Earthworms, Rice Straw, and Plant Interactions Change the Organic Connections in Soil and Promote the Decontamination of Cadmium in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Earthworms and Rice Straw
2.3. Experiment Design
2.4. Analytical Test
2.5. Bioconcentration Factor, Translocation Factor and Kinetics Parameters k1 and k2
2.6. Fourier Transform Infrared Spectra of Rice Straw
2.7. Statistical Analysis
3. Results
3.1. Earthworm Survival and Body Weight Variation
3.2. Soil Physical and Chemical Analysis
3.3. Cd Concentration
3.3.1. Cd Accumulation in Earthworms
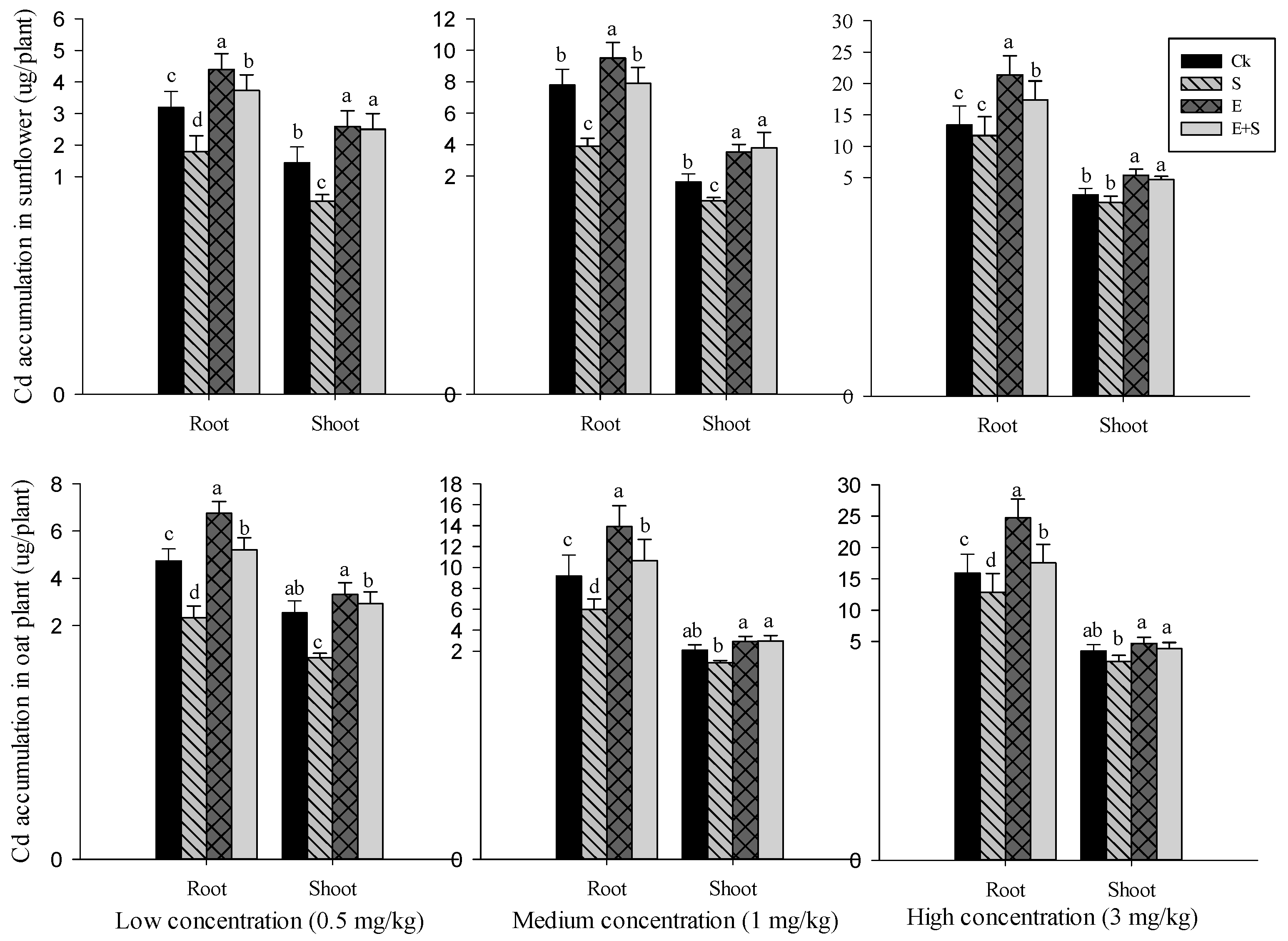
3.3.2. Cd Accumulation in Plant Tissues
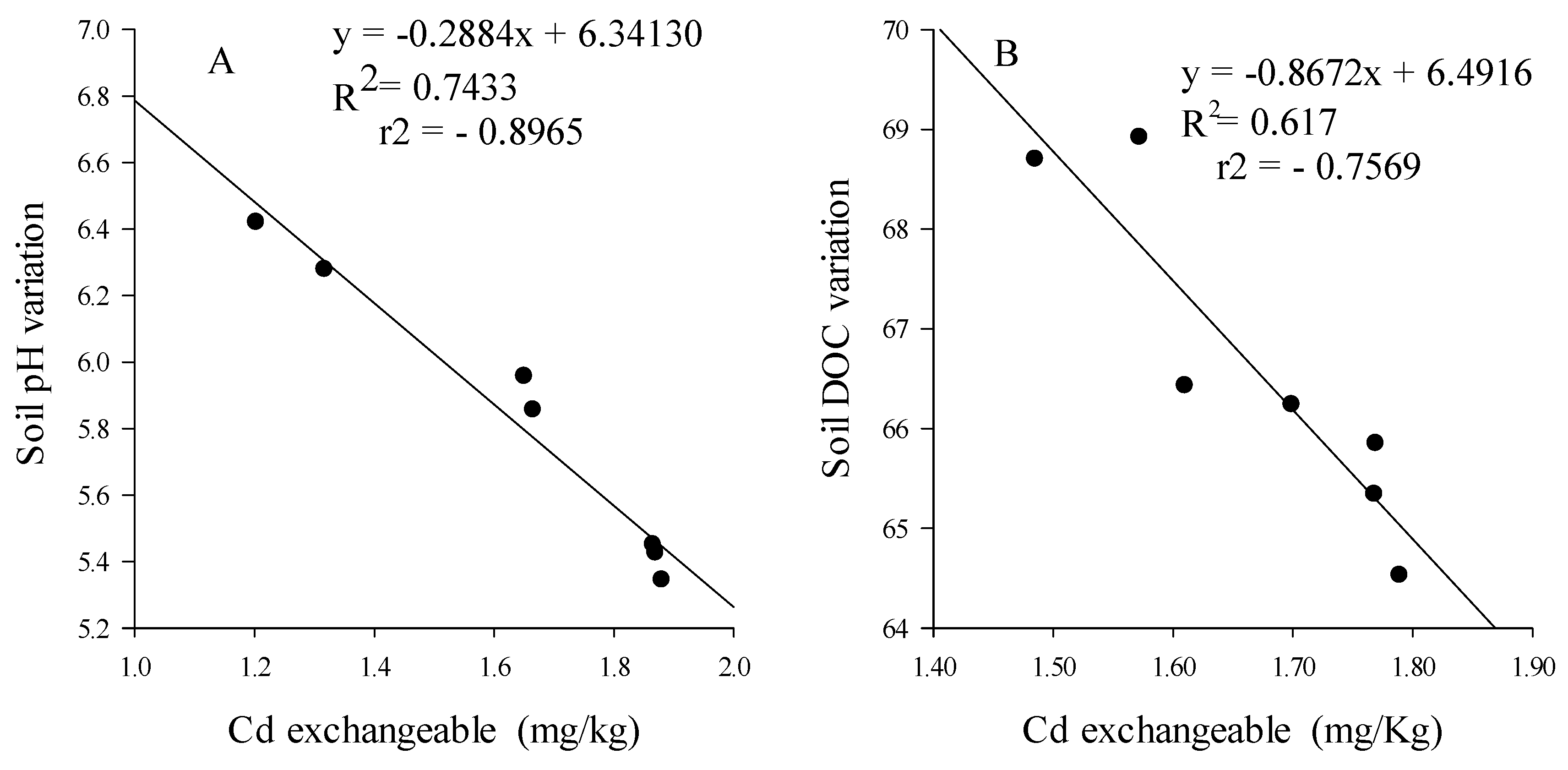
3.3.3. Cd Bioavailability and Mobility
3.4. Fourier Transform Infrared Spectra of Rice Straw
4. Discussion
4.1. Earthworm Survival and Body Weight Variation
4.2. Cd Accumulation
4.2.1. Earthworms
4.2.2. Plants
4.3. Earthworms Change Chemical Form of Cd
4.4. Rice Straw Change Chemical Form of Cd and Sequestrate It in Soil
4.5. Earthworms, Rice Straw and Plants Interaction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kremer, R.J. Environmental implications of herbicide resistance: Soil biology and ecology. Weed Sci. 2014, 62, 415–426. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- de los Reyes, J.A. Mining shareholder value: Institutional shareholders, transnational corporations and the geography of gold mining. Geoforum 2017, 84, 251–264. [Google Scholar] [CrossRef]
- Ignatowicz, K. The impact of sewage sludge treatment on the content of selected heavy metals and their fractions. Environ. Res. 2017, 156, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Xiyili, H.; Çetintaş, S.; Bingöl, D. Removal of some heavy metals onto mechanically activated fly ash: Modeling approach for optimization, isotherms, kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 109, 288–300. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhu, L.; Wang, J. Individual and combined effects of enrofloxacin and cadmium on soil microbial biomass and the ammonia-oxidizing functional gene. Sci. Total Environ. 2018, 624, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Árvay, J.; Tomáš, J.; Hauptvogl, M.; Massányi, P.; Harangozo, Ľ.; Tóth, T.; Stanovič, R.; Bryndzová, Š.; Bumbalová, M. Human exposure to heavy metals and possible public health risks via consumption of wild edible mushrooms from Slovak Paradise National Park, Slovakia. J. Environ. Sci. Health Part B 2015, 50, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Dickinson, N. Phytoremediation. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Brian, T., Brian, G.M., Denis, J.M., Eds.; Elsivier: Amsterdam, The Netherlands, 2017; Volume 3, pp. 321–327. ISBN 978-0-12-394808-3. [Google Scholar]
