Global Identification of HIF-1α Target Genes in Benzene Poisoning Mouse Bone Marrow Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals, Treatments, and Blood Routine Examination
2.3. Chromatin Immunoprecipitation (ChIP) Assay
2.4. Data Analysis
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Benzene Induced Hematopoietic Toxicity and Increased ROS in Mice
3.2. ChIP-Seq Analysis
3.3. Screening of HIF-1α Responsive Genes
3.4. Gene Ontology (GO) Analysis
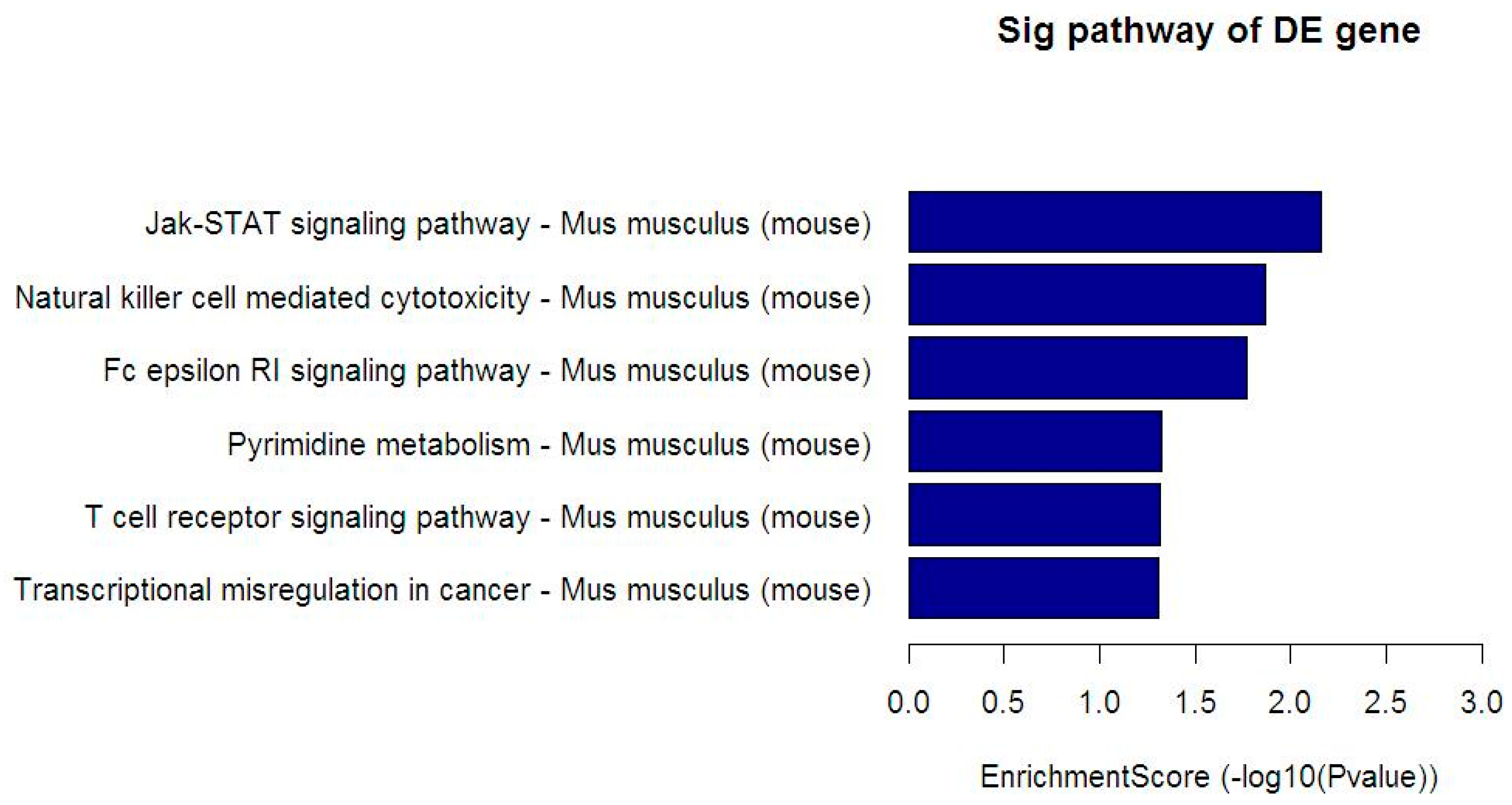
3.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
3.6. Validation of HIF-1α Target Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bird, M.G.; Greim, H.; Kaden, D.A.; Rice, J.M.; Snyder, R. Benzene 2009—Health effects and mechanisms of bone marrow toxicity: Implications for t-AML and the mode of action framework. Chem.-Biol. Interact. 2010, 184, 3–6. [Google Scholar] [CrossRef] [PubMed]
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Pyatt, D.W.; Hays, S.M.; English, C.; Cushing, C.A. United States Voluntary Children’s Chemical Evaluation Program (VCCEP) risk assessment for children exposed to benzene. Toxicol. Mech. Methods 2012, 22, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.; Alves, G.; Otero, U.B.; Tabalipa, M.M.; Scherrer, L.R.; Kosyakova, N.; Ornellas, M.H.; Liehr, T. Monitoring of gas station attendants exposure to benzene, toluene, xylene (BTX) using three-color chromosome painting. Mol. Cytogenet. 2014, 7, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Investigation into variation of endogenous metabolites in bone marrow cells and plasma in C3H/He mice exposed to benzene. Int. J. Mol. Sci. 2014, 15, 4994–5010. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Li, J.; Chen, L.; Xu, Z.; He, D.; Zhou, Y.; Zhu, Y.; Wei, F.; Li, J. Biomass fuels and coke plants are important sources of human exposure to polycyclic aromatic hydrocarbons, benzene and toluene. Environ. Res. 2014, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lai, Y.; Hu, K.; Wei, Q.; Liu, Y. Human CYP2E1-dependent and human sulfotransferase 1A1-modulated induction of micronuclei by benzene and its hydroxylated metabolites in Chinese hamster V79-derived cells. Mutat. Res. 2014, 770, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Yan, S.; Vestal, C.G. Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells. Int. J. Mol. Sci. 2015, 16, 2366–2385. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takubo, K.; Semenza, G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011, 9, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Urao, N.; Ushio-Fukai, M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Rad. Biol. Med. 2013, 54, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, R. The bone marrow niche, stem cells, and leukemia: Impact of drugs, chemicals, and the environment. Ann. N.Y. Acad. Sci. 2014, 1310, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Labbaye, C.; Castelli, G.; Pelosi, E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp. Hematol. 