Meta-Analysis and Systematic Review in Environmental Tobacco Smoke Risk of Female Lung Cancer by Research Type
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Definition of ETS and Never Smoker
2.5. Definition of ETS Exposure Dose
- (1)
- If the ETS exposure was less than 20 pack-years, then the ETS exposure was defined as low pack-year, and if the ETS exposure was 20 or more pack-years then the ETS exposure was defined as high pack-year.
- (2)
- If the ETS exposure was less than 20 years, then the ETS was defined as short-term ETS, and if the exposure was 20 or more years, then it was defined as long-term ETS.
- (3)
- If the ETS exposure was less than 10 cigarettes per day, then the ETS was defined as light ETS, and if the ETS was 10 cigarettes or more, then it was defined as heavy ETS.
2.6. Quality Control
2.7. Statistical Analysis
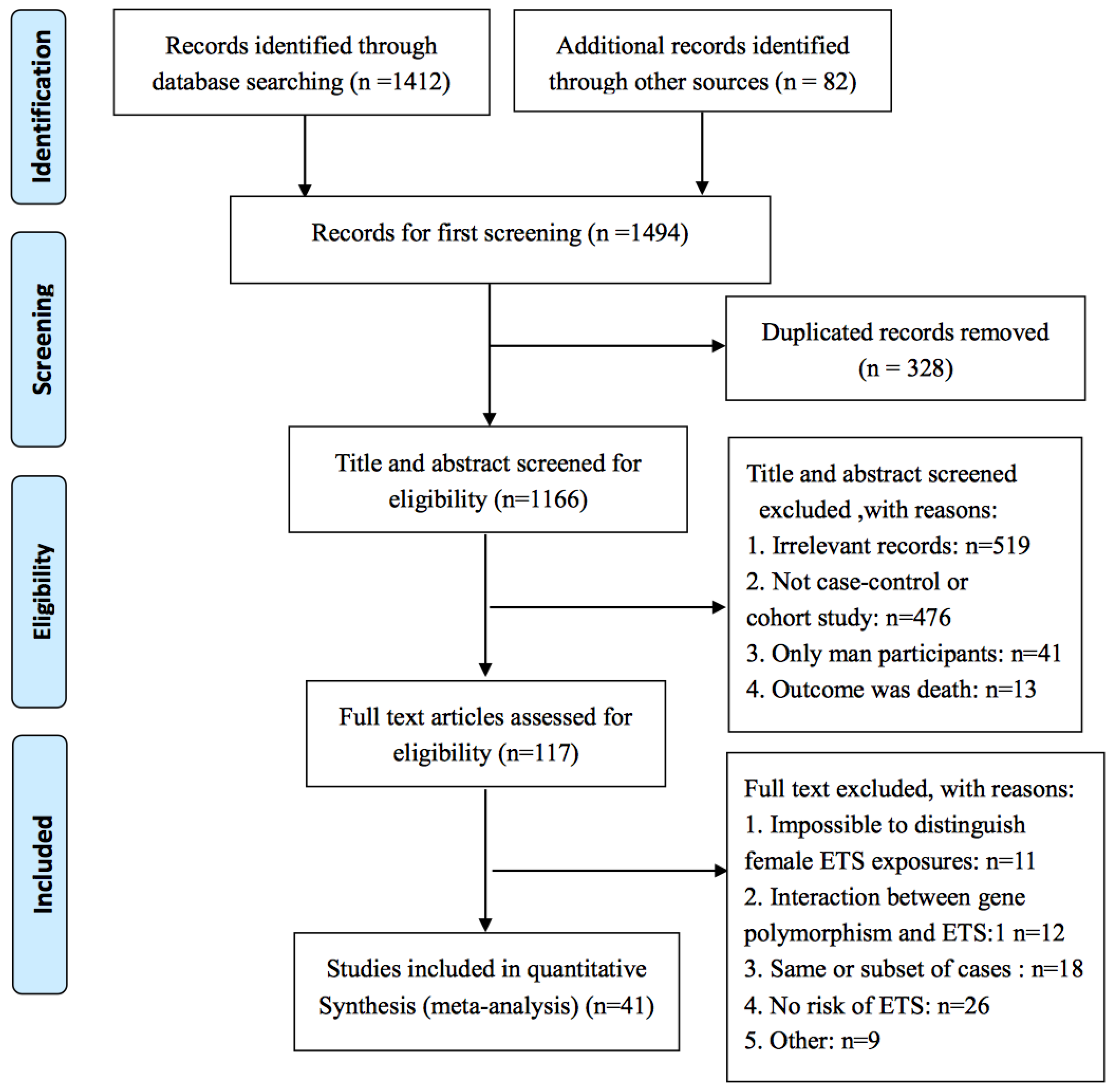
3. Results
3.1. Characteristics of Included Studies
3.2. Association of ETS with Female Lung Cancer by Different Study Type
3.3. Association of Female Lung Cancer with ETS Based on Exposure Source
3.4. Association of Female Lung Cancer with ETS Based on Different Exposure Dose
3.4.1. Association of Female Lung Cancer with ETS Exposure Dose in Cohort Studies
3.4.2. Association of Female Lung Cancer with ETS Exposure Dose in Case-Control Studies
3.5. Bias of Publications
3.6. Heterogeneity
3.7. Previous Meta-Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 83, 1–1438. [Google Scholar]
- Matt, G.E.; Quintana, P.J.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Vitali, M. The new danger of thirdhand smoke: Why passive smoking does not stop at secondhand smoke. Environ. Health Perspect. 2011, 119, A422. [Google Scholar] [CrossRef] [PubMed]
- Öberg, M.; Woodward, A.; Jaakkola, M.S.; Peruga, A.; Prüssüstün, A.; Öberg, M.; Woodward, A.; Jaakkola, M.S.; Peruga, A.; Prüssüstün, A. Global estimate of the burden of disease from second-hand smoke. Available online: http://www.who.int/tobacco/publications/global_estimate_burden_disease_second_hand_smoke/en/ (accessed on 18 May 2018).
