Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch Experiment
2.3. Adsorption Kinetics
2.4. Adsorption Isotherm
2.5. Characterization
2.6. Desorption and Reusability Experiment
3. Results and Discussion
3.1. Characterization of Tea Waste
3.2. Adsorption Behavior
3.2.1. Effect of S/L Ratios and pH on MB Adsorption
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherm
3.3. Desorption and Reusability Performance
3.4. Recommended Adsorption Mechanism
3.5. Environmental Significance of This Work
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Mcmullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qian, K.; Wang, S.; Liang, K.; Yan, W. Polypyrrole-Grafted Coconut Shell Biological Carbon as a Potential Adsorbent for Methyl Tert-Butyl Ether Removal: Characterization and Adsorption Capability. Int. J. Environ. Res. Public Health 2017, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Mayacela Rojas, C.M.; Rivera Velásquez, M.F.; Tavolaro, A.; Molinari, A.; Fallico, C. Use of Vegetable Fibers for PRB to Remove Heavy Metals from Contaminated Aquifers-Comparisons among Cabuya Fibers, Broom Fibers and ZVI. Int. J. Environ. Res. Public Health 2017, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Mu’Azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. Int. J. Environ. Res. Public Health 2017, 14, 1528. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Yang, S.; Xu, H.; Wang, Z.; Chen, Y.; Wang, Y. Adsorption Behavior and Mechanism for the Uptake of Fluoride Ions by Reed Residues. Int. J. Environ. Res. Public Health 2018, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, H.; Yang, S.; Wang, W.; Wang, Y. Adsorption Property and Mechanism of Oxytetracycline onto Willow Residues. Int. J. Environ. Res. Public Health 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Ting, A.S.Y. Common filamentous Trichoderma asperellum for effective removal of triphenylmethane dyes. Desalin. Water Treat. 2016, 57, 13534–13539. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Khan, M.R.; Alothman, Z.A. Adsorptive Removal of Toxic Dye Using Fe3O4–TSC Nanocomposite: Equilibrium, Kinetic, and Thermodynamic Studies. J. Chem. Eng. Data 2016, 61, 3806–3813. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Salleh, L.M.; Azizi, M.; Yunus, C.; Naushad, M. Potassium hydroxide-treated palm kernel shell sorbents for the efficient removal of methyl violet dye. Desalin. Water Treat. 2017, 84, 262–270. [Google Scholar]
- Albadarin, A.B.; Charara, M.; Abu Tarboush, B.J.; Ahmad, M.N.M.; Kurniawan, T.A.; Naushad, M.; Walker, G.M.; Mangwandi, C. Mechanism analysis of tartrazine biosorption onto masau stones; a low cost by-product from semi-arid regions. J. Mol. Liq. 2017, 242, 478–483. [Google Scholar] [CrossRef]
- Xu, H.; Guo, L. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Nasuha, N.; Hameed, B.H.; Din, A.T. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J. Hazard. Mater. 2010, 175, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H. Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 2009, 161, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nasuha, N.; Hameed, B.H. Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem. Eng. J. 2011, 166, 783–786. [Google Scholar] [CrossRef]