2016, 44, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Danet, G.H.; Pan, Y.; Luongo, J.L.; Bonnet, D.A.; Simon, M.C. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Investig. 2003, 112, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forristal, C.E.; Nowlan, B.; Jacobsen, R.N.; Barbier, V.; Walkinshaw, G.; Walkley, C.R.; Winkler, I.G.; Levesque, J.P. HIF-1alpha is required for hematopoietic stem cell mobilization and 4-prolyl hydroxylase inhibitors enhance mobilization by stabilizing HIF-1alpha. Leukemia 2015, 29, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.N.; Lim, J.; Shi, Y.; Joeng, K.S.; Arbeit, J.M.; Shohet, R.V.; Long, F. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 8673–8678. [Google Scholar] [CrossRef] [PubMed]
- Takubo, K.; Nagamatsu, G.; Kobayashi, C.I.; Nakamura-Ishizu, A.; Kobayashi, H.; Ikeda, E.; Goda, N.; Rahimi, Y.; Johnson, R.S.; Soga, T.; et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 2013, 12, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; D’Aprile, A.; Ripoli, M.; Scrima, R.; Lecce, L.; Boffoli, D.; Tabilio, A.; Capitanio, N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem. Biophys. Res. Commun. 2007, 353, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Ria, R.; Scrima, R.; Cela, O.; D’Aprile, A.; Boffoli, D.; Falzetti, F.; Tabilio, A.; Capitanio, N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J. Biol. Chem. 2005, 280, 26467–26476. [Google Scholar] [CrossRef] [PubMed]
- Urao, N.; McKinney, R.D.; Fukai, T.; Ushio-Fukai, M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: Role in reparative mobilization of progenitor cells. Stem Cells 2012, 30, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.Y.; Sharkis, S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007, 110, 3056–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Zhang, J.; Yin, L.; Pu, Y. Involvement of hypoxia-inducible factor-1 alpha (HIF-1alpha) in inhibition of benzene on mouse hematopoietic system. J. Toxicol. Environ. Health A 2016, 79, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, A.V.; Palade, C.; Voinescu, D.; Nica, D.A. Subarachnoid hemorrhage and cerebral vasospasm—Literature review. J. Med. Life 2013, 6, 120–125. [Google Scholar] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.V.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Oxygen Sensing and Homeostasis. Physiology 2015, 30, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Muller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Keswani, S.C.; Bosch-Marce, M.; Reed, N.; Fischer, A.; Semenza, G.L.; Hoke, A. Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc. Natl. Acad. Sci. USA 2011, 108, 4986–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.; Ponka, P.; Prchal, J.T. Hypoxia. 5. Hypoxia and hematopoiesis. Am. J. Physiol. Cell Physiol. 2011, 300, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-G.; Han, Z.-T.; He, S.-H.; Wu, X.-D.; Chen, T.-R.; Shao, C.-H.; Chen, D.-L.; Su, N.; Chen, Y.-M.; Wang, T.; et al. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 2017, 8, 24840–24852. [Google Scholar] [PubMed]
