Potential Health Risk of Endocrine Disruptors in Construction Sector and Plastics Industry: A New Paradigm in Occupational Health
Abstract
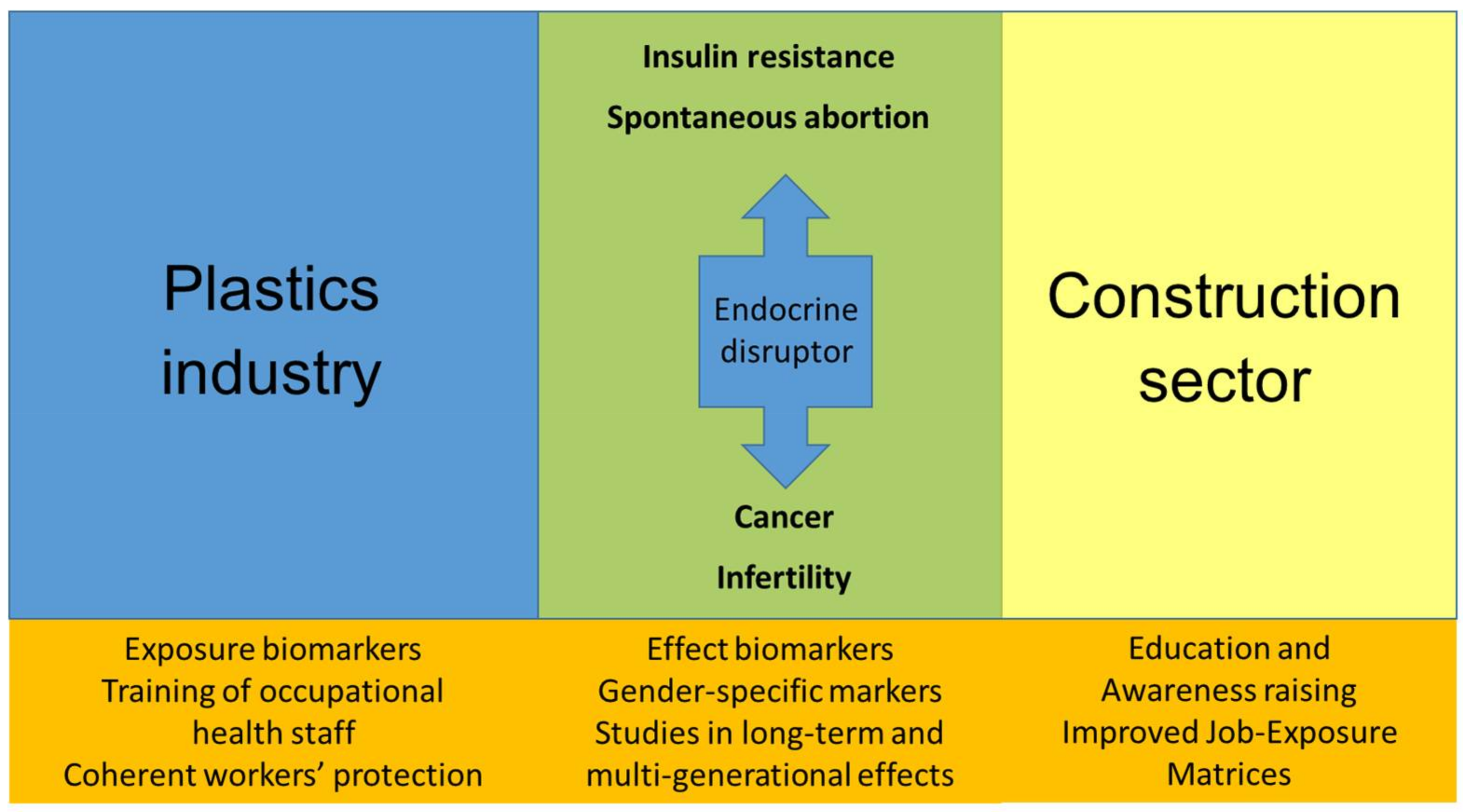
:1. Introduction
2. The Need for a New Paradigm in Occupational Health
3. The Construction and Plastic Industries
4. Discussion on EDs in Construction and Plastic Industry Technologies
4.1. Plastisizers
4.2. Heavy Metals and Metalloestrogens
4.3. Other Agents
5. Conclusions
- (a)
- Fostering of collaborative studies with a view to collecting and sharing data of biomarkers of occupational exposure to EDs initially in construction sector and plastics industry
- (b)
- Promotion of studies to get further insight in estrogen and testosterone levels and fertility problems on workers occupationally exposed to EDs
- (c)
- Inclusion of sex-specific analyses of occupational health risk
- (d)
- Education of occupational physicians and hygienists, employers and employees and other key stakeholders concerning the EDs co-exposures.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fucic, A.; Gamulin, M.; Ferencic, Z.; Katic, J.; Krayer von Krauss, M.; Bartonova, A.; Merlo, D.F. Environmental exposure to xenoestrogens and oestrogen related cancers: Reproductive system, breast, lung, kidney, pancreas, and brain. Environ. Health 2012, 11 (Suppl. 1), S8. [Google Scholar] [CrossRef] [PubMed]
- Bergman, A.; Heindel, J.J.; Kasten, T.; Kidd, K.A.; Jobling, S.; Neira, M.; Zoeller, R.T.; Becher, G.; Bjerregaard, P.; Bornman, R.; et al. The impact of endocrine disruption: A consensus statement on the state of the science. Environ. Health Perspect. 2013, 121, A104–A106. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalfa, N.; Paris, F.; Philibert, P.; Orsini, M.; Broussous, S.; Fauconnet-Servant, N.; Audran, F.; Gaspari, L.; Lehors, H.; Haddad, M.; et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur. Urol. 2015, 68, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef] [PubMed]
- Solecki, R.; Kortenkamp, A.; Bergman, A.; Chahoud, I.; Degen, G.H.; Dietrich, D.; Greim, H.; Håkansson, H.; Hass, U.; Husoy, T.; et al. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Arch. Toxicol. 2017, 91, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; Anderson, O.S.; Montrose, L.; Neier, K.; Dolinoy, D.C. Sexually Dimorphic Effects of Early-Life Exposures to Endocrine Disruptors: Sex-Specific Epigenetic Reprogramming as a Potential Mechanism. Curr. Environ. Health Rep. 2017, 4, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Bergman, A.; Becher, G.; Bjerregaard, P.; Bornman, R.; Brandt, I.; Iguchi, T.; Jobling, S.; Kidd, K.A.; Kortenkamp, A.; et al. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ. Health 2014, 13, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaich, E.M.; Schäfers, C.; Dreier, D.A.; Hecker, M.; Ortego, L.; Kawashima, Y.; Dang, Z.C.; Solomon, K. Challenges in assigning endocrine-specific modes of action: Recommendations for researchers and regulators. Integr. Environ. Assess. Manag. 2017, 13, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Myers, J.P. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Rouillon, S.; Deshayes-Morgand, C.; Enjalbert, L.; Rabouan, S.; Hardouin, J.-B.; DisProSE, G.; Albouy-Llaty, M. Endocrine Disruptors and Pregnancy: Knowledge, Attitudes and Prevention Behaviors of French Women. Int. J. Environ. Res. Public Health 2017, 14, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Suda, K.; Tsuji, E.; Tanemura, K.; Yokota, H.; Inoue, H.; Iwano, H. Late pregnancy is vulnerable period for exposure to BPA. J. Vet. Med. Sci. 2018, 80, 536–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mughal, B.B.; Fini, J.B.; Demeneix, B.A. Thyroid-disrupting chemicals and brain development: An update. Endocr. Connect. 2018, 7, R160–R186. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Nakiwala, D.; Calafat, A.M.; Botton, J.; De Agostini, M.; Heude, B.; Slama, R.; EDEN Mother–Child Study Group. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ. Health Perspect. 2017, 125, 097014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, C.; Johansson, A.L.; Bergdahl, I.A.; Dickman, P.W.; Plato, N.; Adami, J.; Boffetta, P.; Lagergren, J. Occupational exposures and risk of esophageal and gastric cardia cancers among male Swedish construction workers. Cancer Causes Control. 2005, 16, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, J.; Lopez-Abente, G.; Castello, A.; Gonzalez-Sanchez, M.; Fernandez-Navarro, P. Cancer mortality in towns in the vicinity of installations for the production of cement, lime, plaster, and magnesium oxide. Chemosphere 2015, 128, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Beranger, R.; Le Cornet, C.; Schüz, J.; Fervers, B. Occupational and environmental exposures associated with testicular germ cell tumours: Systematic review of prenatal and life-long exposures. PLoS ONE 2013, 8, e77130. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, V.; Eslick, G.D. Forthcoming prognostic markers for esophageal cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2014, 5, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Lin, Y.; Yuan, C.L.; Liu, Z.H.; Li, Y.Q.; Luo, X.L.; Ye, J.Z.; Ye, H.H. High expression of estrogen-related receptor α is significantly associated with poor prognosis in patients with colorectal cancer. Oncol. Lett. 2018, 15, 5933–5939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manente, A.G.; Pinton, G.; Zonca, S.; Tavian, D.; Habib, T.; Jithesh, P.V.; Fennell, D.; Nilsson, S.; Moro, L. KDM6B histone demethylase is an epigenetic regulator of estrogen receptor β expression in human pleural mesothelioma. Epigenomics 2016, 8, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Jukic, Z.; Radulovic, P.; Stojković, R.; Mijic, A.; Grah, J.; Kruslin, B.; Ferencic, Z.; Fucic, A. Gender Difference in Distribution of Estrogen and Androgen Receptors in Intestinal-type Gastric. Cancer Anticancer Res. 2017, 37, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ye, J.; Wang, Z.; Lin, S.X.; Lu, M.; Liang, Y.; Zhu, X.; Olumi, A.F.; Zhong, W.D.; Wu, C.L. Expression of aromatase in tumor related stroma is associated with human bladder cancer progression. Cancer Biol. Ther. 2018, 19, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.; Sakamuru, S.; Huang, R.; Stavreva, D.A.; Varticovski, L.; Hager, G.L.; Judson, R.S.; Houck, K.A.; Kleinstreuer, N.C.; Casey, W.; et al. Identifying environmental chemicals as agonists of the androgen receptor by using a quantitative high-throughput screening platform. Toxicology 2017, 385, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, M.; Nieuwenhuijsen, M.J.; Gardiner, K.; Armstrong, B.; Vrijheid, M.; Dolk, H.; Botting, B. A Job–Exposure Matrix for Potential Endocrine-disrupting Chemicals Developed for a Study into the Association between Maternal Occupational Exposure and Hypospadias. Ann. Occup. Hyg. 2002, 46, 465–477. [Google Scholar] [PubMed]
- Brouwers, M.M.; van Tongeren, M.; Hirst, A.A.; Bretveld, R.W.; Roeleveld, N. Occupational exposure to potential endocrine disruptors: Further development of a job exposure matrix. Occup. Environ. Med. 2009, 66, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Intertek Group (2015) EU—REACH Amendment—Regulation 2015-326-EU on Polycyclic Aromatic Hydrocarbon and Phthalates. Available online: http://www.intertek.com/consumer/insight-bulletins/reach-amendment-regulation-2015-326-eu/ (accessed on 20 March 2018).
- SCOEL (2014) Recommendation from the Scientific Committee on Occupational Exposure Limits for Bisphenol-A. SCOEL/SUM/113. European Commission. Available online: Ec.europa.eu/social/BlobServlet?docId=3873&langId=en (accessed on 20 March 2018).
