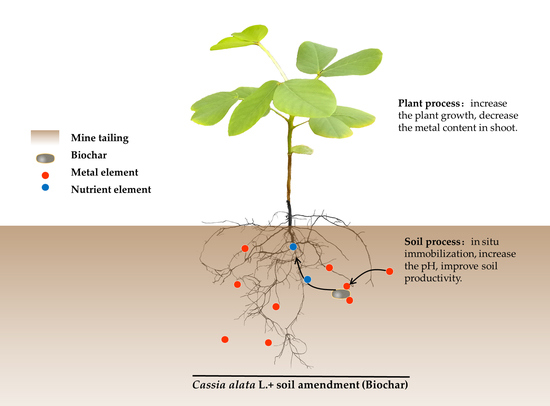
Potential of Cassia alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mine Tailings and Biochar
2.2. Pot Experimental Design
2.3. Sampling and Analysis
2.4. Statistical Analysis
3. Results
3.1. Effects of Different Biochar Treatments on Plant Growth
3.2. Heavy Metal Concentration in Shoots and Roots of Cassia alata L.
3.3. Effects of Different Biochar Treatments on pH, TC, and TN in Mine Tailings
3.4. Effects of Different Biochar Treatments on NH4NO3-Extractable Metal Content in Mine Tailings
3.5. Effects of Different Biochar Treatments on Chemical Fractionation of Metals in Mine Tailings
4. Discussion
4.1. Effects of Biochar on Plant Growth and Heavy Metal Concentration
4.2. Effect of Biochar on Mine Tailings
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, R.; Liu, J.; Zhai, M. Mineral Resources Science in China: A Roadmap to 2050; Science Press: Beijing, China, 2010. [Google Scholar]
- Qiu, R.L.; Qiu, H.; Lei, M.; Ye, Z.H. Advances in research on remediation of multi-metal contaminated soil in mine and surrounding area. J. Agro-Environ. Sci. 2009, 6, 1085–1091. [Google Scholar]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Tapia, Y.; Bustos, P.; Salazar, O.; Casanova, M.; Castillo, B.; Acuna, E.; Masaguer, A. Phytostabilization of Cu in mine tailings using native plant carpobrotus aequilaterus and the addition of potassium humates. J. Geochem. Explor. 2017, 183, 102–113. [Google Scholar] [CrossRef]
- Wu, Q.H.; Wang, S.Z.; Thangavel, P.; Li, Q.F.; Zheng, H.; Bai, J.; Qiu, R.L. Phytostabilization potential of jatropha curcas L. in polymetallic acid mine tailings. Int. J. Phytoremediat. 2011, 13, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.I.S.; Mackowiak, C.; de Almeida, A.Q.; de Carvalho, J.I.T.; Andrade, K.R. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd-Zn hyperaccumulator (Noccaea caerulescens) in contaminated soils amended with biochar. Plant Soil 2015, 395, 57–73. [Google Scholar]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar as plant growth promoter: Better off alone or mixed with organic amendments? Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Shanta, N.; Schwinghamer, T.; Backer, R.; Allaire, S.E.; Teshler, I.; Vanasse, A.; Whalen, J.; Baril, B.; Lange, S.; MacKay, J.; et al. Biochar and plant growth promoting rhizobacteria effects on switchgrass (Panicum virgatum cv. Cave-in-rock) for biomass production in southern quebec depend on soil type and location. Biomass Bioenergy 2016, 95, 167–173. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar]
- Fellet, G.; Marchiol, L.; Delle Vedove, G.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.R.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro clonal propagation and evaluation of genetic fidelity using rapd and issr marker in micropropagated plants of cassia alata L.: A potential medicinal plant. Agrofor. Syst. 2017, 91, 637–647. [Google Scholar] [CrossRef]
- Gao, D.; Wang, X.; Fu, S.; Zhao, J. Legume plants enhance the resistance of soil to ecosystem disturbance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zhou, L.Y.; Yang, M.X.; Liu, W.; Liang, H. Application of ten stress-tolerance plants in ecological restoration of rare earth mine of heping county. Tianjin Agric. Sci. 2013, 7, 92–96. (In Chinese) [Google Scholar]
- Gonzaga, M.I.S.; Mackowiak, C.; Almeida, A.Q.D.; Andrade, K.R. Positive and negative effects of biochar from coconut husks, orange bagasse and pine wood chips on maize (Zea mays L.) growth and nutrition. Catena Suppl. 2018, 162, 414–420. [Google Scholar] [CrossRef]
- Gryschko, R.; Kuhnle, R.; Terytze, K.; Breuer, J.; Stahr, K. Soil extraction of readily soluble heavy metals and as with 1 m NH4NO3-solution—Evaluation of din 19730 (6 pp). J. Soils Sediments 2005, 5, 101–106. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China. Soil and Sediment-Sequential Extraction Procedure of Speciation of 13 Tracement Elements. (GB/T 25282-2010). Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=3CD527C889212919CFEC80932D514455 (accessed on 25 February 2018).