- Kim, C.H.; Lee, Y.C.; Hung, R.J.; McNallan, S.R.; Cote, M.L.; Lim, W.Y.; Chang, S.C.; Kim, J.H.; Ugolini, D.; Chen, Y.; et al. Exposure to secondhand tobacco smoke and lung cancer by histological type: A pooled analysis of the international lung cancer consortium (ilcco). Int. J. Cancer 2014, 135, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.I. The Relationship on Passive Smoking to Various Health Outcomes among Seventh-Day Adventists in California. Ph.D.Thesis, University of California, Los Angeles, CA, USA, 1988. [Google Scholar]
- Cardenas, V.M.; Thun, M.J.; Austin, H.; Lally, C.A.; Clark, W.S.; Greenberg, R.S.; Heath, C.J. Environmental tobacco smoke and lung cancer mortality in the american cancer society’s cancer prevention study. II. Cancer Causes Control 1997, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, L. Time trends in lung cancer mortality among nonsmokers and a note on passive smoking. J. Natl. Cancer Inst. 1981, 66, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Enstrom, J.E.; Kabat, G.C. Environmental tobacco smoke and tobacco related mortality in a prospective study of californians, 1960-98. BMJ 2003, 326, 1057. [Google Scholar] [CrossRef] [PubMed]
- Hole, D.J.; Gillis, C.R.; Chopra, C.; Hawthorne, V.M. Passive smoking and cardiorespiratory health in a general population in the west of scotland. BMJ 1989, 299, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Nishino, Y.; Tsubono, Y.; Tsuji, I.; Komatsu, S.; Kanemura, S.; Nakatsuka, H.; Fukao, A.; Satoh, H.; Hisamichi, S. Passive smoking at home and cancer risk: A population-based prospective study in Japanese nonsmoking women. Cancer Causes Control 2001, 12, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.E.; Blakely, T.; Kawachi, I.; Woodward, A. Mortality among lifelong nonsmokers exposed to secondhand smoke at home: Cohort data and sensitivity analyses. Am. J. Epidemiol. 2007, 165, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Kubo, J.; Luo, J.; Desai, M.; Hedlin, H.; Henderson, M.; Chlebowski, R.; Tindle, H.; Chen, C.; Gomez, S.; et al. Active and passive smoking in relation to lung cancer incidence in the women’s health initiative observational study prospective cohort. Ann. Oncol. 2015, 26, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Lacey, J.J.; Shu, X.O.; Ji, B.T.; Hou, L.; Yang, G.; Li, H.; Rothman, N.; Blair, A.; Gao, Y.T.; et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am. J. Epidemiol. 2008, 168, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Fry, J.S.; Forey, B.A.; Hamling, J.S. Environmental tobacco smoke exposure and lung cancer: A systematic review. World J. Meta-Anal. 2016, 4, 10–43. [Google Scholar] [CrossRef]
- Wells, A.J. Lung cancer from passive smoking at work. Am. J. Public Health 1998, 88, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Tredaniel, J.; Greco, A. Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: A meta-analysis. Environ. Health Perspect. 2000, 108, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Merletti, F.; Richiardi, L.; Boffeta, P. Health effects of passive smoking. Med. del Lavoro 1998, 89, 149–163. [Google Scholar]
- Stayner, L.; Bena, J.; Sasco, A.J.; Smith, R.; Steenland, K.; Kreuzer, M.; Straif, K. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am. J. Public Health 2007, 97, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.G. Lung cancer and environmental tobacco smoke: Occupational risk to nonsmokers. Environ. Health Perspect. 1999, 107, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhou, B.S. Meta-analysis of the potential relationship between exposure to environmental tobacco smoke and lung cancer in nonsmoking chinese women. Lung Cancer 1997, 16, 145–150. [Google Scholar] [CrossRef]
- Taylor, R.; Najafi, F.; Dobson, A. Meta-analysis of studies of passive smoking and lung cancer: Effects of study type and continent. Int. J. Epidemiol. 2007, 36, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Goldberg, M.S.; Parent, M.E.; Hanley, J.A. Exposure to environmental tobacco smoke and the risk of lung cancer: A meta-analysis. Lung Cancer 2000, 27, 3–18. [Google Scholar] [CrossRef]
- Hori, M.; Tanaka, H.; Wakai, K.; Sasazuki, S.; Katanoda, K. Secondhand smoke exposure and risk of lung cancer in Japan: A systematic review and meta-analysis of epidemiologic studies. Jpn. J. Clin. Oncol. 2016, 46, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Cumming, R.; Woodward, A.; Black, M. Passive smoking and lung cancer: A cumulative meta-analysis. Aust. N. Z. J. Public Health 2001, 25, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Rao, K.; Chen, Y. A case-control study of the risk factors of lung cancer in Beijing, Tianjin, Shanghai, Chongqing metropolitan areas. Chin. J. Prev. Med. 2000, 34, 227–231. [Google Scholar]
- Chen, K.X.; Xu, W.L.; Jia, Z.L.; Yu, M.; Wang, Q.S.; Dong, S.F.; Wang, J.F. Risk factors of lung cancer in Tianjin. Chin. J. Oncol. 2003, 25, 575–580. [Google Scholar]
- Villeneuve, P.J.; Jerrett, M.; Brenner, D.; Su, J.; Chen, H.; McLaughlin, J.R. A case-control study of long-term exposure to ambient volatile organic compounds and lung cancer in Toronto, Ontario, Canada. Am. J. Epidemiol. 2014, 179, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Olivo-Marston, S.E.; Yang, P.; Mechanic, L.E.; Bowman, E.D.; Pine, S.R.; Loffredo, C.A.; Alberg, A.J.; Caporaso, N.; Shields, P.G.; Chanock, S.; et al. Childhood exposure to secondhand smoke and functional mannose binding lectin polymorphisms are associated with increased lung cancer risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.Y.; Weng, S.F.; Hernandez, L.; Spitz, M.R.; Forman, M.R. Dietary patterns affect lung cancer risk in never smokers. Nutr. Cancer 2011, 63, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duran, M.; Ruano-Ravina, A.; Kelsey, K.T.; Parente-Lamelas, I.; Leiro-Fernandez, V.; Abdulkader, I.; Provencio, M.; Abal-Arca, J.; Castro-Anon, O.; Montero-Martinez, C.; et al. Environmental tobacco smoke exposure and egfr and alk alterations in never smokers’ lung cancer. Results from the lcrins study. Cancer Lett. 2017, 411, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Ahrens, W.; Nyberg, F.; Mukeria, A.; Bruske-Hohlfeld, I.; Fortes, C.; Constantinescu, V.; Simonato, L.; Batura-Gabryel, H.; Lea, S.; et al. Exposure to environmental tobacco smoke and risk of adenocarcinoma of the lung. Int. J. Cancer 1999, 83, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.Y.; Wu, M.; Han, R.Q.; Zhang, X.F.; Wang, X.S.; Liu, A.M.; Zhou, J.Y.; Lu, Q.Y.; Kim, C.H.; Mu, L.; et al. Household ventilation may reduce effects of indoor air pollutants for prevention of lung cancer: A case-control study in a Chinese population. PLoS ONE 2014, 9, e102685. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Sørensen, M.; Loft, S.; Overvad, K.; Tjønneland, A. Lung cancer incidence and long-term exposure to air pollution from traffic. Environ. Health Perspect. 2011, 119, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Couraud, S.; Debieuvre, D.; Moreau, L.; Dumont, P.; Margery, J.; Quoix, E.; Duvert, B.; Cellerin, L.; Baize, N.; Taviot, B.; et al. No impact of passive smoke on the somatic profile of lung cancers in never-smokers. Eur. Respir. J. 2015, 45, 1415–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, L.; Su, J.; Qiu, X.; Palepu, P.R.; Hon, H.; Fadhel, E.; Harland, L.; La Delfa, A.; Habbous, S.; Kashigar, A.; et al. Second-hand smoke as a predictor of smoking cessation among lung cancer survivors. J. Clin. Oncol. 2014, 32, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Gao, Y.; Zhong, L.; Jin, F.; Sun, L.; Cheng, J.; Zhai, Y. A case-control study on relationship between body mass index and lung cancer in non-smoking women. Chin. J. Prev. Med. 1999, 33, 9–12. [Google Scholar]
- Neuberger, M.; Moshammer, H. Suspended particulates and lung health. Wien. Klin. Wochenschr. Suppl. 2004, 116, 8–12. [Google Scholar]
- Bromen, K.; Pohlabeln, H.; Jahn, I.; Ahrens, W.; Jockel, K.H. Aggregation of lung cancer in families: Results from a population-based case-control study in Germany. Am. J. Epidemiol. 2000, 152, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Seersholm, N.; Hertz, H.; Olsen, J.H. Cancer in the offspring of parents with lung cancer. Eur. J. Cancer 1997, 33, 2376–2379. [Google Scholar] [CrossRef]
- Gunbatar, H.; Sertogullarindan, B.; Ozbay, B.; Avcu, S.; Bulut, G.; Kosem, M. Chronic effects of environmental biomass smoke on lung histopathology in Turkish non-smoking women: A case series. Arh. Hig. Rada Toksikol. 2012, 63, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Sarihan, S.; Ercan, I.; Karadag, M. Evaluating quality of life and pulmonary function of long-term survivors of non-small cell lung cancer treated with radical or postoperative radiotherapy. Am. J. Clin. Oncol. 2009, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wünsch-Filho, V.; Boffetta, P.; Colin, D.; Moncau, J.E. Familial cancer aggregation and the risk of lung cancer. Sao Paulo Med. J. 2002, 120, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, S.; Thorsteinsdottir, U.; Gudbjartsson, D.F.; Jonsson, H.H.; Kristjansson, K.; Arnason, S.; Gudnason, V.; Isaksson, H.J.; Hallgrimsson, J.; Gulcher, J.R.; et al. Familial risk of lung carcinoma in the icelandic population. JAMA 2004, 292, 2977–2983. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; He, X. Molecular epidemiological studies on the relationship between indoor coal burning and lung cancer in Xuan Wei, China. Toxicology 2004, 198, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Masago, K.; Takeshita, J.; Togashi, Y.; Hata, A.; Kaji, R.; Kokubo, M.; Katakami, N. Multiple primary malignancies in patients with non-small cell lung cancer. Intern. Med. 2015, 54, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Seow, A.; Zhao, B.; Poh, W.T.; Teh, M.; Eng, P.; Wang, Y.T.; Tan, W.C.; Lee, E.J.; Lee, H.P. Nat2 slow acetylator genotype is associated with increased risk of lung cancer among non-smoking chinese women in Singapore. Carcinogenesis 1999, 20, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, M.R.; Naghan, P.A.; Taslimi, S.; Yousefifard, M.; Ebrahimi, S.M.; Khosravi, A.; Karimi, S.; Hosseini, M.; Mortaz, E. Opium could be considered an independent risk factor for lung cancer: A case-control study. Respiration 2013, 85, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Vähäkangas, K.H.; Bennett, W.P.; Castrén, K.; Welsh, J.A.; Khan, M.A.; Blömeke, B.; Alavanja, M.C.R.; Harris, C.C. P53 and K-ras mutations in lung cancers from former and never-smoking women. Cancer Res. 2001, 61, 4350–4356. [Google Scholar] [PubMed]
- Fang, X.; Yin, Z.; Li, X.; Xia, L.; Zhou, B. Polymorphisms in GEMIN4 and AGO1 genes are associated with the risk of lung cancer: A case-control study in Chinese female non-smokers. Int. J. Environ. Res. Public Health 2016, 13, 939. [Google Scholar] [CrossRef] [PubMed]
- Laurila, A.L.; Anttila, T.; Läärä, E.; Bloigu, A.; Virtamo, J.; Albanes, D.; Leinonen, M.; Saikku, P. Serological evidence of an association between chlamydia pneumoniae infection and lung cancer. Int. J. Cancer 1997, 74, 31–34. [Google Scholar] [CrossRef]
- Das, A.; Krishnamurthy, A.; Ramshankar, V.; Sagar, T.G.; Swaminathan, R. The increasing challenge of never smokers with adenocarcinoma lung: Need to look beyond tobacco exposure. Indian J. Cancer 2017, 54, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Comstock, G.W.; Alberg, A.J.; Huang, H.Y.; Wu, K.; Burke, A.E.; Hoffman, S.C.; Norkus, E.P.; Gross, M.; Cutler, R.G.; Morris, J.S.; et al. The risk of developing lung cancer associated with antioxidants in the blood: Ascorbic acid, carotenoids, alpha-tocopherol, selenium, and total peroxyl radical absorbing capacity. Cancer Epidemiol. Biomark. Prev. 1997, 6, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Ravina, A.; Torres-Durán, M.; Barros-Dios, J.; Raíces, M.; Pérez-Ríos, M.; Abal-Arca, J.; Parente, I.; Leiro, V.; Montero-Martínez, C.; Pena, C.; et al. Residential radon and lung cancer in never smokers. Preliminary results of the lung cancer risk factors in never smokers (LCRINS) study. Am. J. Epidemiol. 2013, 177, S126. [Google Scholar] [CrossRef]
- García-Sancho, C.F.; Fernández-Plata, R.; de la Garza, M.S.R.; Mora-Pizano, M.A.; Martínez-Briseño, D.; Franco-Marina, F.; Pérez-Padilla, J.R. Wood smoke as a risk factor for lung cancer nonsmoking population hospitalized. Rev. Inst. Nac. Enferm. Respir. 2012, 71, 325–332. [Google Scholar]
- Leung, C.C.; Hui, L.; Lam, T.H.; Yew, W.W.; Law, W.S.; Tam, C.M. Tuberculosis increases the risk of lung cancer death in the elderly. Am. J. Respir. Crit. Care Med. 2012, 185, A6781. [Google Scholar]
- Mulcahy, M.; Evans, D.S.; Hammond, S.K.; Repace, J.L.; Byrne, M. Secondhand smoke exposure and risk following the irish smoking ban: An assessment of salivary cotinine concentrations in hotel workers and air nicotine levels in bars. Tob. Control 2005, 14, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.S.; Gesell, T.F. Residential radon exposure and lung cancer: Risk in nonsmokers. Health Phys. 2002, 83, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Bonner, M.; Irla, A.; Jin, J.; Olson, J.; Ellison, C.; Zhang, Z.F. Mitochondrial DNA copy number and lung cancer risk among Chinese female non-smoker. Cancer Res. 2012, 72, 5481. [Google Scholar] [CrossRef]