- Harms, H. 6.09 Bioavailability and Bioaccessibility as Key Factors in Bioremediation. In Comprehensive Biotechnology, 2nd ed.; Murray, M.Y., Ed.; Elsivier: Amsterdam, The Netherlands, 2011; Volume 6, pp. 83–94. ISBN 978-0-08-088504-9. [Google Scholar]
- Al-Wabel, M.I.; Usman, A.R.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M. Mobility of heavy metals in sandy soil after application of composts produced from maize straw, sewage sludge and biochar. J. Environ. Manag. 2018, 210, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Piron, D.; Boizard, H.; Heddadj, D.; Pérès, G.; Hallaire, V.; Cluzeau, D. Indicators of earthworm bioturbation to improve visual assessment of soil structure. Soil Tillage Res. 2017, 173, 53–63. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Robinson, B.; Lee, K.-A.; Boyer, S.; Dickinson, N. Interactions between earthworm burrowing, growth of a leguminous shrub and nitrogen cycling in a former agricultural soil. Appl. Soil Ecol. 2017, 110, 79–87. [Google Scholar] [CrossRef]
- Lin, Z.; Zhen, Z.; Ren, L.; Yang, J.; Luo, C.; Zhong, L.; Hu, H.; Zhang, Y.; Li, Y.; Zhang, D. Effects of two ecological earthworm species on atrazine degradation performance and bacterial community structure in red soil. Chemosphere 2017, 196, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Garg, V. Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia fetida (Sav.). Bioresour. Technol. 2018, 250, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Kai, X.; Li, R.; Yang, T.; Shen, S.; Ji, Q.; Zhang, T. Study on the co-pyrolysis of rice straw and high density polyethylene blends using TG-FTIR-MS. Energy Convers. Manag. 2017, 146, 20–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Han, H.; Qiu, Z.; Achal, V. Stimulatory effect of in-situ detoxification on bioethanol production by rice straw. Energy 2017, 135, 32–39. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Zhao, L.; Liu, B.; Ren, N. Alkali/Urea pretreatment of rice straw at low temperature for enhanced biological hydrogen production. Bioresour. Technol. 2018, 267, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.G.; Zaia, D.A.M.; da Silva Alfaya, R.V.; da Silva Alfaya, A.A. Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J. Hazard. Mater. 2009, 166, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating binding characteristics of cadmium and copper to DOM derived from compost and rice straw using EEM-PARAFAC combined with two-dimensional FTIR correlation analyses. J. Hazard. Mater. 2018, 344, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.-F.; Lin, X.-G.; Bai, J.-F.; Shao, Y.-F.; Yin, R.; Jiang, Q. Effects of arbuscular mycorrhizal fungi and earthworm on nematode communities and arsenic uptake by maize in arsenic-contaminated soils. Pedosphere 2010, 20, 163–173. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Sim, E.Y.S.; Lim, P.N.; Clarke, C. Biotransformation of rice husk into organic fertilizer through vermicomposting. Ecol. Eng. 2012, 41, 60–64. [Google Scholar] [CrossRef]
- Dittbrenner, N.; Moser, I.; Triebskorn, R.; Capowiez, Y. Assessment of short and long-term effects of imidacloprid on the burrowing behaviour of two earthworm species (Aporrectodea caliginosa and Lumbricus terrestris) by using 2D and 3D post-exposure techniques. Chemosphere 2011, 84, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, D.; Wang, P.; Peijnenburg, W.J. Kinetics of cadmium uptake and subcellular partitioning in the earthworm Eisenia fetida exposed to cadmium-contaminated soil. Arch. Environ. Contam. Toxicol. 2009, 57, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Z.; Zhou, D.-M.; Wang, P.; Allen, H.E.; Sauvé, S. Predicting Cd partitioning in spiked soils and bioaccumulation in the earthworm Eisenia fetida. Appl. Soil Ecol. 2009, 42, 118–123. [Google Scholar] [CrossRef]
- ASTM D4972, 2007 C. Standard Test Method for pH of Soils; ASTM International: West Conshohocken, PA, USA, 2007.
- Sakan, S.; Đorđević, D.; Dević, G.; Relić, D.; Anđelković, I.; Ðuričić, J. A study of trace element contamination in river sediments in Serbia using microwave-assisted aqua regia digestion and multivariate statistical analysis. Microchem. J. 2011, 99, 492–502. [Google Scholar] [CrossRef]
- Fabrizio de Iorio, A. Sorption Capacity of Cu and Zn in Natraquols of Depressed Pampa (Argentina). Relationship with Geochemical Phases. Ph.D. Thesis, University of Vigo, Pontevedra, Spain, 2010. [Google Scholar]
- Spurgeon, D.; Hopkin, S. Comparisons of metal accumulation and excretion kinetics in earthworms (Eisenia fetida) exposed to contaminated field and laboratory soils. Appl. Soil Ecol. 1999, 11, 227–243. [Google Scholar] [CrossRef]