- Henson, M.C.; Chedrese, P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004, 229, 383–392. [Google Scholar] [CrossRef]
- Jurasovic, J.; Cvitković, P.; Pizent, A.; Colak, B.; Telisman, S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals 2004, 17, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Yoshihara, S. New aspects of cadmium as endocrine disruptor. Environ. Sci. 2006, 13, 107–116. [Google Scholar] [PubMed]
- Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The effects of nanomaterials as endocrine disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and Breast Cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.V. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Chalmey, C.; Modick, H.; Jensen, L.S.; Dierkes, G.; Weiss, T.; Jensen, B.A.; Nørregård, M.M.; Borkowski, K.; Styrishave, B.; et al. Aniline is rapidly converted into paracetamol impairing male reproductive development. Toxicol. Sci. 2015, 148, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modick, H.; Weiss, T.; Dierkes, G.; Koslitz, S.; Käfferlein, H.U.; Brüning, T.; Koch, H.M. Human metabolism and excretion kinetics of aniline after a single oral dose. Arch. Toxicol. 2016, 90, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Burghardt, R.C. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 2016, 303, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plastics Europe (2017) Plastics—The Facts 2017. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf (accessed on 20 March 2018).
- Sartorius, I.; Joachim Wuttke, J. Sustainable Management and Recovery Potential of Plastic Waste from the Commercial and Private Household Sectors. Available online: https://www.wastematters.eu/uploads/media/SMM_Presentation_Ingo_Sartorius_Plastics.pdf (accessed on 27 April 2018).
- Plastics in Building and Construction. Available online: https://www.plasticseurope.org/en/about-plastics/building-construction (accessed on 9 June 2018).
- COM/2014/0398. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Towards a Circular Economy: A Zero Waste Programme for Europe. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:50edd1fd-01ec-11e4-831f-01aa75ed71a1.0001.01/DOC_1&format=PDF (accessed on 27 April 2018).
- EU Directive on the Energy Performance of Buildings Directive 2010/31/EU. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:153:0013:0035:EN:PDF (accessed on 27 April 2018).
- Directive 2009/28/EC Of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX: 32009L0028&from=EN (accessed on 27 April 2018).
- Fucic, A. The main health hazards from building materials. In Toxicity of Building Materials; Pacheco-Torgal, F., Jalali, S., Fucic, A., Eds.; Woodhead: Cambridge, UK, 2012; pp. 1–22. [Google Scholar]
- Isnin, Z.; Ahmad, S.; Yahya, Z. Challenges of the Unknown Building Material Substances for Greener Adaptation Projects. Procedia Soc. Behav. Sci. 2012, 68, 53–62. [Google Scholar] [CrossRef]
- Liew, K.M.; Sojobi, A.O.; Zhang, L.W. Green concrete: Prospects and challenges. Constr. Build. Mater. 2017, 156, 1063–1095. [Google Scholar] [CrossRef]
- Rudel, R.A.; Perovich, L.J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrawarthi, V.; Darmar, B.; Elangovan, A. Copper slag concrete admixed with polypropylene fibres. Gradevinar 2016, 68, 95–178. [Google Scholar] [CrossRef]
- European Commission. EU Construction and Demolition Waste Protocol. Available online: https://ec.europa.eu/growth/content/eu-construction-and-demolition-waste-protocol-0_en (accessed on 27 April 2018).