- Lagarde, F.; Axelsson, G.; Damber, L.; Mellander, H.; Nyberg, F.; Pershagen, G. Residential radon and lung cancer among never-smokers in Sweden. Epidemiology 2001, 12, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Maoa, Y.; Hua, J.; Ugnata, A.M.; Semenciwa, R.; Finchamb, S. Socioeconomic status and lung cancer risk in canada. Int. J. Epidemiol. 2001, 30, 809–817. [Google Scholar] [CrossRef]
- Nyberg, F.; Isaksson, I.; Harris, J.R.; Pershagen, G. Misclassification of smoking status and lung cancer risk from environmental tobacco smoke in never-smokers. Epidemiology 1997, 8, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhou, B.; Xu, Z. A case-control study on risk factor of lung cancer in female nonsmokers. Chin. J. Lung Cancer 2002, 5, 98–100. [Google Scholar]
- Nyberg, F.; Agudo, A.; Boffetta, P.; Fortes, C.; González, C.A.; Pershagen, G. A european validation study of smoking and environmental tobacco smoke exposure in nonsmoking lung cancer cases and controls. Cancer Causes Control 1998, 9, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cai, L. Environmental and dietary factors and lung cancer risk among chinese women: A case-control study in Southeast China. Nutr. Cancer 2012, 64, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, M.; Molitor, J.; Richardson, S.; Riboli, E.; Vineis, P. Examining the joint effect of multiple risk factors using exposure risk profiles: Lung cancer in nonsmokers. Environ. Health Perspect. 2011, 119, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Hsiao, C.F.; Chang, G.C.; Tsai, Y.H.; Su, W.C.; Perng, R.P.; Huang, M.S.; Hsiung, C.A.; Chen, C.J.; Yang, P.C. Hormone replacement therapy and lung cancer risk in Chinese. Cancer 2007, 110, 1768–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Mao, Y.; Dryer, D.; White, K.; Paulse, B.; Dewar, R.; Kreiger, N.; Whittaker, H.; Robson, D.; Fincham, S.; et al. Risk factors for lung cancer among canadian women who have never smoked. Cancer Detect. Prev. 2002, 26, 129–138. [Google Scholar] [CrossRef]
- Kreuzer, M.; Heinrich, J.; Kreienbrock, L.; Rosario, A.S.; Gerken, M.; Wichmann, H.E. Risk factors for lung cancer among nonsmoking women. Int. J. Cancer 2002, 100, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.J.; Xiang, Y.B.; Yang, G.; Li, H.L.; Lan, Q.; Gao, Y.T.; Zheng, W.; Shu, X.O.; Fowke, J.H. Vitamin E intake and the lung cancer risk among female nonsmokers: A report from the shanghai women’s health study. Int. J. Cancer 2015, 136, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Zatloukal, P.; Kubík, A.; Pauk, N.; Tomášek, L.; Petruzelka, L. Adenocarcinoma of the lung among women: Risk associated with smoking, prior lung disease, diet and menstrual and pregnancy history. Lung Cancer 2003, 41, 283–293. [Google Scholar] [CrossRef]
- Wang, X.R.; Chiu, Y.L.; Qiu, H.; Au, J.S.; Yu, I.T. The roles of smoking and cooking emissions in lung cancer risk among chinese women in Hong Kong. Ann. Oncol. 2009, 20, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y. Lung cancer in the Chinese passive smoking populations. Value Health 2009, 12, A37. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hsiung, C.N.; Li, Y.J.; Chang, G.C.; Tsai, Y.H.; Chen, K.Y.; Huang, M.S.; Su, W.C.; Chen, Y.M.; Hsiung, C.A.; et al. Fanconi anemia genes in lung adenocarcinoma- a pathway-wide study on cancer susceptibility. J. Biomed. Sci. 2016, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Veglia, F.; Garte, S.; Malaveille, C.; Matullo, G.; Dunning, A.; Peluso, M.; Airoldi, L.; Overvad, K.; Raaschou-Nielsen, O.; et al. Genetic susceptibility according to three metabolic pathways in cancers of the lung and bladder and in myeloid leukemias in nonsmokers. Ann. Oncol. 2007, 18, 1230–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veglia, F.; Vineis, P.; Overvad, K.; Boeing, H.; Bergmann, M.M.; Trichopoulou, A.; Trichopoulos, D.; Palli, D.; Krogh, V.; Tumino, R.; et al. Occupational exposures, environmental tobacco smoke, and lung cancer. Epidemiology 2007, 18, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Baltar, V.T.; Xun, W.W.; Chuang, S.C.; Relton, C.; Ueland, P.M.; Vollset, S.E.; Midttun, O.; Johansson, M.; Slimani, N.; Jenab, M.; et al. Smoking, secondhand smoke, and cotinine levels in a subset of EPIC cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Shu, X.O.; Yang, G.; Li, H.; Dai, Q.; Gao, J.; Cai, Q.; Chow, W.H.; Gao, Y.; Zheng, W. Association between dietary calcium intake and lung cancer risk in female non-smokers: A report from the shanghai women’s health study. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Cai, Q.; Gao, Y.T.; Wen, W.; Milne, G.L.; Yang, G.; Ji, B.T.; Rothman, N.; Li, H.L.; Shu, X.O.; Chow, W.H.; et al. Prospective study of urinary prostaglandin E2 metabolite and lung cancer risk. Cancer Res. 2010, 70. [Google Scholar] [CrossRef]
- Fowke, J.H.; Gao, Y.T.; Chow, W.H.; Cai, Q.; Shu, X.O.; Li, H.L.; Ji, B.T.; Rothman, N.; Yang, G.; Chung, F.L.; et al. Urinary isothiocyanate levels and lung cancer risk among non-smoking women: A prospective investigation. Lung Cancer 2011, 73, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soo, R.A.; Kubo, A.; Ando, M.; Kawaguchi, T.; Ahn, M.J.; Ou, S.H.I. Association between environmental tobacco smoke exposure and the occurrence of egfr mutations and alk rearrangements in never-smokers with non–small-cell lung cancer: Analyses from a prospective multinational ets registry. Clin. Lung Cancer 2017, 18, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.P.; Alavanja, M.C.R.; Blomeke, B.; Vähäkangas, K.H.; Castrén, K.; Welsh, J.A.; Bowman, E.D.; Khan, M.A.; Flieder, D.B.; Harris, C.C. Environmental tobacco smoke, genetic susceptibility, and risk of lung cancer in never-smoking women. J. Natl. Cancer Inst. 1999, 91, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lee, C.K.; Pang, H.; Chan, H.T.; Lo, I.L.; Lam, S.K.; Cheong, T.H.; Ho, J.C. Genetic predisposition to lung adenocarcinoma among never-smoking chinese with different epidermal growth factor receptor mutation status. Lung Cancer 2017, 114, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Bennett, W.P.; Xiong, W.; Lan, Q.; Brownson, R.C.; Harris, C.C.; Field, R.W.; Lubin, J.H.; Alavanja, M.C. Radon, secondhand smoke, glutathione-s-transferase m1 and lung cancer among women. Int. J. Cancer 2006, 119, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Hedley, A.J.; McGhee, S.M.; Repace, J.L.; Wong, L.C.; Yu, M.Y.; Wong, T.W.; Lam, T.H. Risks for heart disease and lung cancer from passive smoking by workers in the catering industry. Toxicol. Sci. 2006, 90, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Talaska, G.; Kahn, R.S.; Schumann, B.; Khoury, J.; Leonard, A.C.; Lanphear, B.P. White blood cell DNA adducts in a cohort of asthmatic children exposed to environmental tobacco smoke. Int. Arch. Occup. Environ. Health 2011, 84, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Ando, M.; Soo, R.; Kawaguchi, T.; Ou, S.H.I.; Ahn, M.J. Impacts of environmental tobacco smoke on EGFR mutations and ALK rearrangements in never smokers with non-small cell lung cancer: Analyses on a prospective multinational ETS registry. J. Thorac. Oncol. 2013, 8. [Google Scholar] [CrossRef]