- Ren, H.; Accili, D.; Duan, C. Hypoxia converts the myogenic action of insulin-like growth factors into mitogenic action by differentially regulating multiple signaling pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 5857–5862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.; Xia, L.; Ning, X.; Liu, L.; Sun, W.; Huang, C.; Wang, H.; Sun, S. Hypoxia-induced Bmi1 promotes renal tubular epithelial cell-mesenchymal transition and renal fibrosis via PI3K/Akt signal. Mol. Biol. Cell 2014, 25, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-K.; Qian, D.; Kiel, M.; Becker, M.W.; Pihalja, M.; Weissman, I.L.; Morrison, S.J.; Clarke, M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003, 423, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Esplugues, A.; Estarlich, M.; Llop, S.; Cases, A.; Mantilla, E.; Ballester, F.; Iniguez, C. Infants’ indoor and outdoor residential exposure to benzene and respiratory health in a Spanish cohort. Environ. Pollut. 2017, 222, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Kocabas, F.; Zheng, J.; Deberardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hu, H.; Chang, R.; Zhong, J.; Knabel, M.; O’Meally, R.; Cole, R.N.; Pandey, A.; Semenza, G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011, 145, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Kusubata, K.; Tashiro, Y.; Gritli, I.; Sato, A.; Ohki-Koizumi, M.; Morita, Y.; Nagano, M.; Sakamoto, T.; Koshikawa, N.; et al. MT1-MMP plays a critical role in hematopoiesis by regulating HIF-mediated chemokine/cytokine gene transcription within niche cells. Blood 2012, 119, 5405–5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin. Cancer Biol. 2009, 19, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Chachami, G.; Paraskeva, E.; Mingot, J.M.; Braliou, G.G.; Gorlich, D.; Simos, G. Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem. Biophys. Res. Commun. 2009, 390, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Naora, H.; Naora, H. Down-regulated RPS3a/nbl Expression during Retinoid-induced Differentiation of HL-60 Cell: A Close Association with Diminished Susceptibility to Actinomycin D-stimulated Apoptosis. Cell Struct. Funct. 2000, 25, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Penna, I.; Du, H.; Ferriani, R.; Taylor, H.S. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Mol. Hum. Reprod. 2008, 14, 613–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiusolo, V.; Jacquemin, G.; Yonca Bassoy, E.; Vinet, L.; Liguori, L.; Walch, M.; Kozjak-Pavlovic, V.; Martinvalet, D. Granzyme B enters the mitochondria in a Sam50-, Tim22- and mtHsp70-dependent manner to induce apoptosis. Cell Death Differ. 