- Huang, D.L.; Xue, W.J.; Zeng, G.M.; Wan, J.; Chen, G.M.; Huang, C.; Zhang, C.; Cheng, M.; Xu, P.A. Immobilization of Cd in river sediments by sodium alginate modified nanoscale zero-valent iron: Impact on enzyme activities and microbial community diversity. Water Res. 2016, 106, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, M.; Lorestani, B.; Khorasani, N.; Yousefi, N.; Karami, M. Findings on the phytoextraction and phytostabilization of soils contaminated with heavy metals. Biol. Trace Elem. Res. 2011, 144, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Usman, A.R.A.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Cea, M.; Eugenia, G.M.; Cornejo, P.; Ok, Y.S.; Borie, F. Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil. J. Soils Sediments 2017, 17, 741–750. [Google Scholar] [CrossRef]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.Y.; Deng, X.; Herbert, S.; Xing, B.S. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.G.; Titirici, M.M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.D.; Pullammanappallil, P.; Yang, L.Y. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Liu, L.S.; Zeng, G.M.; Xu, P.; Huang, C.; Deng, L.J.; Wang, R.Z.; Wan, J. The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 2017, 174, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Hmid, A.; Al Chami, Z.; Sillen, W.; De Vocht, A.; Vangronsveld, J. Olive mill waste biochar: A promising soil amendment for metal immobilization in contaminated soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.L.; Cai, C.; Liang, J.H.; Huang, Q.; Chen, Z.; Huang, Y.Z.; Arp, H.P.H.; Sun, G.X. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 2012, 89, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M.; Pagano, L.; Pigoni, V.; Fellet, G.; Fresno, T.; Vamerali, T.; Bandiera, M.; Marmiroli, N. Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci. Total Environ. 2013, 454, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Zhang, W.H.; Qiu, H.; Tsang, D.C.W.; Morel, J.L.; Qiu, R.L. Effect of coexisting Al(III) ions on Pb(II) sorption on biochars: Role of pH buffer and competition. Chemosphere 2016, 161, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon N. Y. 