- Ryan, B.M.; Robles, A.I.; McClary, A.C.; Bowman, E.; Vahakangas, K.; Olivo-Marston, S.; Yang, P.; Jen, J.; Harris, C.C. Interaction between drd1 and childhood exposure to environmental tobacco smoke modulates lung cancer risk in smokers and never smokers. Cancer Res. 2013, 73. [Google Scholar] [CrossRef]
- Yang, L.; Lv, X.X.; Ling, X.X.; Song, J.L.; Ji, W.D.; Bin, X.N.; Lv, J.C. Association between the genetic variant in CHRNA3 promoter and lung cancer risk in passive smoking population. Chin. J. Cancer Prev. Treat. 2010, 17, 972–975, 990. [Google Scholar]
- Kiyohara, C.; Wakai, K.; Mikami, H.; Sido, K.; Ando, M.; Ohno, Y. Risk modification by CYP1A1 and GSTM1 polymorphisms in the association of environmental tobacco smoke and lung cancer: A case-control study in Japanese nonsmoking women. Int. J. Cancer 2003, 107, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Hou, S.M.; Hemminki, K.; Lambert, B.; Pershagen, G. Glutathione S-transferase μ1 and N-acetyltransferase 2 genetic polymorphisms and exposure to tobacco smoke in nonsmoking and smoking lung cancer patients and population controls. Cancer Epidemiol. Biomark. Prev. 1998, 7, 875–883. [Google Scholar]
- Miller, D.P.; De Vivo, I.; Neuberg, D.; Wain, J.C.; Lynch, T.J.; Su, L.; Christiani, D.C. Association between self-reported environmental tobacco smoke exposure and lung cancer: Modification by GSTP1 polymorphism. Int. J. Cancer 2003, 104, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Seow, A.; Poh, W.T.; Teh, M.; Eng, P.; Wang, Y.T.; Tan, W.C.; Chia, K.S.; Yu, M.C.; Lee, H.P. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: Evidence for a protective effect of soy in nonsmokers. Int. J. Cancer 2002, 97, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R. Spousal smoking as an indicator of total secondhand smoke exposure. Nicot. Tob. Res. 2009, 11, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, H.; Wang, D.; Liu, D.; Zeng, J.; Wang, Y.; Zhang, S.; Gao, J.; Yu, J.; Li, W. Design and application of a self-evaluation questionnaire for individuals at a high-risk of lung cancer. Thorac. Cancer 2012, 3, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.G.; Cote, M.L.; Wenzlaff, A.S.; Van Dyke, A.; Chen, W.; Ruckdeschel, J.C.; Gadgeel, S.; Soubani, A.O. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J. Thorac. Oncol. 2009, 4, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ferreccio, C.; Yuan, Y.; Calle, J.; Benitez, H.; Parra, R.L.; Acevedo, J.; Smith, A.H.; Liaw, J.; Steinmaus, C. Arsenic, tobacco smoke, and occupation: Associations of multiple agents with lung and bladder cancer. Epidemiology 2013, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Inoue, M.; Liu, Y.; Iwasaki, M.; Sasazuki, S.; Sobue, T.; Tsugane, S. Passive smoking and lung cancer in Japanese non-smoking women: A prospective study. Int. J. Cancer 2008, 122, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Gan, D.K.; Zheng, S.H.; Zhang, H.W. A case-control study of the risk factors for lung cancer among chinese women who have never smoked. J. Hyg. Res. 2006, 35, 464–467. [Google Scholar]
- Zhou, B.S.; Wang, T.J.; Guan, P.; Wu, J.M. Indoor air pollution and pulmonary adenocarcinoma among females: A case-control study in Shenyang, China. Oncol. Rep. 2000, 7, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Liu, L.; Niu, R.; Zhao, B.; Shi, J.; Li, Y.; Swanson, M.; Scheider, W.; Su, J.; Chang, S.C.; et al. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Causes Control 2013, 24, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.L.; Hsiao, C.F.; Chang, G.C.; Tsai, Y.H.; Huang, M.S.; Su, W.C.; Chen, Y.M.; Hsin, C.W.; Chang, C.H.; Yang, P.C.; et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control 2013, 24, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Goldberg, M.S.; Gao, Y.T.; Jin, F. A case-control study of lung cancer and environmental tobacco smoke among nonsmoking women living in Shanghai, China. Cancer Causes Control 1999, 10, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Lee, C.H.; Chen, M.J.; Huang, C.C.; Chang, W.Y.; Lin, H.J.; Wang, H.Z.; Chang, P.Y. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int. J. Epidemiol. 1997, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ko, Y.C.; Goggins, W.; Huang, J.J.; Huang, M.S.; Kao, E.L.; Wang, H.Z. Lifetime environmental exposure to tobacco smoke and primary lung cancer of non-smoking Taiwanese women. Int. J. Epidemiol. 2000, 29, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaridze, D.; Maximovitch, D.; Zemlyanaya, G.; Aitakov, Z.N.; Boffetta, P. Exposure to environmental tobacco smoke and risk of lung cancer in non- smoking women from Moscow, Russia. Int. J. Cancer 1998, 75, 335–338. [Google Scholar] [CrossRef]
- Kreuzer, M.; Gerken, M.; Kreienbrock, L.; Wellmann, J.; Wichmann, H.E. Lung cancer in lifetime nonsmoking men—Results of a case-control study in Germany. Br. J. Cancer 2001, 84, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Agudo, A.; Ahrens, W.; Benhamou, E.; Benhamou, S.; Darby, S.C.; Ferro, G.; Fortes, C.; Gonzalez, C.A.; Jöckel, K.H.; et al. Multicenter case-control study of exposure to environmental tobacco smoke and lung cancer in Europe. J. Natl. Cancer Inst. 1998, 90, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.Y.; Zhang, Y.; Schabath, M.B.; Lei, L.; Zhang, Q.; Amos, C.I.; Spitz, M.R. Never smokers and lung cancer risk: A case-control study of epidemiological factors. Int. J. Cancer 2006, 118, 1798–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rylander, R.; Axelsson, G. Lung cancer risks in relation to vegetable and fruit consumption and smoking. Int. J. Cancer 2006, 118, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Agrenius, V.; Svartengren, K.; Svensson, C.; Pershagen, G. Environmental tobacco smoke and lung cancer in nonsmokers: Does time since exposure play a role? Epidemiology 1998, 9, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Ohrr, H.; Kim, I.S. Effects of husbands’ smoking on the incidence of lung cancer in Korean women. Int. J. Epidemiol. 1999, 28, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Li, X.L.; Yu, X.S.; Guan, P.; Yin, Z.H.; He, Q.C.; Zhou, B.S. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: A systematic review. Int. J. Cancer 2009, 125, 2936–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan-Yeung, M.; Koo, L.C.; Ho, J.C.M.; Tsang, K.W.T.; Chau, W.S.; Chiu, S.W.; Ip, M.S.M.; Lam, W.K. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 2003, 40, 131–140. [Google Scholar] [CrossRef]