2017, 24, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radke, I.; Gotte, M.; Smollich, M.; Scharle, N.; Kiesel, L.; Wulfing, P. Expression of PRL-3 regulates proliferation and invasion of breast cancer cells in vitro. Arch. Gynecol. Obstet. 2017, 296, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Moreira, S.; Moreno, S.G.; Ghinatti, G.; Lewandowski, D.; Hoffschir, F.; Ferri, F.; Gallouet, A.S.; Gay, D.; Motohashi, H.; Yamamoto, M.; et al. Low-Dose Irradiation Promotes Persistent Oxidative Stress and Decreases Self-Renewal in Hematopoietic Stem Cells. Cell Rep. 2017, 20, 3199–3211. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, N.; Kousari, A.; Kovalik, T.; Li, Y.; Bowser, R. RBM45 Modulates the Antioxidant Response in Amyotrophic Lateral Sclerosis through Interactions with KEAP1. Mol. Cell. Biol. 2015, 35, 2385–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Reagent | Volume |
|---|---|
| DNase/RNase-Free Water | 6 μL |
| SYBR Green PCR Master mix (Toyobo) | 10 μL |
| Forward /Reverse primer (100 μM) | 0.8 μL |
| ROX Reference Dye | 0.4 μL |
| cDNA | 2 μL |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | CTATGCTCTCCCTCACGCCA | TCACGCACGATTTCCCTCTC |
| Ptp4a3 | CCTGTAAGGCAGCCCCAACTA | GTGTCTTAGCCAGGGTTTTATG |
| Samd4 | CAGACGAGGAAGAGTAGAGGG | ACAGACGCATTACTATCACCAA |
| Ifitm3 | GAGGACCAAGGTGCTGATGTT | TAGCCTATGCCTACTCCGTGAA |
| Gzmb | GCCAGTCTTTGCAGTCCTTTA | CTCTGATTACCCATCGTCCCT |
| Acta1 | CCTTCTGACCCATACCTACCAT | AAGCCTCACTTCCTACCCTCG |
| Rbm45 | TTTAGGTTCAGCCAAGAGTGC | CGGGAGAAGTTCAAGGTGTAT |
| Capn5 | TGATTCCTCTTAGCCTCGTCA | GTGGATTTCACAGGTGGTGTT |
| Rps3a1 | AGCAAGGCTCACTTCAAACAC | TTAGGAACATCGGGAAGACAC |
| Ipo4 | AGCCACTCCTCCATGTCTTCC | CATCTTTGGGTTGGGCGTACT |
| Asb15 | GAGCCTCAGCATAATCTCATC | TATACTTCGCCGTCTCCAATA |
| Rabgap1l | AGAGGCGGCTTAGTTGTTTGG | GCGGTCTACCTGTTGATTGCC |
| Name | Raw Reads | Mapped to Reference Genome | Mapped Percentage |
|---|---|---|---|
| C-Input | 13,294,498 | 13,049,446 | 98.16% |
| C1-IP | 16,759,284 | 16,585,336 | 98.96% |
| C2-IP | 15,330,270 | 14,798,086 | 96.53% |
| L-Input | 14,177,435 | 13,932,833 | 98.27% |
| L1-IP | 17,959,618 | 17,666,744 | 98.37% |
| L2-IP | 16,528,262 | 15,512,603 | 93.86% |
| Sample Name | Size (bp) | Concentration (ng/μL) | Concentration (nmol/L) | Volume (μL) | Total Amount (ng) |
|---|---|---|---|---|---|
| C-Input | 304 | 7.98 | 39.8 | 20 | 159.6 |
| C1-IP | 336 | 9.71 | 43.7 | 20 | 194.2 |
| C2-IP | 301 | 10.02 | 50.5 | 20 | 200.4 |
| L-Input | 296 | 7.44 | 38.1 | 20 | 148.8 |
| L1-IP | 298 | 10.74 | 54.6 | 20 | 214.8 |
| L2-IP | 298 | 10.83 | 55.1 | 20 | 216.6 |
| Down-Regulated Gene Name | Fold Change | FDR | Up-Regulated Gene Name | Fold Change | FDR |
|---|---|---|---|---|---|
| Olfr1120 | −138.5 | 0.000 | Fpgt | 128.8 | 0.005 |
| Hilpda | −116.3 | 0.003 | Lrriq3 | 128.8 | 0.005 |