- González-Alcaraz, M.N.; Loureiro, S.; van Gestel, C.A. Toxicokinetics of Zn and Cd in the earthworm Eisenia andrei exposed to metal-contaminated soils under different combinations of air temperature and soil moisture content. Chemosphere 2018, 197, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-L.; He, M.-M.; Xu, M.; Yan, Z.-G.; Zhou, Y.-Y.; Guo, G.-L.; Nie, J.; Wang, L.-Q.; Hou, H.; Li, F.-S. Interactive effects between earthworms and maize plants on the accumulation and toxicity of soil cadmium. Soil Biol. Biochem. 2014, 72, 193–202. [Google Scholar] [CrossRef]
- Wu, S.; Xu, X.; Zhao, S.; Shen, F.; Chen, J. Evaluation of phenanthrene toxicity on earthworm (Eisenia fetida): An ecotoxicoproteomics approach. Chemosphere 2013, 93, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.P.; Schmidt, O. The feeding ecology of earthworms—A review. Pedobiologia 2007, 50, 463–477. [Google Scholar] [CrossRef]
- Zhou, D.; Ning, Y.; Wang, B.; Wang, G.; Su, Y.; Li, L.; Wang, Y. Study on the influential factors of Cd2+ on the earthworm Eisenia fetida in oxidative stress based on factor analysis approach. Chemosphere 2016, 157, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bielská, L.; Hovorková, I.; Kuta, J.; Machát, J.; Hofman, J. The variability of standard artificial soils: Cadmium and phenanthrene sorption measured by a batch equilibrium method. Ecotoxicol. Environ. Saf. 2017, 135, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; White, R.A., III; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Matejicek, A.; Philippot, L.; Moreau, D. Positive effects of plant association on rhizosphere microbial communities depend on plant species involved and soil nitrogen level. Soil Biol. Biochem. 2017, 114, 1–4. [Google Scholar] [CrossRef]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; Van Hees, P.A.W. Organic acid behavior in soils–misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Kitturmath, M.; Giraddi, R.; Viraktamath, S.; Sattigi, H. Evaluation of different feeding additives for bio-degradation of groundnut shell and rice husk using earthworm, Eudrilus eugeniae (Kingberg). Karnataka J. Agric. Sci. 2010, 17, 52–56. [Google Scholar]
