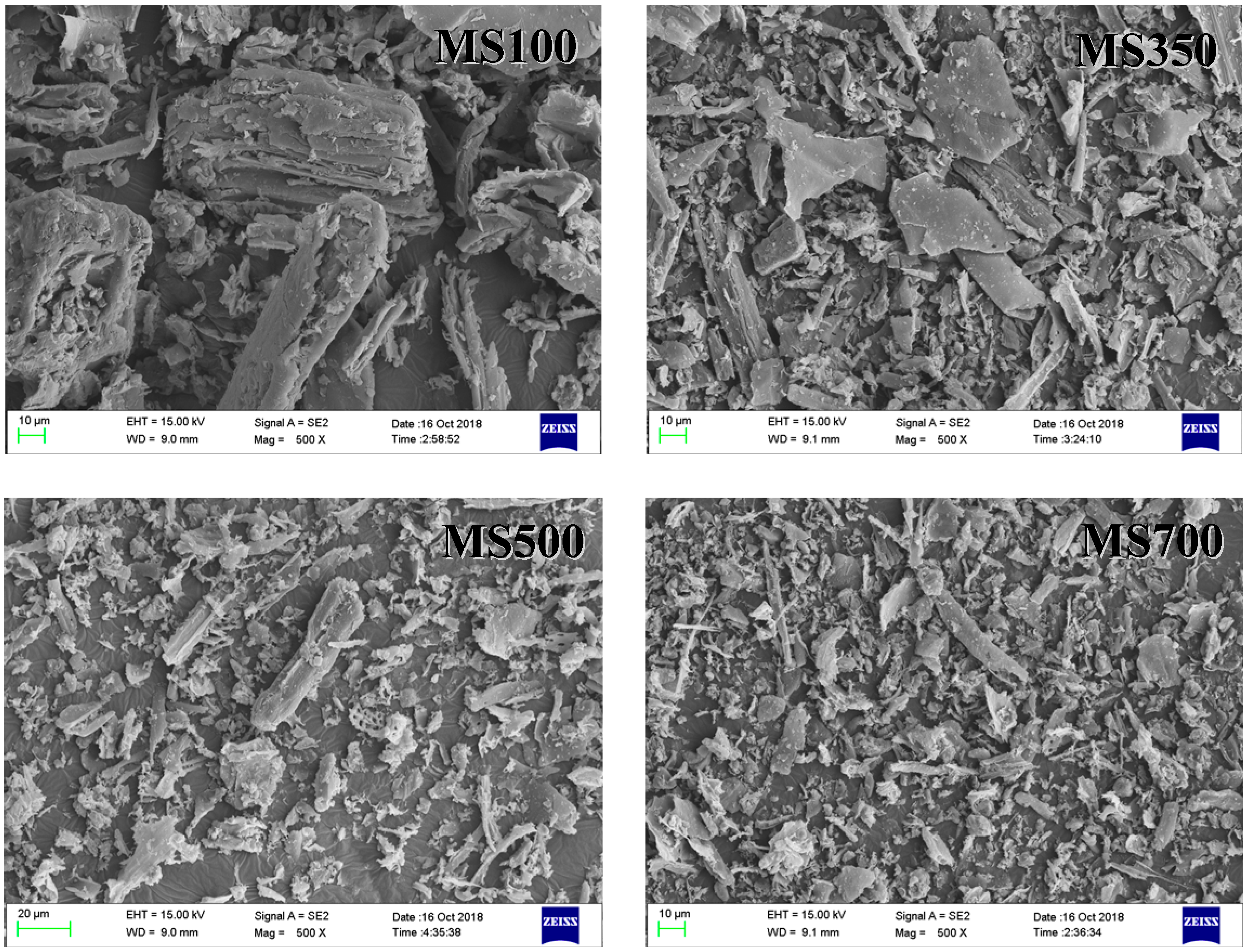



| Soil | Geographic Location | Moisture Content/% | pH | SSA/(m2·g−1) | TOC/(g·kg−1) |
|---|---|---|---|---|---|
| Peat 1) | N 33°10′23.0″, E 102°37′2.4″ | 88.26 | 5.72 | 3.10 | 92.7 |
| NDS 1) | N 33°10′47.555″, E 102°37′34.172″ | 48.14 | 5.73 | 2.02 | 73.1 |
| LDSI | N 33°19′36.0″, E 102°33′58.9″ | 33.47 | 6.03 | 2.52 | 45.2 |
| LDSII 1) | N 33°10′45.710″, E 102°37′34.016″ | 10.53 | 6.40 | 2.71 | 15.7 |
| MDS 1) | N 33°10′43.667″, E 102°37′33.488″ | 8.77 | 6.59 | 3.06 | 8.90 |
| SDS | N 33°19′41.5″, E 102°33′42.5″ | 4.47 | 6.91 | 3.11 | 5.60 |
| Biochar | Yield/wt% | Ash/% | d50 1)/μm | SSA/(m2·g−1) | Elemental Composition and Atomic Ratio of Biochar Organic Components 2) | pH | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C/wt% | H/wt% | N/wt% | O/wt% | (O+N)/C | H/C | ||||||
| MS100 | 100.00 | 14.61 | 11.36 | 3.09 | 44.75 | 6.32 | 1.36 | 47.57 | 0.82 | 1.70 | 6.32 |
| MS350 | 47.58 | 34.90 | 9.31 | 3.82 | 63.97 | 4.66 | 2.56 | 28.81 | 0.37 | 0.87 | 6.49 |
| MS500 | 33.70 | 36.20 | 9.09 | 6.03 | 66.38 | 2.93 | 2.40 | 28.29 | 0.35 | 0.53 | 8.50 |
| MS700 | 30.40 | 41.67 | 8.04 | 133.4 | 72.49 | 2.16 | 1.83 | 23.51 | 0.26 | 0.36 | 9.08 |
| Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·kg−1) | KL/(L·mg−1) | R2 | Kf/(mg·kg−1)(mg/L−1)-N | N | R2 | ||
| Soil 1) | Peat | 6309 | 0.025 | 0.99 | 343 | 0.567 | 0.97 |
| NDS | 5361 | 0.022 | 0.99 | 261 | 0.583 | 0.98 | |
| LDSI | 1710 | 0.054 | 0.99 | 126 | 0.555 | 0.98 | |
| LDSII | 1028 | 0.035 | 0.99 | 58 | 0.584 | 0.98 | |
| MDS | 553 | 0.037 | 0.99 | 33 | 0.583 | 0.99 | |
| SDS | 170 | 0.047 | 0.99 | 15 | 0.500 | 0.99 | |
| Biochar 2) | MS 100 | 2622 | 0.024 | 0.92 | 132 | 0.586 | 0.86 |