| Ebag9 | −116.3 | 0.003 | Pitpnm2 | 113.6 | 0.007 |
| Ptp4a3 | −105.4 | 0.002 | Rspry1 | 111 | 0.006 |
| Rgs1 | −105.4 | 0.002 | Fam192a | 111 | 0.006 |
| Tmc1 | −99.9 | 0.005 | Otud1 | 106 | 0.007 |
| Snx33 | −94.4 | 0.007 | Rab5c | 104.3 | 0.010 |
| Spred3 | −94.3 | 0.007 | Edn3 | 102.3 | 0.004 |
| Snhg17 | −94.3 | 0.007 | Ccdc88a | 97.3 | 0.007 |
| Olfr1113 | −94.3 | 0.007 | Sod2 | 96.7 | 0.012 |
| Commd9 | −89 | 0.005 | Sept9 | 96.7 | 0.012 |
| Rhno1 | −89 | 0.005 | C330013E15Rik | 96.1 | 0.012 |
| Foxm1 | −89 | 0.005 | Dynll1 | 95.5 | 0.012 |
| Pgm5 | −88.9 | 0.005 | Gm13830 | 95.5 | 0.012 |
| Mug1 | −88.8 | 0.009 | Asnsd1 | 94.1 | 0.007 |
| Samd4 | −88.8 | 0.009 | 4930486L24Rik | 90.4 | 0.012 |
| Pathway | Total | Hits | Target Gene Name |
|---|---|---|---|
| Jak-STAT signaling pathway | 155 | 5 | CSF2RA, GRB2, PIK3CA, SPRED2, SPRED3 |
| Natural killer cell mediated cytotoxicity | 119 | 4 | GRB2, GZMB, LCP2, PIK3CA |
| Fc epsilon RI signaling pathway | 70 | 3 | GRB2, LCP2, PIK3CA |
| Pyrimidine metabolism | 104 | 3 | CTPS, NT5C3B, TXNRD1 |
| T cell receptor signaling pathway | 105 | 3 | GRB2, LCP2, PIK3CA |
| Transcriptional misregulation in cancer | 178 | 4 | BCL2A1C, GZMB, JMJD1C, LYL1 |
| Gene | 2−ΔΔC(t) | |
|---|---|---|
| LB/C | HB/C | |
| Ptp4a3 | 0.11985 *** | 0.07554 *** |
| Samd4 | 0.10351 *** | 0.04879 *** |
| Ifitm3 | 0.39707 * | 0.53172 |
| Gzmb | 0.41548 | 0.35731 * |
| Acta1 | 0.06175 ** | 0.11185 * |
| Rbm45 | 0.70175 | 0.49878 ** |
| Capn5 | 0.08935 *** | 0.06172 *** |
| Rps3a1 | 0.48881 *** | 0.57754 ** |
| Ipo4 | 0.39123 ** | 0.31920 ** |
| Asb15 | 0.09352 ** | 0.25600 |
| Rabgap1l | 0.23461 *** | 0.41362 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, Z.; Meng, X.; Sun, F.; Pu, Y.; Xu, K.; Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Global Identification of HIF-1α Target Genes in Benzene Poisoning Mouse Bone Marrow Cells. Int. J. Environ. Res. Public Health 2018, 15, 2531. https://doi.org/10.3390/ijerph15112531
Man Z, Meng X, Sun F, Pu Y, Xu K, Sun R, Zhang J, Yin L, Pu Y. Global Identification of HIF-1α Target Genes in Benzene Poisoning Mouse Bone Marrow Cells. International Journal of Environmental Research and Public Health. 2018; 15(11):2531. https://doi.org/10.3390/ijerph15112531
Chicago/Turabian StyleMan, Zhaodi, Xing Meng, Fengxia Sun, Yunqiu Pu, Kai Xu, Rongli Sun, Juan Zhang, Lihong Yin, and Yuepu Pu. 2018. "Global Identification of HIF-1α Target Genes in Benzene Poisoning Mouse Bone Marrow Cells" International Journal of Environmental Research and Public Health 15, no. 11: 2531. https://doi.org/10.3390/ijerph15112531
APA StyleMan, Z., Meng, X., Sun, F., Pu, Y., Xu, K., Sun, R., Zhang, J., Yin, L., & Pu, Y. (2018). Global Identification of HIF-1α Target Genes in Benzene Poisoning Mouse Bone Marrow Cells. International Journal of Environmental Research and Public Health, 15(11), 2531. https://doi.org/10.3390/ijerph15112531