- Fong, J.P.; Lee, F.J.; Lu, I.S.; Uang, S.N.; Lee, C.C. Relationship between urinary concentrations of di(2-ethylhexyl) phthalate (DEHP) metabolites and reproductive hormones in polyvinyl chloride production workers. Occup. Environ. Med. 2015, 72, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.P.; Lee, C.C.; Hsu, P.C.; Shih, T.S. The association between semen quality in workers and the concentration of di(2-ethylhexyl) phthalate in polyvinyl chloride pellet plant air. Fertil. Steril. 2011, 96, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, C.G.; Hardell, L. Testicular cancer and occupational exposures with a focus on xenoestrogens in polyvinyl chloride plastics. Chemosphere 2000, 40, 1277–1282. [Google Scholar] [CrossRef]
- Hougaard, K.S.; Hannerz, H.; Feveile, H.; Bonde, J.P. Increased incidence of infertility treatment among women working in the plastics industry. Reprod. Toxicol. 2009, 27, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Ladeira, C.; Viegas, S. EDCs mixtures: A stealthy hazard for human health? Toxics 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Yuan, W.; Zhu, G.; He, X.; Li, D.K. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod. Toxicol. 2011, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Ladeira, C.; Viegas, S. Occupational exposure to Bisphenol A (BPA): A reality that still needs to be unveiled. Toxics 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of Bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Phedonos, A.; Arno, M.; Balu, S.; Corton, J.C.; Antoniou, M.N. Editor’s Highlight: Transcriptome profiling reveals Bisphenol A alternatives activate estrogen receptor alpha in human breast cancer cells. Toxicol. Sci. 2017, 158, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Hu, Y.; Guo, J.; Yu, T.; Sun, Y.L.; Xiao, X.; Desheng, D.; Nakanishi, T.; Hiromori, Y.; Li, J.; et al. Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy outcomes in mice. Nat. Comm. 2017, 8, 14585. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Y.; Ren, X.M.; Li, C.H.; Zhang, J.; Qin, W.P.; Yang, Y.; Wan, B.H.; Bisphenol, A.F.; Bisphenol, B. Exert Higher Estrogenic Effects than Bisphenol A via G Protein-Coupled. Estrogen Receptor Pathway. Environ. Sci. Technol. 2017, 51, 11423–11430. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Crump, D. A review of the emission of VOCs from polymeric materials used in buildings. Build. Environ. 1998, 33, 357–374. [Google Scholar] [CrossRef]
- Xu, Y.; Hubal, E.A.; Clausen, P.A.; Little, J.C. Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: A mechanistic analysis. Environ. Sci. Technol. 2009, 43, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Blesson, C.S.; Yallampalli, C. Pregnancy is a New Window of Susceptibility for Bisphenol A Exposure. Endocrinology 2015, 156, 1611–1612. [Google Scholar] [CrossRef] [PubMed]
- KEMI (Swedish Chemicals Agency). Phthalates Which Are Toxic for Reproduction and Endocrine-Disrupting—Proposals for a Phase-Out in Sweden Report from a Government Assignment; Report 4/15; Swedish Chemical Agency: Sundbyberg, Sweden, 2015; ISSN 0284-1185. Available online: https://www.kemi.se/global/rapporter/2015/report-4-15-phatalates.pdf (accessed on 20 March 2018).
- Sheikh, I.A.; Yasir, M.; Abu-Elmagd, M.; Dar, T.A.; Abuzenadah, A.M.; Damanhouri, G.A.; Al-Qahtani, M.; Beg, M.A. Human sex hormone-binding globulin as a potential target of alternate plasticizers: An in silico study. BMC Struct. Biol. 2016, 16 (Suppl. 1), 15. [Google Scholar] [CrossRef] [PubMed]
- Attina, T.M.; Trasande, L. Association of Exposure to Di-2-Ethylhexylphthalate Replacements with increased insulin resistance in adolescents from NHANES 2009-2012. J. Clin. Endocrinol. Metab. 2015, 100, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Sunghoon, L.; Lau, M.; Marques, L.; Papadopoulos, V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Sci. Rep. 2017, 7, 11072. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinol. 2003, 144, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Lederer, J.; Trinkel, V.; Fellner, J. Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: The case of Austria. Waste Manag. 2017, 60, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Kearl, E.R.; Solman, K.R. Lead and other toxic metals in playground paints from South West England. Sci. Total Environ. 2016, 544, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Sorgato, M.J.; Schneider, K.; Rüther, R. Technical and economic evaluation of thin-film CdTe building-integrated photovoltaics (BIPV) replacing façade and rooftop materials in office buildings in a warm and sunny climate. Renew. Energy 2018, 118, 84–98. [Google Scholar] [CrossRef]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Duan, X.; Li, M.; Huang, C.; Li, J.; Chu, R.; Ying, H.; Song, H.; Jia, X.; Ba, Q.; et al. Systematic network assessment of the carcinogenic activities of cadmium. Toxicol. Appl. Pharmacol. 