- Wang, L.; Lubin, J.H.; Zhang, S.R.; Metayer, C.; Xia, Y.; Brenner, A.; Shang, B.; Wang, Z.; Kleinerman, R.A. Lung cancer and environmental tobacco smoke in a non-industrial area of China. Int. J. Cancer 2000, 88, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Rapiti, E.; Jindal, S.K.; Gupta, D.; Boffetta, P. Passive smoking and lung cancer in Chandigarh, India. Lung Cancer 1999, 23, 183–189. [Google Scholar] [CrossRef]
- Johnson, K.C.; Hu, J.; Mao, Y. Lifetime residential and workplace exposure to environmental tobacco smoke and lung cancer in never-smoking women, Canada 1994-97. Int. J. Cancer 2001, 93, 902–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachtan, J. Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer 2002, 35, 129–136. [Google Scholar] [CrossRef]
- Franco-Marina, F.; Villalba Caloca, J.; Corcho-Berdugo, A.; Pérez, C.I.; Morales, F.M.; Sabido, R.C.; Casanova Ma, E.R.; Schnweeiss, L.G.; Acevedo, E.C.; Díaz, E.T.; et al. Role of active and passive smoking on lung cancer etiology in Mexico City. Salud Publica Mex. 2006, 48 (Suppl. 1), S75–S82. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.S.; Mahnken, J.D.; Mayo, M.S.; Field, R.W. Risk factors for lung cancer in Iowa women: Implications for prevention. Cancer Detect. Prev. 2006, 30, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubik, A.K.; Zatloukal, P.; Tomasek, L.; Petruzelka, L. Lung cancer risk among Czech women: A case-control study. Prev. Med. 2002, 34, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Nishino, Y.; Tanji, F.; Maemondo, M.; Takahashi, S.; Sato, I.; Kawai, M.; Minami, Y. Cigarette smoking and lung cancer risk according to histologic type in Japanese men and women. Cancer Sci. 2013, 104, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.C.; Wang, S.Y.; Chen, Y. Fraction analysis of the involvement of multiple risk factors in the etiology of lung cancer: Risk factor interactions in a case-control study for lung cancer in females. Zhonghua Liu Xing Bing Xue Za Zhi 1997, 18, 341–344. [Google Scholar] [PubMed]
- Yu, I.T.; Chiu, Y.L.; Au, J.S.; Wong, T.W.; Tang, J.L. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006, 66, 4961–4967. [Google Scholar] [CrossRef] [PubMed]
- Speizer, F.E.; Colditz, G.A.; Hunter, D.J.; Rosner, B.; Hennekens, C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes Control 1999, 10, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Airoldi, L.; Veglia, F.; Olgiati, L.; Pastorelli, R.; Autrup, H.; Dunning, A.; Garte, S.; Gormally, E.; Hainaut, P.; et al. Environmental tobacco smoke and risk of respiratory cancer and chronic obstructive pulmonary disease in former smokers and never smokers in the epic prospective study. BMJ 2005, 330, 277. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yan, W.; Dai, X. Indoor air pollution and women lung cancer. J. Environ. Health 1999, 16, 201–202. [Google Scholar]
- He, F.; Xie, J.X.; Liu, C.L.; Xiong, W.M.; Xu, Q.P.; Liu, Z.Q.; Lin, T.; Xiao, R.D.; Li, X.; Cai, L. The relationship of lung cancer with menstrual and reproductive factors may be influenced by passive smoking, cooking oil fumes, and tea intake: A case-control study in Chinese women. Medicine 2017, 96, e8816. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Fan, R.; Wu, Z. Studies on relationship between passive smoking and lung cancer in non-smoking women. Chin. J. Prev. Med. 1997, 31, 163–165. [Google Scholar]
- Yin, Z.H.; Cui, Z.G.; Ren, Y.W.; Su, M.; Ma, R.; He, Q.C.; Zhou, B.S. Tp63 gene polymorphisms, cooking oil fume exposure and risk of lung adenocarcinoma in Chinese non-smoking females. Asian Pac. J. Cancer Prev. 2014, 14, 6519–6522. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, Y.C.; Hung, R.J.; Boffetta, P.; Xie, D.; Wampfler, J.A.; Cote, M.L.; Chang, S.C.; Ugolini, D.; Neri, M.; et al. Secondhand tobacco smoke exposure and lung adenocarcinoma in situ/minimally invasive adenocarcinoma (AIS/MIA). Cancer Epidemiol. Biomark. Prev. 2015, 24, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Naghan, P.A.; Karimi, S.; SeyedAlinaghi, S.; Bahadori, M.; Khodadad, K.; Mohammadi, F.; Kaynama, K.; Masjedi, M.R. Environmental risk factors for lung cancer in Iran: A case-control study. Int. J. Epidemiol. 2009, 38, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yin, Z.; Li, K.; Wan, Y.; Li, X.; Wu, W.; Guan, P.; Zhou, B. TGFβ-1 and TGFBR2 polymorphisms, cooking oil fume exposure and risk of lung adenocarcinoma in Chinese nonsmoking females: A case control study. BMC Med. Genet. 2015, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Behera, D.; Balamugesh, T. Indoor air pollution as a risk factor for lung cancer in women. J. Assoc. Phys. India 2005, 53, 190–192. [Google Scholar]
- Phukan, R.K.; Saikia, B.J.; Borah, P.K.; Zomawia, E.; Sekhon, G.S.; Mahanta, J. Role of household exposure, dietary habits and glutathione S-transferases M1, T1 polymorphisms in susceptibility to lung cancer among women in Mizoram India. Asian Pac. J. Cancer Prev. 2014, 15, 3253–3260. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey, S.G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boffetta, P. Involuntary smoking and lung cancer. Scand. J. Work Environ. Health 2002, 28, 30–40. [Google Scholar] [PubMed]
- Hackshaw, A.K.; Law, M.R.; Wald, N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. Br. Med. J. 1997, 315, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Gross, A.J. Presentation: The risk of lung cancer in nonsmokers in the united states and its reported association with environmental tobacco smoke. J. Clin. Epidemiol. 1995, 48, 587–598. [Google Scholar] [CrossRef]
- Tweedie, R.L.; Scott, D.J.; Biggerstaff, B.J.; Mengersen, K.L. Bayesian meta-analysis, with application to studies of ETS and lung cancer. Lung Cancer 1996, 14 (Suppl. 1), S171–S194. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Passive Smoking. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects; National Academies Press (US): Washington, DC, USA, 1986; ISBN-10: 0-309-03730-1. [Google Scholar]
- Blot, W.J.; Fraumeni, J.J. Passive smoking and lung cancer. J. Natl. Cancer Inst. 1986, 77, 993–1000. [Google Scholar] [PubMed]