- Vijver, M.G.; Vink, J.P.; Miermans, C.J.; van Gestel, C.A. Oral sealing using glue: A new method to distinguish between intestinal and dermal uptake of metals in earthworms. Soil Biol. Biochem. 2003, 35, 125–132. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Z.; Xu, Y.; Li, D.; Li, M. Combined toxicity of cadmium and lead on the earthworm Eisenia fetida (Annelida, Oligochaeta). Ecotoxicol. Environ. Saf. 2012, 81, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Maleri, R.; Reinecke, A.; Reinecke, S. Metal uptake of two ecophysiologically different earthworms (Eisenia fetida and Aporrectodea caliginosa) exposed to ultramafic soils. Appl. Soil Ecol. 2008, 38, 42–50. [Google Scholar] [CrossRef]
- Ma, L.; Xie, Y.; Han, Z.; Giesy, J.P.; Zhang, X. Responses of earthworms and microbial communities in their guts to Triclosan. Chemosphere 2017, 168, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, F.J.; McGrath, S.P.; Luo, Y.M. Influence of soil properties and aging on arsenic phytotoxicity. Environ. Toxicol. Chem. 2006, 25, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsic, T.; Rengel, Z. The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol. Plant. 2010, 139, 303–312. [Google Scholar] [PubMed]
- Liang, S.-H.; Chen, S.-C.; Chen, C.-Y.; Kao, C.-M.; Yang, J.-I.; Shieh, B.-S.; Chen, J.-H.; Chen, C.-C. Cadmium-induced earthworm metallothionein-2 is associated with metal accumulation and counteracts oxidative stress. Pedobiologia 2011, 54, 333–340. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Convers. Manag. 2007, 48, 937–941. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Maqbool, S.; Zhong, H.; El-Maghraby, Y.; Ahmad, A.; Chai, B.; Wang, W.; Sabzikar, R.; Sticklen, M. Competence of oat (Avena sativa L.) shoot apical meristems for integrative transformation, inherited expression, and osmotic tolerance of transgenic lines containing hva1. Theor. Appl. Genet. 2002, 105, 201–208. [Google Scholar] [PubMed]
- Czerwiński, J.; Bartnikowska, E.; Leontowicz, H.; Lange, E.; Leontowicz, M.; Katrich, E.; Gorinstein, S. Oat (Avena sativa L.) and amaranth (Amaranthus hypochondriacus) meals positively affect plasma lipid profile in rats fed cholesterol-containing diets. J. Nutr. Biochem. 2004, 15, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Chirakkara, R.A.; Reddy, K.R. Biomass and chemical amendments for enhanced phytoremediation of mixed contaminated soils. Ecol. Eng. 2015, 85, 265–274. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant. Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Y.; Shen, M.; Zeng, G.; Zhou, M.; Li, M. Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresour. Technol. 2016, 218, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cheng, J.; Wong, M.H. Earthworm–mycorrhiza interaction on Cd uptake and growth of ryegrass. Soil Biol. Biochem. 2005, 37, 195–201. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Vega, F.; Silva, L.; Andrade, M. Soil interaction and fractionation of added cadmium in some Galician soils. Microchem. J. 2013, 110, 681–690. [Google Scholar] [CrossRef]