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Reverchon, F.; Yang, H.; Ho, T.Y.; Yan, G.J.; Wang, J.; Xu, Z.H.; Chen, C.R.; Zhang, D.K. A preliminary assessment of the potential of using an acacia-biochar system for spent mine site rehabilitation. Environ. Sci. Pollut. Res. Int. 2015, 22, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Anawar, H.M.; Akter, F.; Solaiman, Z.M.; Strezov, V. Biochar: An emerging panacea for remediation of soil contaminants from mining, industry and sewage wastes. Pedosphere 2015, 25, 654–665. [Google Scholar] [CrossRef]
- Zhang, X.K.; Wang, H.L.; He, L.Z.; Lu, K.P.; Sarmah, A.; Li, J.W.; Bolan, N.; Pei, J.C.; Huang, H.G. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. Int. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.D.; Ma, L.N.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Zhang, W.H.; Yang, Y.X.; Huang, X.F.; Wang, S.Z.; Qiu, R.L. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.D.; Ma, L.Q.; Chen, M.; Singh, S.P.; Harris, W.G. Impacts of phosphate amendments on lead biogeochemistry at a contaminated site. Environ. Sci. Technol. 2002, 36, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Neupane, G.; Donahoe, R.J.; Arai, Y. Kinetics of competitive adsorption/desorption of arsenate and phosphate at the ferrihydrite—Water interface. Chem. Geol. 2014, 368, 31–38. [Google Scholar] [CrossRef]
- Tian, H.; Shi, Q.; Jing, C. Arsenic biotransformation in solid waste residue: Comparison of contributions from bacteria with arsenate and iron reducing pathways. Environ. Sci. Technol. 2016, 49, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol. 2014, 162, 308–315. [Google Scholar] [CrossRef] [PubMed]



| Material | HB | SB | MB | MT | SEQS |
|---|---|---|---|---|---|
| Pb (mg·kg−1) | 35 | 71.4 | 12.4 | 3642.7 | 500 |
| Zn (mg·kg−1) | 100.3 | 813.4 | 328 | 981 | 500 |
| Cu (mg·kg−1) | 0.2 | 203.5 | 40.4 | 70.5 | 400 |
| Cd (mg·kg−1) | 0.3 | 2.6 | 0.2 | 31.8 | 1 |
| As (mg·kg−1) | 1.8 | 18.7 | 3.4 | 1587.1 | 40 |
| pH | 7.5 | 7.1 | 9.1 | 6.5 | - |
| C (%) | 75.1 | 19.3 | 7 | 0.3 | - |
| H (%) | 2.9 | 1.3 | 0.5 | 0.3 | - |
| N (%) | 0.39 | 2.75 | 0.71 | 0.01 | - |
| S (%) | 0.21 | 0.35 | 0.47 | 1.3 | - |
| BET Surface (m2·g−1) | 117.61 | 14.10 | 6.52 | - | - |
| Langmuir Surface (m2·g−1) | 232.63 | 71.46 | 20.74 | - | - |
| Micro-pore volume (cm3·g−1) | 0.024 | −0.00073 | −0.0013 | - | - |
| Median pore width (nm) | 0.73 | 0.74 | 0.74 | - | - |