- An, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar]
- Eroğlu, H.; Yapici, S.; Nuhoğlu, Ç.; Varoğlu, E. An environmentally friendly process; adsorption of radionuclide Tl-201 on fibrous waste tea. J. Hazard. Mater. 2008, 163, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Özbaş, E.E.; Öngen, A.; Gökçe, C.E. Removal of astrazon red 6B from aqueous solution using waste tea and spent tea bag. Desalin. Water Treat. 2013, 51, 7523–7535. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Li, Y.; Tang, J.; Wang, Z.; Tang, J.; Li, X.; Hu, K. Facile synthesis of tea waste/Fe3O4 nanoparticle composite for hexavalent chromium removal from aqueous solution. RSC Adv. 2017, 7, 7576–7590. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.D.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Shu, Y.; Zhang, R.; Li, X.; Song, J.; Li, B.; Zhang, Y.; Ou, D. Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv. 2017, 7, 16092–16103. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Chen, Y.-L.; Jiang, H. Slow Pyrolysis Magnetization of Hydrochar for Effective and Highly Stable Removal of Tetracycline from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 3059–3066. [Google Scholar] [CrossRef]
- Rodrigues, R.; Gonçalves, M.; Mandelli, D.; Pescarmona, P.P.; Carvalho, W.A. Solvent-free conversion of glycerol to solketalcatalysed by activated carbons functionalised with acid groups. Catal. Sci. Technol. 2014, 4, 2293–2301. [Google Scholar] [CrossRef]
- Milczarek, G.; Ciszewski, A.; Stepniak, I. Oxygen-doped activated carbon fiber cloth as electrode material for electrochemical capacitor. J. Power Sources 2011, 196, 7882–7885. [Google Scholar] [CrossRef]
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787. [Google Scholar] [CrossRef]
- Wohlgemuth, S.A.; Vilela, F.; Titirici, M.M.; Antonietti, M. A one-pot hydrothermal synthesis of tunable dual heteroatom-doped carbon microspheres. Green Chem. 2012, 14, 741–749. [Google Scholar] [CrossRef]
- Mo, Z.; Liao, S.; Zheng, Y.; Fu, Z. Preparation of nitrogen-doped carbon nanotube arrays and their catalysis towards cathodic oxygen reduction in acidic and alkaline media. Carbon 2012, 50, 2620–2627. [Google Scholar] [CrossRef]
- Archanjo, B.S.; Araujo, J.R.; Silva, A.M.; Capaz, R.B.; Falcão, N.P.S.; Jorio, A.; Achete, C.A. Chemical Analysis and Molecular Models for Calcium–Oxygen–Carbon Interactions in Black Carbon Found in Fertile Amazonian Anthrosoils. Environ. Sci. Technol. 2014, 48, 7445–7452. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.T.; Feng, S.; Ma, Q.; Peng, X.; Wu, D. Preparation and Application of Carboxylated Graphene Oxide Sponge in Dye Removal. Int. J. Environ. Res. Public Health 2017, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jiang, C.; Lin, Z.; Zou, Z. Microwave-Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal. Int. J. Environ. Res. Public Health 2018, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Ventenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. A kinetic study of dye sorption by biosorbent waste product pith. Resour. Conserv. Recycl. 1999, 25, 171–193. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, B.; Chiou, C.T. Fast and Slow Rates of Naphthalene Sorption to Biochars Produced at Different Temperatures. Environ. Sci. Technol. 2012, 46, 11104–11111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, C.; Cheng, W.; Wang, X. Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Khraisheh, M.A.; Ahmad, M.N.; Allen, S. Adsorption behaviour of methylene blue onto Jordanian diatomite: A kinetic study. J. Hazard. Mater. 2008, 165, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Malash, G.F.; Elkhaiary, M.I. Methylene blue adsorption by the waste of Abu-Tartour phosphate rock. J. Colloid Interface Sci. 2010, 348, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, N.; Liu, Y.; Jiang, L.; Zeng, G.; Tan, X.; Liu, S.; Yin, Z.; Tian, S.; Li, J. Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry. Int. J. Environ. Res. Public Health 2017, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Chen, Z. Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study. Int. J. Environ. Res. Public Health 2017, 14, 1453. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, Y.; Wang, H.; Tan, X.; Yang, Y.; Liu, Y. Efficient Removal of Tetracycline from Aqueous Media with a Fe3O4Nanoparticles@graphene Oxide Nanosheets Assembly. Int. J. Environ. Res. Public Health 2017, 14, 1495. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part II.—Liquids. J. Am. Chem. Soc. 1916, 38, 102–105. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen: ZeitschriftfürphysikalischeChemie. J. Am. Chem. Soc. 1906, 62, 121–125. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physicochim. USSR 1940, 12, 217–225. [Google Scholar]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind. Eng. Chem. Fundam. 1966, 5, 587–594. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tang, Y.K.; Chen, C.; Wu, J.T.; Huang, Z.; Mo, Y.Y.; Zhang, K.X.; Chen, J.B. Regeneration of magnetic biochar derived from eucalyptus leaf residue for lead(II) removal. Bioresour. Technol. 2015, 186, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Zhu, S.; Fang, S.; Huo, M.; Yu, Y.; Chen, Y.; Yang, X.; Geng, Z.; Wang, Y.; Bian, D.; Huo, H. A novel conversion of the groundwater treatment sludge to magnetic particles for the adsorption of methylene blue. J. Hazard. Mater. 2015, 292, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Bao, C.; Ma, J.; Liu, M.; Wang, F. Magnetic responsive metal–organic frameworks nanosphere with core–shell structure for highly efficient removal of methylene blue. Chem. Eng. J. 2016, 283, 1127–1136. [Google Scholar] [CrossRef]
- Altenor, S.; Carene, B.; Emmanuel, E.; Lambert, J.; Ehrhardt, J.J.; Gaspard, S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J. Hazard. Mater. 2008, 165, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, W.; Zhang, C.; Zhang, S.; Liu, L.; Zhu, L.; Zhao, W. Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Bioresour. Technol. 2016, 206, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2012, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
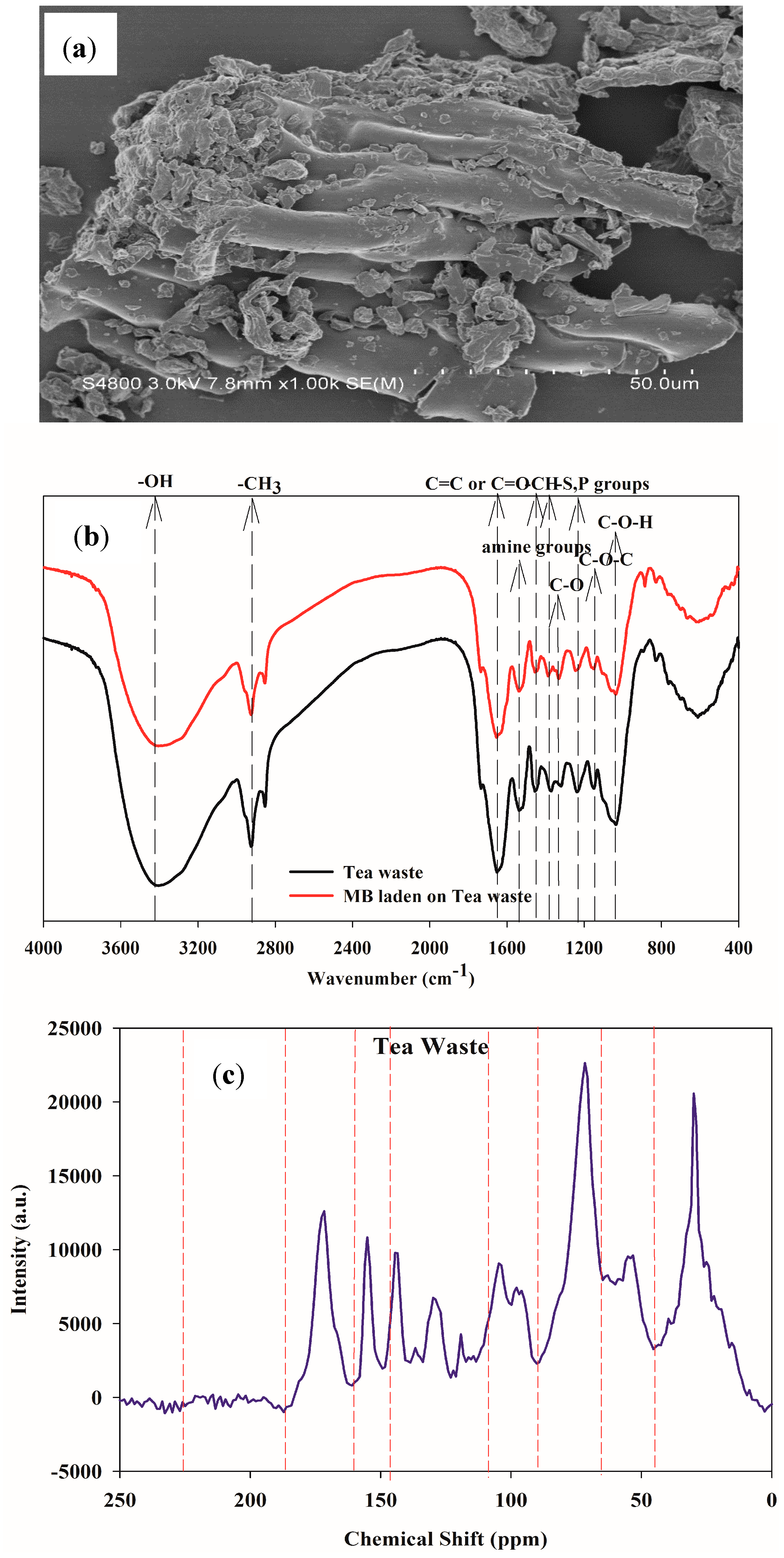





| Tea Waste | Assignment | ||
|---|---|---|---|
| Before Adsorption | After Adsorption | Difference | |