- Wells, A.J. An estimate of adult mortality in the united states from passive smoking. Environ. Int. 1998, 14, 249–265. [Google Scholar] [CrossRef]
- Lee, P.N. Environmental Tobacco Smoke and Mortality; Karger: New York, NY, USA; Basel, Switzerland, 1992; Volume 1, pp. 110–118(119). [Google Scholar]
- U.S. Environmental Protection Agency; Office of Health and Environmental Assessment. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders, 1st ed.; National Academies Press: Washington, DC, USA, 1993.
- Pershagen, G. Epidemiology of Lung Cancer, 1st ed.; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Mengersen, K.L.; Tweedie, R.L.; Biggerstaff, B. The impact of method choice on meta-analysis. Aust. N. Z. J. Stat. 2010, 37, 19–44. [Google Scholar] [CrossRef]
- Dockery, D. Environmental Tobacco Smoke and Lung Cancer: Environmental Smoke Screen; CRC/Lewis: New York, NY, USA, 1996; Volume 90, pp. 309–323. [Google Scholar]
- Zhao, H.; Gu, J.; Xu, H.; Yang, B.; Han, Y.; Li, L.; Liu, S.; Yao, H. Meta-analysis of the relationship between passive smoking population in China and lung cancer. Zhongguo Fei Ai Za Zhi 2010, 13, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J.; Nanchahal, K.; Thompson, S.G.; Cuckle, H.S. Does breathing other people’s tobacco smoke cause lung cancer? Br. Med. J. (Clin. Res. Ed.) 1986, 293, 1217–1222. [Google Scholar] [CrossRef]
- Saracci, R.; Riboli, E. Passive smoking and lung cancer: Current evidence and ongoing studies at the international agency for research on cancer. Mutat. Res. 1989, 222, 117–127. [Google Scholar] [CrossRef]
- Law, M.R.; Hackshaw, A.K. Environmental tobacco smoke. Br. Med. Bull. 1996, 52, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tweedie, R.L.; Mengersen, K.L. Lung cancer and passive smoking: Reconciling the biochemical and epidemiological approaches. Br. J. Cancer 1992, 66, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Zhang, L. The relationship of indoor coal use and environmental tobacco smoke exposure with lung cancer in China: A meta-analysis. J. Cancer Res. Ther. 2018, 14, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Wang, C.P.; Han, Y.F.; Niu, J.J.; Zhang, Y.Z.; Fang, Y. Meta-analysis on related risk factors regarding lung cancer in non-smoking Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi 2016, 37, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, T.; Wu, M.; Zhang, L.; Jiang, C. Relationship between environmental tobacco smoke and lung cancer risk among nonsmokers in China: A meta-analysis. Chin. J. Prev. Med. 2015, 49, 644–648. [Google Scholar]
- Levois, M.E.; Layard, M.W. Inconsistency between workplace and spousal studies of environmental tobacco smoke and lung cancer. Regul. Toxicol. Pharmacol. 1994, 25, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bero, L.A.; Glantz, S.A.; Rennie, D. Publication bias and public health policy on environmental tobacco smoke. JAMA 1994, 272, 133–136. [Google Scholar] [CrossRef] [PubMed]

| ETS Category | Definition | References |
|---|---|---|
| Workplace ETS | ETS from smoking colleagues who worked in the same office or workplace | [13,98,99,100,101,102,103,104,105,106,107,108,109,110,111] |
| Family ETS | ETS from parents in childhood, husbands of current smokers or ever smokers, or other family smokers | [11,13,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123] |
| Family and Workplace ETS | ETS both from family and workplace | [13,98,102,103,109,114,117,121,124] |
| Unknown ETS | ETS source was not specified | [13,14,99,101,102,103,105,107,108,109,113,114,115,117,121,124,125,126,127,128,129,130,131,132,133,134,135] |
| Author | Year | Country | RR | 95% CI | Adjustment |
|---|---|---|---|---|---|
| Jee et al. [112] | 1999 | Korea | 1.90 | 1.00–3.50 | Yes: age, socioeconomic status, residency, vegetable consumption, occupation |
| Speize et al. [125] | 1999 | U.S. | 1.50 | 0.30–6.30 | Yes: age |
| Nishino et al. [11] | 2001 | Japan | 1.80 | 0.67–4.60 | Yes: age, study area, alcohol, diet, history of lung diseases |
| Vineis et al. [126] | 2005 | Europe | 1.20 | 0.71–2.02 | Yes: age, sex, smoking, country, school years |
| Weiss et al. [14] | 2008 | China | 0.94 | 0.65–1.35 | No |
| Kurahashi et al. [98] | 2008 | Japan | 1.45 | 0.86–2.44 | No |
| Wang et al. [13] | 2015 | U.S. | 0.88 | 0.52–1.49 | Yes: age, body mass index (BMI), ethnicity, history of lung cancer, family history of cancer, education, occupation, hormone therapy use, oral contraceptive use, fruit servings per day, vegetable servings per day, red meat serving per day, alcohol, physical activity |
| Pooled RR (Fixed effect) RR: 1.17, 95% CI: 0.94–1.44 | |||||
| Author | Year | Country | OR | 95% CI | Adjustment |
|---|---|---|---|---|---|
| Zheng et al. [129] | 1997 | China | 1.04 | 0.59–1.85 | No |
| Ko 1 et al. [104] | 1997 | Taiwan | 0.80 | 0.40–1.60 | Yes: socioeconomic status, residential area, education |
| Dai et al. [123] | 1997 | China | 3.14 | 1.97–5.01 | No |
| Boffetta et al. [108] | 1998 | Europe | 1.15 | 0.86–1.55 | Yes: age, sex |
| Nyberg 1 et al. [111] | 1998 | Sweden | 0.76 | 0.42–1.37 | Yes: age, gender, catchment area, occasional smoking, vegetable consumption, degree of urban residence, years of exposure to risk occupation |
| Song et al. [127] | 1999 | China | 2.31 | 1.36–3.90 | No |
| Zhong et al. [103] | 1999 | China | 1.20 | 0.80–1.80 | Yes: age, income, intake of vitamin C, respondent status, smokiness of cooking, family history of lung cancer, occupation |
| Zaridze 1 et al. [106] | 1999 | Russia | 0.88 | 0.55–1.41 | Yes: age, education |
| Rapiti 1 et al. [116] | 1999 | India | 1.20 | 0.50–2.90 | Yes: age, residence, religion |
| Zhou 1 et al. [100] | 2000 | China | 0.89 | 0.25–3.16 | No |
| Wang et al. [115] | 2000 | China | 1.15 | 0.60–2.10 | No |
| Lee et al. [105] | 2000 | Taiwan | 1.88 | 1.36–2.60 | No |
| Kreuzer et al. [107] | 2000 | Germany | 1.09 | 0.79–1.50 | No |
| Johnson et al. [117] | 2001 | Canada | 1.32 | 0.66–2.63 | No |
| Fang et al. [99] | 2002 | China | 2.95 | 1.60–5.47 | No |
| Rachtan [118] | 2002 | Poland | 2.49 | 1.36–4.54 | Yes: age, diet, siblings with cancer, tuberculosis, place of residence, occupational exposure, pack-years smoking |
| Kubík et al. [121] | 2002 | Czech | 1.05 | 0.59–1.86 | No |
| Chan-Yeung et al. [114] | 2003 | Hong Kong | 1.57 | 0.92–2.68 | No |
| Phukan et al. [135] | 2005 | India | 1.56 | 1.02–2.39 | Yes: age, education, occupational status |
| Yu et al. [124] | 2006 | Hong Kong | 1.39 | 0.80–2.41 | No |
| Francomarina et al. [119] | 2006 | Mexico | 1.70 | 1.10–2.80 | Yes: age, educational level, access to social security |
| Neuberger 1 et al. [120] | 2006 | U.S. | 0.37 | 0.26–0.54 | No |