| Group | Number | Mine Tailings (kg) | Amendment (w/w) | Repetition |
|---|---|---|---|---|
| Control group | CK | 1 | - | 3 |
| Amendment Group | HB0.4 | 1 | 0.4% HB | 3 |
| HB1 | 1 | 1% HB | 3 | |
| HB3 | 1 | 3% HB | 3 | |
| SB0.4 | 1 | 0.4% SB | 3 | |
| SB1 | 1 | 1% SB | 3 | |
| SB3 | 1 | 3% SB | 3 | |
| MB0.4 | 1 | 0.4% MB | 3 | |
| MB1 | 1 | 1% MB | 3 | |
| MB3 | 1 | 3% MB | 3 |
| Treatment | pH 1 | TC 1 (g·kg−1) | TN 1 (g·kg−1) |
|---|---|---|---|
| CK | 6.53 ± 0.02f | 3.41 ± 0.07d | 0.1 ± 0.01f |
| HB0.4 | 7.13 ± 0.01a | 8.84 ± 0.46cd | 0.22 ± 0.02c |
| HB1 | 7.05 ± 0.03b | 15.52 ± 4.47b | 0.19 ± 0.05cd |
| HB3 | 7.08 ± 0.04ab | 23.7 ± 1.51a | 0.2 ± 0.02cd |
| SB0.4 | 6.89 ± 0.01de | 4.52 ± 0.2cd | 0.18 ± 0.01cde |
| SB1 | 6.88 ± 0.03e | 5.32 ± 0.12cd | 0.31 ± 0.01b |
| SB3 | 6.83 ± 0.02e | 8.28 ± 0.21c | 0.75 ± 0.03a |
| MB0.4 | 7.03 ± 0.01b | 4.03 ± 0.26cd | 0.12 ± 0.01ef |
| MB1 | 7.01 ± 0.02bc | 4.12 ± 0.08cd | 0.14 ± 0def |
| MB3 | 6.95 ± 0.03cd | 5.47 ± 0.04cd | 0.24 ± 0.01c |
| Treatment | NH4NO3-Extractable Metal Contents (mg·kg−1) | ||||
|---|---|---|---|---|---|
| Pb 1 | Zn 1 | Cu 1 | Cd 1 | As 1 | |
| CK | 0.35 ± 0.02ab | 3.42 ± 0.1d | 0.39 ± 0.00abc | 0.1 ± 0.00de | 0.39 ± 0.04cd |
| HB0.4 | 0.34 ± 0.01ab | 3.17 ± 0.09d | 0.39 ± 0.00abc | 0.08 ± 0.00e | 0.38 ± 0.02cd |
| HB1 | 0.35 ± 0.01ab | 4.62 ± 0.33a | 0.39 ± 0.01abc | 0.13 ± 0.01ab | 0.34 ± 0.01d |
| HB3 | 0.37 ± 0.01ab | 4.17 ± 0.26abc | 0.38 ± 0.00bcd | 0.12 ± 0.01abc | 0.43 ± 0.02abcd |
| SB0.4 | 0.34 ± 0.02ab | 4.32 ± 0.17ab | 0.37 ± 0.00cd | 0.12 ± 0.01abcd | 0.35 ± 0.01d |
| SB1 | 0.46 ± 0.11a | 4.14 ± 0.1abc | 0.38 ± 0.00cd | 0.14 ± 0.01a | 0.42 ± 0.04abcd |
| SB3 | 0.34 ± 0.00ab | 4.32 ± 0.16ab | 0.36 ± 0.00d | 0.13 ± 0.01ab | 0.41 ± 0.03bcd |
| MB0.4 | 0.35 ± 0.01ab | 3.78 ± 0.14bcd | 0.4 ± 0.02ab | 0.1 ± 0.00de | 0.48 ± 0.05abc |
| MB1 | 0.33 ± 0.00b | 3.68 ± 0.16cd | 0.4 ± 0.01ab | 0.11 ± 0.01bcd | 0.51 ± 0.06ab |
| MB3 | 0.36 ± 0.01ab | 3.7 ± 0.22cd | 0.41 ± 0.01a | 0.1 ± 0.00cde | 0.53 ± 0.02a |
| Metal | Treatment | Mild Acid-Soluble 1 | Reducible Fraction 1 | Oxidizable Fraction 1 | Residual 1 |
|---|---|---|---|---|---|
| Pb | CK | 1.45 ± 0.26ab | 22.34 ± 0.87b | 0.61 ± 0.15b | 75.6 ± 0.66a |
| HB0.4 | 1.36 ± 0.1ab | 23.38 ± 0.32ab | 0.63 ± 0.1b | 74.63 ± 0.18ab | |