- Clemente, R.; Escolar, Á.; Bernal, M.P. Heavy metals fractionation and organic matter mineralisation in contaminated calcareous soil amended with organic materials. Bioresour. Technol. 2006, 97, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jing, D.; Gong, H.; Zhou, L.; Yang, X. Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresour. Technol. 2012, 114, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-L.; Li, J.-T.; Li, X.-J.; Ye, Y.-C.; Liu, S.-S.; Hallett, P.D.; Ogden, M.R.; Naveed, M. Physical protection by soil aggregates stabilizes soil organic carbon under simulated N deposition in a subtropical forest of China. Geoderma 2017, 285, 323–332. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Lv, B.; Xing, M.; Yang, J. Speciation and transformation of heavy metals during vermicomposting of animal manure. Bioresour. Technol. 2016, 209, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Role of earthworms in phytoremediation of cadmium (Cd) by modulating the antioxidative potential of Brassica juncea L. Appl. Soil Ecol. 2017, 124, 306–316. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, F.; Li, H.; Li, X. Influence of earthworm mucus and amino acids on tomato seedling growth and cadmium accumulation. Environ. Pollut. 2009, 157, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2017, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]





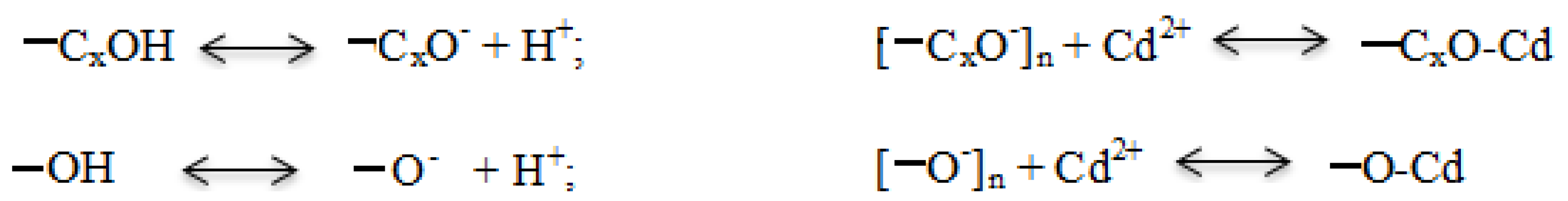
| Eisenia fetida Body Weight (mg) n = 6 | Aporrectodea caliginosa Body Weight (mg) n = 6 | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial Weight | Final Body Weight after 60 d | Initial Weight | Final Body Weight after 60 d | |||||
| Treat | 0 mg.kg | Low | Medium | High | 0 mg/kg | Low | Medium | High |
| E0 | 1835 ± 8.4 a | 1832 ± 4.8 a | 1841 ± 5.3 a | 1830 ± 6.1 a | 3044 ± 7.6 a | 3049 ± 5.8 a | 3039 ± 6.2 ab | 3045 ± 6.5 a |
| E0 + S | 1845 ± 6.8 ab | 1883 ± 7.1 a | 1856 ± 5.7 b | 1853 ± 7.8 b | 3025 ± 5.7 b | 3043 ± 5.6 a | 3038 ± 8.1 ab | 3045 ± 5.9 a |
| E1 | 1825 ± 6.3 ab | 1839 ± 5.7 a | 1829 ± 6.3 ab | 1827 ± 6.7 ab | 3026 ± 4.8 ab | 3028 ± 8.2 ab | 3037 ± 7.6 a | 3028 ± 4 ab |