2016, 310, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Daoud, S.; Sellami, A.; Bouassida, M.; Kebaili, S.; Ammar Keskes, L.; Rebai, T.; Chakroun Feki, N. Routine assessment of occupational exposure and its relation to semen quality in infertile men: A cross-sectional study. Turk. J. Med. Sci. 2017, 47, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Rotter, I.; Kosik-Bogacka, D.I.; Dołęgowska, B.; Safranow, K.; Kuczynska, M.; Laszczynska, M. Analysis of the relationship between the sblood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Environ. Geochem. Health 2016, 38, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.; Pesch, B.; Vermeulen, R.; Olsson, A.; Schüz, J.; Portengen, L.; Kendzia, B.; Kromhout, H.; Straif, K.; Brüning, T.; et al. Exposure to hexavalent chromium and nickel and lung cancer risk: A pooled analysis of case-control studies from Europe and Canada. Occup. Environ. Med. 2017, 74 (Suppl. 1), A152. [Google Scholar]
- Khadem, M.; Golbabaei, F.; Rahmani, A. Occupational exposure assessment of Chromium (VI): A review of environmental and biological monitoring. Int. J. Occup. Hygene 2017, 9, 118–131. [Google Scholar]
- Hjollund, N.H.; Bonde, J.P.; Jensen, T.K.; Henriksen, T.B.; Andersson, A.M.; Kolstad, H.A.; Ernst, E.; Giwercman, A.; Skakkebaek, N.E.; Olsen, J. Male-mediated spontaneous abortion among spouses of stainless steel welders. Scand. J. Work Environ. Health 2000, 26, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giergiczny, Z.; Krol, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard Mater. 2008, 160, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. DIRECTIVE 2003/53/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 June 2003 Amending for the 26th Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Nonylphenol, Nonylphenol Ethoxylate and Cement). 2003. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:178:0024:0027:en:PDF (accessed on 21 March 2018).
- Bensefa-Colas, L.; Stocks, S.J.; McNamee, R.; Faye, S.; Pontin, F.; Agius, R.M.; Lasfargues, G.; RNV3P members; Telle-Lamberton, M.; Momas, I. Effectiveness of the European chromium(VI) directive for cement implementation on occupational allergic contact dermatitis occurrence: Assessment in France and the U.K. Br. J. Dermatol. 2017, 177, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Park, J.B.; Lee, K.J. Exposure assessment of particulate matter and blood chromium levels in people living near a cement plant. Environ. Geochem. Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Gamboni, S.E.; Palmer, A.M.; Nixon, R.L. Occupational allergic contact dermatitis to chromium from cement: Estimating the size of the problem in Australia. Australas J. Dermatol. 2015, 56, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, D.; Katyal, N.K. Study of total chromium and water soluble chromium in Indian cement samples. IJOART 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Jolly, J.; Lawrence, D.A. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol. Appl. Pharmacol. 2017, 334, 142–157. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Global Report on the Status of Legal Limits on Lead in Paint. United Nations Environmental Program, 2016. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/11348/ Limits-Lead-Paint-2016%20Report-Final.pdf?sequence=1&isAllowed=y (accessed on 21 March 2018).
- Petersen, A.K.; Solberg, B. Environmental and economic impacts of substitution between wood products and alternative materials: A review of micro-level analyses from Norway and Sweden. For. Policy Econ. 2005, 7, 249–259. [Google Scholar] [CrossRef]
- Ritter, M.A.; Skog, K.; Bergman, R. Science Supporting the Economic and Environmental Benefits of Using Wood and Wood Products in Green Building Construction; General Technic Report FPL-GTR-206; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2011.
- Herczeg, M.; McKinnon, D.; Milios, L.; Bakas, I.; Klaassens, E.; Svatikova, K.; Widerberg, O. Resource Efficiency in the Building Sector; Final Report; Prepared for European Commission by ECORYS and Copenhagen Resource Institute: Rotterdam, The Netherlands, 2014. [Google Scholar]
- Commission Directive 2003/2/EC. Relating to Restrictions on the Marketing and Use of Arsenic (Tenth Adaptation to Technical Progress to Council Directive 76/769/EEC. Available online: https://www.tid.gov.hk/english/aboutus/tradecircular/cic/eu/2003/files/ci552003a.pdf (accessed on 8 June 2018).