| HB1 | 1.27 ± 0.18ab | 25.99 ± 1.31ab | 0.56 ± 0.08b | 72.19 ± 1.53ab | |
| HB3 | 1.71 ± 0.12a | 33.44 ± 1.37a | 0.63 ± 0.26b | 64.22 ± 1.38b | |
| SB0.4 | 1.11 ± 0.11ab | 20.77 ± 6.47b | 0.94 ± 0.14ab | 77.19 ± 6.58a | |
| SB1 | 1.01 ± 0.11b | 20.39 ± 1.79b | 0.66 ± 0.05b | 77.94 ± 1.69a | |
| SB3 | 0.95 ± 0.03b | 15.02 ± 0.25b | 0.82 ± 0.15ab | 83.21 ± 0.4a | |
| MB0.4 | 0.96 ± 0.11b | 20.53 ± 1.52b | 0.9 ± 0.1ab | 77.6 ± 1.73a | |
| MB1 | 1.38 ± 0.31ab | 19.71 ± 1.56b | 1.27 ± 0.29ab | 77.64 ± 1.83a | |
| MB3 | 1.22 ± 0.29ab | 22.83 ± 7.17b | 1.51 ± 0.58a | 74.45 ± 8ab | |
| Zn | CK | 31.81 ± 4.85a | 16.65 ± 0.32a | 6.69 ± 0.22c | 44.85 ± 4.37a |
| HB0.4 | 38.64 ± 1.95a | 17.96 ± 0.58a | 9.17 ± 1.12ab | 34.24 ± 1.32abc | |
| HB1 | 41.31 ± 6.56a | 18.71 ± 1.33a | 9.61 ± 0.47a | 30.37 ± 8.31bc | |
| HB3 | 42.72 ± 1.71a | 20.42 ± 0.52a | 9.56 ± 1.11a | 27.3 ± 2.96c | |
| SB0.4 | 32.06 ± 1.38a | 17.94 ± 5.63a | 7.07 ± 0.3bc | 42.93 ± 4.87ab | |
| SB1 | 32.89 ± 0.94a | 19.92 ± 3.01a | 7.84 ± 0.6abc | 39.35 ± 1.8abc | |
| SB3 | 32.06 ± 1.82a | 16.06 ± 0.85a | 7.56 ± 0.3abc | 44.32 ± 1.57a | |
| MB0.4 | 35.41 ± 3.22a | 17.29 ± 0.37a | 9.12 ± 0.17abc | 38.18 ± 3.69abc | |
| MB1 | 34.32 ± 1.28a | 13.89 ± 0.55a | 7.97 ± 0.62abc | 43.83 ± 1.34ab | |
| MB3 | 33.88 ± 3.4a | 13.75 ± 0.31a | 8.71 ± 0.82abc | 43.66 ± 4.45ab | |
| Cu | CK | 10.2 ± 1.65ab | 16.57 ± 0.25ab | 14.64 ± 3.96a | 58.59 ± 4.69a |
| HB0.4 | 10.32 ± 0.68ab | 16.34 ± 1.28ab | 12.65 ± 2.3a | 60.7 ± 3.03a | |
| HB1 | 9.93 ± 1.3ab | 19.98 ± 0.97ab | 11.31 ± 2.79a | 58.78 ± 3.99a | |
| HB3 | 10.46 ± 0.14ab | 22.39 ± 0.6a | 12.29 ± 1.56a | 54.86 ± 1.69a | |
| SB0.4 | 7.36 ± 0.71bc | 15.59 ± 4.59b | 10.45 ± 1.87a | 66.59 ± 2.85a | |
| SB1 | 7.23 ± 0.72bc | 19.15 ± 2.71ab | 18.66 ± 2.28a | 54.96 ± 3.77a | |
| SB3 | 4.17 ± 0.44c | 14.71 ± 0.34b | 19.98 ± 3.16a | 61.15 ± 3.22a | |
| MB0.4 | 10.77 ± 1.1ab | 18.2 ± 1.2ab | 19.21 ± 2.94a | 51.82 ± 5.22a | |
| MB1 | 12.61 ± 2.7a | 16.71 ± 1.84ab | 18.77 ± 5.12a | 51.91 ± 9.49a | |
| MB3 | 8.32 ± 1.39ab | 16.17 ± 1.34ab | 20.16 ± 4.61a | 55.35 ± 7.33a | |
| Cd | CK | 7.56 ± 1.21a | 13.17 ± 0.37ab | 3.1 ± 0.58a | 76.18 ± 0.28a |
| HB0.4 | 8.37 ± 0.34a | 13.74 ± 0.15ab | 4.08 ± 0.14a | 73.8 ± 0.11ab | |
| HB1 | 8.88 ± 0.91a | 14.73 ± 0.94ab | 4.48 ± 0.4a | 71.91 ± 0.96ab | |
| HB3 | 9.52 ± 0.24a | 17.63 ± 0.41a | 4.38 ± 0.48a | 68.47 ± 1.12b | |