| MS 350 | 22987 | 0.030 | 0.99 | 1483 | 0.549 | 0.99 | |
| MS 500 | 28391 | 0.061 | 0.97 | 3637 | 0.425 | 0.91 | |
| MS 700 | 49016 | 0.047 | 0.98 | 4813 | 0.470 | 0.96 | |
| Adsorbent | Adsorption Capacity (Qm, mg·kg−1) | References |
|---|---|---|
| Vinegar residue biochar 700 °C | 2910 | [21] |
| Wheat straw biochar 450 °C | 5000 | [40] |
| Wheat straw biochar 600 °C | 1960 | [40] |
| Swine manure biochar 300 °C | 42440 | [40] |
| Peanut hull biochar 450 °C | 6740 | [41] |
| Maize stalk biochar 700 °C | 49016 | This study |
| Sample | RC1) (%) | D2) (%) | Q’m,soil+MS3)/(mg·kg−1) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RCsoil | RCbiochar | Qm/(mg·kg−1) | KL/(L·mg−1) | R2 | Kf/(mg·kg−1)(mg·L−1)−N | N | R2 | |||
| MDS | 100 | -- | -- | -- | 553 | 0.037 | 0.99 | 33.3 | 0.583 | 0.99 |
| MDS+1%MS 700 | 67.7 | 60.6 | 22.1 | 1038 | 809 | 0.047 | 0.95 | 93.7 | 0.434 | 0.93 |
| MDS+2%MS 700 | 48.5 | 87.7 | 26.5 | 1522 | 1118 | 0.063 | 0.99 | 153 | 0.413 | 0.97 |
| MDS+4%MS 700 | 30.5 | 113 | 30.1 | 2492 | 1742 | 0.072 | 0.99 | 231 | 0.432 | 0.95 |
| Sample | RC1) (%) | D2) (%) | Q’m,soil+MS3)/(mg·kg−1) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RCsoil | RCbiochar | Qm/(mg·kg−1) | KL/(L·mg−1) | R2 | Kf/(mg·kg−1)(mg·L−1)−N | N | R2 | |||
| MDS | 100 | -- | -- | -- | 553 | 0.037 | 0.99 | 33.3 | 0.583 | 0.99 |
| MDS+2%MS 100 | 90.2 | 8.73 | −1.2 | 594 | 601 | 0.027 | 0.99 | 41.1 | 0.509 | 0.95 |
| MDS+2%MS 350 | 62.4 | 52.9 | 13.3 | 1002 | 869 | 0.046 | 0.98 | 86.7 | 0.480 | 0.93 |
| MDS+2%MS 500 | 53.9 | 56.4 | 9.4 | 1110 | 1006 | 0.058 | 0.99 | 129 | 0.424 | 0.97 |
| MDS+2%MS 700 | 48.5 | 87.7 | 26.5 | 1522 | 1118 | 0.063 | 0.99 | 153 | 0.413 | 0.97 |