| E1 + S | 1834 ± 5.9 bc | 1862 ± 4.5 a | 1855 ± 5.4 b | 1836 ± 5.7 bc | 3032 ± 5.9 b | 3045 ± 5.3 a | 3048 ± 5.2 a | 3039 ± 6.4 ab |
| E2 | 1842 ± 7.3 ab | 1851 ± 4.8 a | 1840 ± 7.6 ab | 1845 ± 8.2 ab | 3028 ± 7.3 ab | 3037 ± 6.2 a | 3027 ± 7.4 ab | 3025 ± 7.1 ab |
| E2 + S | 1859 ± 6.3 c | 1871 ± 5.4 a | 1864 ± 5.2 ab | 1867 ± 5.1 ab | 3041 ± 5.8 ab | 3054 ± 6.5 a | 3049 ± 5.7 a | 3042 ± 8.3 ab |
| Treatments | CEC | % OM | Planted Pots | Unplanted Pots | ||
|---|---|---|---|---|---|---|
| pH | DOC | pH | DOC | |||
| Ck | 12.83 | 10.23 | 7.4 | 38.356 | 7.28 | 30.569 |
| S | 11.58 | 37.24 *** | 7.32 | 45.569 * | 7.15 | 37.542 * |
| E | 16.74 * | 25.01 ** | 6.78 * | 42.659 * | 6.89 * | 35.247 * |
| E + S | 13.41 | 27.51 ** | 5.91 ** | 47.586 * | 6.042 * | 37.421 * |
| Eisenia fetida (n = 6) | Aporrectodea caliginosa (n = 6) | |||||
|---|---|---|---|---|---|---|
| Treatment | Low | Medium | High | Low | Medium | High |
| E0 | 1.69 ± 0.3 bc | 2.85 ± 0.4 c | 5.55 ± 0.3 ab | 2.15 ± 0.2 bc | 4.29 ± 0.4 b | 7.83 ± 0.3 b |
| E0 + S | 2.52 ± 0.2 a | 5.06 ± 0.6 a | 7.73 ± 0.5 a | 4.68 ± 0.4 a | 6.37 ± 0.5 a | 9.09 ± 0.8 a |
| E1 | 1.16 ± 0.4 c | 2.35 ± 0.5 d | 3.53 ± 0.6 c | 1.95 ± 0.3 bc | 2.74 ± 0.3 c | 4.74 ± 0.4 d |
| E1 + S | 1.97 ± 0.2 b | 3.63 ± 0.4 b | 5.72 ± 0.3 ab | 2.69 ± 0.5 b | 3.84 ± 0.7 bc | 5.39 ± 0.6 c |
| E2 | 1.12 ± 0.3 c | 2.47 ± 0.7 d | 3.64 ± 0.6 c | 1.85 ± 0.4 c | 2.85 ± 0.3 c | 3.95 ± 0.5 e |
| E2 + S | 1.84 ± 0.4 b | 2.97 ± 0.5 c | 4.74 ± 0.4 b | 2.58 ± 0.6 b | 3.74 ± 0.5 bc | 5.41 ± 0.6 c |
| Cd Concentration | E. fetida | A. caliginosa | ||||
|---|---|---|---|---|---|---|
| BCF | k1 (d−1) | k2 (d−1) | BCF | k1 (d−1) | k2 (d−1) | |
| Low | 1.31 ± 0.03 | 0.024 ± 0.01 | 0.018 ± 0.06 | 1.72 ± 0.04 | 0.022 ± 0.02 | 0.013 ± 0.04 |
| Medium | 1.20 ± 0.01 | 0.05 ± 0.02 | 0.041 ± 0.04 | 1.38 ± 0.02 | 0.05 ± 0.03 | 0.019 ± 0.01 |
| High | 0.64 ± 1.01 | 0.03 ± 0.02 | 0.048 ± 0.07 | 0.83 ± 0.01 | 0.03 ± 0.01 | 0.021 ± 1.02 |
| Sunflower Plant | Oat Plant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |||||||
| Exp | BCF | TF | BCF | TF | BCF | TF | BCF | TF | BCF | TF | BCF | TF |
| Ck | 1.21 ± 0.02 | 0.45 ± 0.04 | 0.65 ± 0.03 | 0.21 ± 0.02 | 0.35 ± 0.01 | 0.17 ± 0.04 | 1.67 ± 0.02 | 0.54 ± 0.03 | 1.12 ± 0.04 | 0.23 ± 0.02 | 0.59 ± 0.02 | 0.22 ± 0.01 |
| S | 0.64 ± 0.01 | 0.13 ± 0.02 | 0.38 ± 0.02 | 0.11 ± 0.03 | 0.31 ± 0.03 | 0.09 ± 0.03 | 0.78 ± 0.03 | 0.27 ± 0.02 | 0.53 ± 0.03 | 0.15 ± 0.03 | 0.37 ± 0.03 | 0.14 ± 0.03 |