- Kjærstad, M.B.; Taxvig, C.; Nellemann, C.; Vinggaard, A.M.; Andersen, H.R. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod. Toxicol. 2010, 30, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Taxvig, C.; Vinggaard, A.M.; Hass, U.; Axelstad, M.; Metzdorff, S.; Nellemann, C. Endocrine-disrupting properties in vivo of widely used azole fungicides. Int. J. Androl. 2008, 31, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Fukunaga, K.; Sasaki, M.; Nakamura, M.; Tsuji, M.; Nishiyama, T. Evaluation of estrogenic activities of pesticides using an in vitro reporter gene assay. Int. J. Environ. Health Res. 2005, 15, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Blume, B.; Kietzmann, M.; Kranke, P.; Moder, M.; Schrader, S.; Wahren, M. Deuterium labelled nonylphenols in an in-vitro model of percutaneous absorption of environmental xenoestrogens. Isot. Environ. Health Stud. 2000, 36, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Noorimotlagh, Z.; Haghighi, N.J.; Ahmadimoghadam, M.; Rahim, F. An updated systematic review on the possible effect of nonylphenol on male fertility. Environ. Sci. Pollut. Res. Int. 2017, 24, 3298–3314. [Google Scholar] [CrossRef] [PubMed]
- Hay-Schmidt, A.; Finkielman, O.T.E.; Jensen, B.A.H.; Høgsbro, C.F.; Bak Holm, J.; Johansen, K.H.; Jensen, T.K.; Andrade, A.M.; Swan, S.H.; Bornehag, C.G.; et al. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction 2017, 154, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporossi, L.; Papaleo, B. Bisphenol A and Metabolic Diseases: Challenges for Occupational Medicine. Int. J. Environ. Res. Public Health 2017, 14, 959. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Ogawa, Y.; Endo, Y.; Kawai, T.; Namera, A.; Yamamuro, K.; Sumino, K.; Endo, G. Evaluation of urinary cyclohexanediols and cyclohexanol as biomarkers of occupational exposure to cyclohexane. J. Occup. Health 2015, 57, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fucic, A.; Galea, K.S.; Duca, R.C.; El Yamani, M.; Frery, N.; Godderis, L.; Halldorsson, T.I.; Iavicoli, I.; Ndaw, S.; Ribeiro, E.; et al. Potential Health Risk of Endocrine Disruptors in Construction Sector and Plastics Industry: A New Paradigm in Occupational Health. Int. J. Environ. Res. Public Health 2018, 15, 1229. https://doi.org/10.3390/ijerph15061229
Fucic A, Galea KS, Duca RC, El Yamani M, Frery N, Godderis L, Halldorsson TI, Iavicoli I, Ndaw S, Ribeiro E, et al. Potential Health Risk of Endocrine Disruptors in Construction Sector and Plastics Industry: A New Paradigm in Occupational Health. International Journal of Environmental Research and Public Health. 2018; 15(6):1229. https://doi.org/10.3390/ijerph15061229
Chicago/Turabian StyleFucic, Aleksandra, Karen S. Galea, Radu Corneliu Duca, Mounia El Yamani, Nadine Frery, Lode Godderis, Thórhallur Ingi Halldorsson, Ivo Iavicoli, Sophie Ndaw, Edna Ribeiro, and et al. 2018. "Potential Health Risk of Endocrine Disruptors in Construction Sector and Plastics Industry: A New Paradigm in Occupational Health" International Journal of Environmental Research and Public Health 15, no. 6: 1229. https://doi.org/10.3390/ijerph15061229
APA StyleFucic, A., Galea, K. S., Duca, R. C., El Yamani, M., Frery, N., Godderis, L., Halldorsson, T. I., Iavicoli, I., Ndaw, S., Ribeiro, E., Viegas, S., & Moshammer, H. (2018). Potential Health Risk of Endocrine Disruptors in Construction Sector and Plastics Industry: A New Paradigm in Occupational Health. International Journal of Environmental Research and Public Health, 15(6), 1229. https://doi.org/10.3390/ijerph15061229