| Gorlova et al. [109] | 2006 | U.S. | 1.27 | 0.82–1.97 | No |
| Rylander 1 et al. [110] | 2006 | Sweden | 1.37 | 0.72–2.61 | No |
| Liang et al. [113] | 2009 | China | 1.43 | 1.00–2.07 | Yes: age, marital status, years of schooling, ethnicity, BMI, 5 years ago |
| Hosseini et al. [132] | 2009 | Iran | 1.50 | 0.80–3.00 | No |
| Mu et al. [101] | 2013 | China | 1.48 | 0.93–2.35 | No |
| Lo et al. [102] | 2013 | Taiwan | 1.39 | 1.17–1.67 | Yes: age, years of education |
| Seki et al. [122] | 2013 | Japan | 1.31 | 0.99–1.72 | Yes: age, year of recruitment, area of residence, referral status, occupation, alcohol drinking, family history of lung cancer |
| Yin et al. [130] | 2014 | China | 1.28 | 0.92–1.79 | Yes: age |
| Behera et al. [134] | 2014 | India | 2.01 | 0.83–4.92 | Yes: smoking, cooking fuel, residence, occupational history |
| Kim et al. [131] | 2015 | U.S. | 1.37 | 0.89–2.10 | Yes: age, sex, race/ethnicity |
| Ren et al. [133] | 2015 | China | 1.10 | 0.79–1.53 | No |
| He et al. [128] | 2017 | China | 2.16 | 1.67–2.80 | No |
| Pooled OR (Random effect) OR: 1.35, 95% CI: 1.17–1.56 | |||||
| Exposure Source | Number | RR (95% CI) | I2 (95% UI) | p Value | Model |
|---|---|---|---|---|---|
| Cohort Studies | |||||
| Family | 4 | 1.40 (1.08–1.82) | 0 (0–85) | 0.61 | Fixed |
| Workplace | 2 | 1.54 (0.61–3.91) | 74 (0–94) | 0.05 | Random |
| Family and Workplace | 2 | 1.10 (0.71–1.69) | 55 (0–89) | 0.14 | Fixed |
| Unknown | 4 | 0.99 (0.77–1.29) | 0 (0–79) | 0.79 | Fixed |
| Case-Control Studies | |||||
| Family | 24 | 1.27 (1.05–1.53) | 75 (64–83) | <0.01 | Random |
| Workplace | 13 | 1.36 (1.21–1.53) | 37 (0–67) | 0.09 | Fixed |
| Family and Workplace | 7 | 1.75 (1.43–2.14) | 0 (0–61) | 0.05 | Random |
| Unknown | 23 | 1.43 (1.32–1.55) | 38 (0–71) | 0.86 | Fixed |
| Exposure | Study | Exposure Categories | RR (95% CI) | p Trend |
|---|---|---|---|---|
| Pack-year 1 | Kurahashi | <30 | 1.05 (0.55–2.02) | 0.03 |
| ≥30 | 1.46 (0.85–2.50) | |||
| Duration(year) | Wang | <20 | 1.11 (0.74–1.65) | 0.24 |
| 20–30 | 1.11 (0.63–1.96) | |||
| ≥30 | 1.61 (1.00–2.58) | |||
| Jee | 1–29 | 1.60 (0.80–3.00) | <0.01 | |
| ≥30 | 3.10 (1.40–6.60) | |||
| Cigarettes/day | Kurahashi | <20 | 1.02 (0.51–2.04) | 0.02 |
| ≥20 | 1.47 (0.87–2.49) | |||
| Jee | 1–19 | 2.00 (1.10–3.90) | <0.10 | |
| ≥20 | 1.50 (0.70–3.30) |
| Exposures | Number | OR (95% CI) | I2 (95% UI) | p Value | Model |
|---|---|---|---|---|---|
| Pack-Year | |||||
| <20 | 4 | 0.93 (0.77–1.13) | 25 (0–71) | 0.26 | Fixed |
| ≥20 | 3 | 1.74 (1.04–2.90) | 74 (11–92) | 0.02 | Random |
| Years of Exposure | |||||
| <20 | 7 | 1.71 (1.01–2.90) | 79 (58–90) | <0.01 | Random |
| ≥20 | 6 | 1.57 (1.05–2.35) | 70 (31–87) | <0.01 | Random |
| Cigarettes/Day | |||||
| <10 | 4 | 1.23 (0.90–1.69) | 61 (0–87) | 0.05 | Random |
| ≥10 | 4 | 1.53 (0.69–3.40) | 88 (72–95) | <0.01 | Random |
| ID | Author | Number of Studies | Sex | Pooled OR or RR (95% CI) | Exposure Source |
|---|---|---|---|---|---|
| 1 | Boffetta et al. [139] | 45 | F | 1.25 (1.14–1.38) | Spouse |
| 15 | F | 1.17 (1.02–1.33) | Work | ||
| 2 | Taylor et al. [22] | 43 | F | 1.29 (1.17–1.43) | Spouse |
| 3 | Lee et al. [15] | 93 | F | 1.22 (1.14–1.31) | Spouse |
| 47 | F and M | 1.22 (1.15–1.30) | Work | ||
| 41 | F and M | 1.15 (1.02–1.29) | Childhood | ||
| 4 | Zhong et al. [23] | 40 | F | 1.20 (1.12–1.29) | Spouse |
| 14 | F | 1.15 (1.04–1.28) | Work | ||
| 18 | F | 0.89 (0.81–0.98) | Childhood | ||
| 5 | Hackshaw et al. [140] | 37 | F | 1.24 (1.13–1.36) | Spouse |
| 6 | Taylor et al. [25] | 55 | F | 1.27 (1.17–1.37) | Spouse |
| 7 | Gross [141] | 31 | F | 1.18 (1.06–1.28) | Spouse |
| 8 | Wang [21] | 6 | F | 0.91 (0.75–1.10) | Spouse |
| 9 | Tweedie et al. [142] | 36 | F | 1.22 (1.08–1.37) | Spouse |
| 9 | F | 1.10 (0.90–1.32) | Work | ||
| 10 | U.S. National Research Council [143] | 13 | F | 1.32 (1.16–1.52) | Spouse |
| 11 | Blot et al. [144] | 12 | F | 1.30 (1.10–1.50) | Spouse |
| 12 | Wells [145] | 17 | F | 1.44 (1.26–1.66) | Spouse |
| 13 | Lee [146] | 28 | F | 1.18 (1.07–1.30) | Spouse |
| 14 | U.S. Environmental Protective Agency [147] | 11 | F | 1.19 (1.01–1.39) | Spouse |
| 15 | Pershagen [148] | 25 | F | 1.23 (1.11–1.36) | Spouse |
| 16 | Mengersen et al. [149] | 34 | F | 1.23 (1.08–1.41) | Spouse |
| 17 | Dockery [150] | 33 | F | 1.27 (1.18–1.38) | Spouse |
| 18 | Zhao et al. [151] | 4 | F and M | 1.18 (0.80–1.74) | Spouse |
| 5 | F and M | 1.41 (1.19–1.66) | Work | ||
| 3 | F and M | 1.04 (0.86–1.27) | Childhood | ||
| 19 | Wald et al. [152] | 13 | F and M | 1.35 (1.20–1.53) | Spouse |
| 20 | Saracci et al. [153] | 14 | F and M | 1.35 (1.20–1.53) | Spouse |
| 21 | Law et al. [154] | 34 | F and M | 1.24 (1.11–1.38) | Spouse |
| 22 | Merletti et al. [18] | 39 | F and M | 1.24 (1.15–1.34) | Spouse |
| 23 | Tweedie et al. [155] | 26 | F | 1.17 (1.06–1.28) | Spouse |
| 24 | Li et al. [156] | 5 | F and M | 1.15 (1.00–1.33) | Spouse |
| 2 | F and M | 1.21 (1.09–1.34) | Childhood | ||
| 25 | Yu et al. [157] | 8 | F | 1.47 (1.28–1.69) | Work |
| 8 | F | 0.99 (0.85–1.15) | Childhood | ||
| 26 | Stayner et al. [19] | 22 | F and M | 1.24 (1.18–1.29) | Work |
| 27 | Fu et al. [158] | 12 | F and M | 1.38 (1.13–1.69) | Work |
| 5 | F and M | 1.37 (0.98–1.91) | Childhood | ||
| 28 | Wells [16] | 5 | F and M | 1.39 (1.15–1.68) | Work |
| 29 | Brown et al. [20] | 14 | F | 1.25 (1.08–1.41) | Work |
| 30 | Levois et al. [159] | 12 | F and M | 1.01 (0.92–1.11) | Work |
| 31 | Boffetta et al. [17] | 10 | F and M | 0.91 (0.80–1.05) | Childhood |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Xu, N.; Wang, Q. Meta-Analysis and Systematic Review in Environmental Tobacco Smoke Risk of Female Lung Cancer by Research Type. Int. J. Environ. Res. Public Health 2018, 15, 1348. https://doi.org/10.3390/ijerph15071348
Ni X, Xu N, Wang Q. Meta-Analysis and Systematic Review in Environmental Tobacco Smoke Risk of Female Lung Cancer by Research Type. International Journal of Environmental Research and Public Health. 2018; 15(7):1348. https://doi.org/10.3390/ijerph15071348
Chicago/Turabian StyleNi, Xue, Ning Xu, and Qiang Wang. 2018. "Meta-Analysis and Systematic Review in Environmental Tobacco Smoke Risk of Female Lung Cancer by Research Type" International Journal of Environmental Research and Public Health 15, no. 7: 1348. https://doi.org/10.3390/ijerph15071348
APA StyleNi, X., Xu, N., & Wang, Q. (2018). Meta-Analysis and Systematic Review in Environmental Tobacco Smoke Risk of Female Lung Cancer by Research Type. International Journal of Environmental Research and Public Health, 15(7), 1348. https://doi.org/10.3390/ijerph15071348