| E | 1.38 ± 0.03 | 0.59 ± 0.03 | 0.82 ± 0.02 | 0.37 ± 0.01 | 0.67 ± 0.02 | 0.25 ± 0.02 | 1.43 ± 0.02 | 0.49 ± 0.04 | 0.88 ± 0.02 | 0.21 ± 0.03 | 0.43 ± 0.03 | 0.19 ± 0.02 |
| E + S | 1.01 ± 0.02 | 0.67 ± 0.04 | 0.46 ± 0.04 | 0.48 ± 0.03 | 0.39 ± 0.04 | 0.27 ± 0.01 | 1.21 ± 0.03 | 0.56 ± 0.02 | 0.66 ± 0.03 | 0.28 ± 0.02 | 0.40 ± 0.01 | 0.22 ± 0.03 |
| Wavenumber (cm−1) | Functional Groups | Compounds |
|---|---|---|
| 3708 | O–H stretching | H2O |
| 3430 | O–H stretching | cellulose and lignin |
| 2919 and 2853 | C–H stretching vibration | Aliphatic materials |
| 1640–1500 | C=O | Ketone, carbonyl group |
| 1450–1407 | C=C stretching vibration | Aromatic skeletal |
| 1388 | C–H blending vibration | alkanes |
| 1321–1302 | C–O stretching and O–H blending | phenols, alcohols and esters |
| 1242–1162 | C–O–C stretching | aryl-alkyl ether |
| 1070 | C–O–C stretching vibration or C–O stretching and C–O deformation | ethanol group |
| 1009 | C–O–H and O–H blending | Decomposition of hemicellulose and cellulose |
| 900–700 | C–H | Aromatic hydrogen |
| 700–400 | C–C stretching |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyamine, A.M.; Moussa, M.G.; Ismael, M.A.; Wei, J.; Zhao, Y.; Wu, Y.; Hu, C. Earthworms, Rice Straw, and Plant Interactions Change the Organic Connections in Soil and Promote the Decontamination of Cadmium in Soil. Int. J. Environ. Res. Public Health 2018, 15, 2398. https://doi.org/10.3390/ijerph15112398
Elyamine AM, Moussa MG, Ismael MA, Wei J, Zhao Y, Wu Y, Hu C. Earthworms, Rice Straw, and Plant Interactions Change the Organic Connections in Soil and Promote the Decontamination of Cadmium in Soil. International Journal of Environmental Research and Public Health. 2018; 15(11):2398. https://doi.org/10.3390/ijerph15112398
Chicago/Turabian StyleElyamine, Ali Mohamed, Mohamed G. Moussa, Marwa A. Ismael, Jia Wei, Yuanyuan Zhao, Yupeng Wu, and Chengxiao Hu. 2018. "Earthworms, Rice Straw, and Plant Interactions Change the Organic Connections in Soil and Promote the Decontamination of Cadmium in Soil" International Journal of Environmental Research and Public Health 15, no. 11: 2398. https://doi.org/10.3390/ijerph15112398
APA StyleElyamine, A. M., Moussa, M. G., Ismael, M. A., Wei, J., Zhao, Y., Wu, Y., & Hu, C. (2018). Earthworms, Rice Straw, and Plant Interactions Change the Organic Connections in Soil and Promote the Decontamination of Cadmium in Soil. International Journal of Environmental Research and Public Health, 15(11), 2398. https://doi.org/10.3390/ijerph15112398