| 3416 | 3406 | +17 | bonded –OH groups |
| 2924 | 2925 | 0 | aliphatic C–H group |
| 2852 | 2852 | 0 | aliphatic C–H group |
| 1651 | 1644 | +7 | C=O stretching, Aromatic C=C, C=O/C=C stretching Amid Igroup |
| 1530 | 1537 | −7 | secondary amine II group |
| 1455 | 1455 | 0 | C–H alkanes in aromatic rings |
| 1371 | 1385 | −14 | C–H bending,–CH3 symmetric bending of CH3 |
| 1320 | 1331 | −11 | C–O stretching |
| 1237 | 1244 | −4 | –SO3 stretching/P=O or COO vibration |
| 1150 | 1150 | 0 | C–O–C of polysaccharides |
| 1036 | 1036 | 0 | C–O–H stretching |
| Tea Waste | Chemical Shift (ppm), δ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Relative proportion | 0–46 | 46–65 | 65–90 | 90–108 | 108–145 | 145–160 | 160–185 | 185–225 | 225–250 |
| (%) | 22.61 | 12.90 | 23.76 | 10.46 | 14.21 | 5.71 | 9.96 | 0.13 | 0.26 |
| Pseudo-First-Order | Pseudo-Second-Order | |||||
| k1 (L·min−1) | qe (mg·g−1) | R2 | k2 (L·min−1) | qe (mg·g−1) | R2 | |
| 39.726 | 23.323 | 0.9625 | 0.2317 | 24.077 | 0.9908 | |
| TC models | Elovich | |||||
| R2 | R2 | |||||
| Ffast | Fslow | 0.9847 | 0.0071 | 11.9523 | 0.9159 | |
| 0.7899 | 0.2101 | |||||
| kfast | kslow | |||||
| 852.8291 | 20.3117 | |||||
| Langmuir | Freundlich | |||||
| b (L·mg−1) | Q0 (mg·g−1) | R2 | Kf | 1/n | R2 | |
| 0.08372 | 113.1461 | 0.9748 | 21.3460 | 0.3524 | 0.9159 | |
| Temkin | D-R | |||||
| bT (J·mol−1) | KT (L·mg−1) | R2 | qm (mg·g−1) | B (mol2·kJ−1) | E (kJ·mol−1) | R2 |
| 103.466 | 0.7790 | 0.9727 | 124.1802 | 1.0330 × 10−8 | 6.956 | 0.9638 |
| Adsorbent | Q0 (mg·g−1) | pH | References |
|---|---|---|---|
| rejected tea (particle size in the range 250–355 μm) | 147 (30 °C), 154 (40 °C), 156 (50 °C) | pH of 6–7 | [11] |
| spent tea leaves (0.5–1.0 mm) | 300.052 | without changing the solution pH | [12] |
| tea waste (180–300 μm) | 85.16 | pH of 8 | [13] |
| NaOH-modified rejected tea | 242.11 | pH of 7 | [14] |
| Tea waste (less than 150 μm) | 113.1461 | pH unadjusted | This study |
| Samples | The Net Amountof Released Cations (Mequivg−1) | Sum | |||
|---|---|---|---|---|---|
| Ca2+ | K+ | Mg+ | Na+ | ||
| 100 mg·L−1–35 °C | 0.02545 | −0.00679 | 0.001938 | 0.073848 | 0.09444 |
| 100 mg·L−1–45 °C | 0.0157188 | −0.00955 | 0.002167 | 0.05962 | 0.067954 |
| 100 mg·L−1–45 °C | 0.0186688 | −0.01212 | 0.002333 | 0.055554 | 0.064441 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Fan, S.; Li, Y. Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism. Int. J. Environ. Res. Public Health 2018, 15, 1321. https://doi.org/10.3390/ijerph15071321
Liu L, Fan S, Li Y. Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism. International Journal of Environmental Research and Public Health. 2018; 15(7):1321. https://doi.org/10.3390/ijerph15071321
Chicago/Turabian StyleLiu, Li, Shisuo Fan, and Yang Li. 2018. "Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism" International Journal of Environmental Research and Public Health 15, no. 7: 1321. https://doi.org/10.3390/ijerph15071321
APA StyleLiu, L., Fan, S., & Li, Y. (2018). Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism. International Journal of Environmental Research and Public Health, 15(7), 1321. https://doi.org/10.3390/ijerph15071321