| SB0.4 | 7.03 ± 0.25a | 13.8 ± 4.21ab | 4.12 ± 0.58a | 75.05 ± 4.35a | |
| SB1 | 7.75 ± 0.19a | 16.05 ± 1.68ab | 4.63 ± 0.42a | 71.56 ± 1.57ab | |
| SB3 | 7.57 ± 0.4a | 13.02 ± 0.3ab | 3.9 ± 0.4a | 75.51 ± 0.41a | |
| MB0.4 | 8.57 ± 1.02a | 13.05 ± 0.99ab | 4.11 ± 0.09a | 74.27 ± 1.93ab | |
| MB1 | 9.16 ± 0.8a | 12.39 ± 0.27b | 4.21 ± 0.55a | 74.25 ± 1.25ab | |
| MB3 | 9.1 ± 1.35a | 12.37 ± 0.66b | 4.04 ± 0.56a | 74.49 ± 2.42ab | |
| As | CK | 0.49 ± 0.1d | 16.55 ± 0.62a | 3.69 ± 0.9a | 79.28 ± 1.42a |
| HB0.4 | 0.52 ± 0.05d | 17.5 ± 0.51a | 4.6 ± 0.38a | 77.37 ± 0.4ab | |
| HB1 | 0.53 ± 0.09d | 18.51 ± 1.5a | 5.18 ± 0.54a | 75.78 ± 0.91ab | |
| HB3 | 1.3 ± 0.2b | 22.94 ± 0.69a | 5.21 ± 0.56a | 70.55 ± 1.42b | |
| SB0.4 | 0.63 ± 0.09cd | 17.77 ± 5.41a | 5.28 ± 0.93a | 76.33 ± 5.94ab | |
| SB1 | 0.88 ± 0.06cd | 20.05 ± 1.94a | 5.53 ± 0.66a | 73.55 ± 2.14ab | |
| SB3 | 1.49 ± 0.07ab | 16.43 ± 0.33a | 4.73 ± 0.51a | 77.34 ± 0.71ab | |
| MB0.4 | 0.57 ± 0.06cd | 16.2 ± 1.12a | 4.78 ± 0.31a | 78.44 ± 0.97ab | |
| MB1 | 0.92 ± 0.07c | 16.97 ± 1.01a | 5.43 ± 1.06a | 76.68 ± 2.13ab | |
| MB3 | 1.73 ± 0.23a | 16.94 ± 2.06a | 4.99 ± 0.61a | 76.33 ± 2.65ab |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Li, Y.; Zhao, M.; Chao, Y.; Qiu, R.; Yang, Y.; Wang, S. Potential of Cassia alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings. Int. J. Environ. Res. Public Health 2018, 15, 494. https://doi.org/10.3390/ijerph15030494
Huang L, Li Y, Zhao M, Chao Y, Qiu R, Yang Y, Wang S. Potential of Cassia alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings. International Journal of Environmental Research and Public Health. 2018; 15(3):494. https://doi.org/10.3390/ijerph15030494
Chicago/Turabian StyleHuang, Lige, Yuanyuan Li, Man Zhao, Yuanqing Chao, Rongliang Qiu, Yanhua Yang, and Shizhong Wang. 2018. "Potential of Cassia alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings" International Journal of Environmental Research and Public Health 15, no. 3: 494. https://doi.org/10.3390/ijerph15030494
APA StyleHuang, L., Li, Y., Zhao, M., Chao, Y., Qiu, R., Yang, Y., & Wang, S. (2018). Potential of Cassia alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings. International Journal of Environmental Research and Public Health, 15(3), 494. https://doi.org/10.3390/ijerph15030494