| Sample | RC1) (%) | D2) (%) | Q’m,soil+MS3)/(mg·kg−1) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RCsoil | RCbiochar | Qm/(mg·kg−1) | KL/(L·mg−1) | R2 | Kf/(mg·kg−1)(mg·L−1)-N | N | R2 | |||
| NDS+2%MS 700 | 72 | 13.4 | −17.1 | 6234 | 7300 | 0.018 | 0.99 | 265 | 0.639 | 0.98 |
| LDSI+2%MS 700 | 67.9 | 39.7 | 7.1 | 2656 | 2468 | 0.033 | 0.96 | 170 | 0.540 | 0.92 |
| LDSII+2%MS 700 | 63.5 | 61.8 | 20.2 | 1988 | 1586 | 0.051 | 0.97 | 171 | 0.461 | 0.92 |
| MDS+2%MS 700 | 48.5 | 87.7 | 26.5 | 1522 | 1118 | 0.063 | 0.99 | 153 | 0.413 | 0.97 |
| SDS+2%MS 700 | 22.9 | 134.8 | 36.6 | 1147 | 727 | 0.134 | 0.89 | 180 | 0.299 | 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Li, Y.; Chen, M.; Luo, X.; Chen, Y.; Belzile, N.; Huang, S. Adsorption of Cadmium on Degraded Soils Amended with Maize-Stalk-Derived Biochar. Int. J. Environ. Res. Public Health 2018, 15, 2331. https://doi.org/10.3390/ijerph15112331
Wu C, Li Y, Chen M, Luo X, Chen Y, Belzile N, Huang S. Adsorption of Cadmium on Degraded Soils Amended with Maize-Stalk-Derived Biochar. International Journal of Environmental Research and Public Health. 2018; 15(11):2331. https://doi.org/10.3390/ijerph15112331
Chicago/Turabian StyleWu, Caixia, Yungui Li, Mengjun Chen, Xiang Luo, Yuwei Chen, Nelson Belzile, and Sheng Huang. 2018. "Adsorption of Cadmium on Degraded Soils Amended with Maize-Stalk-Derived Biochar" International Journal of Environmental Research and Public Health 15, no. 11: 2331. https://doi.org/10.3390/ijerph15112331
APA StyleWu, C., Li, Y., Chen, M., Luo, X., Chen, Y., Belzile, N., & Huang, S. (2018). Adsorption of Cadmium on Degraded Soils Amended with Maize-Stalk-Derived Biochar. International Journal of Environmental Research and Public Health, 15(11), 2331. https://doi.org/10.3390/ijerph15112331








