Tetanus Vaccination and Extra-Immunization among Adult Populations: Eight-Year Follow Up Cohort Study of 771,443 Adults in Taiwan, 2006–2013
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Ethical Concerns
2.2. Identification of Study Cohort
2.3. Ascertaining Immunity Status Against Tetanus
2.4. Estimation of Vaccination Coverage (VC) and Extra-Immunization
2.5. Characteristics of Visits for Tetanus Boosters
2.6. Statistical Analysis
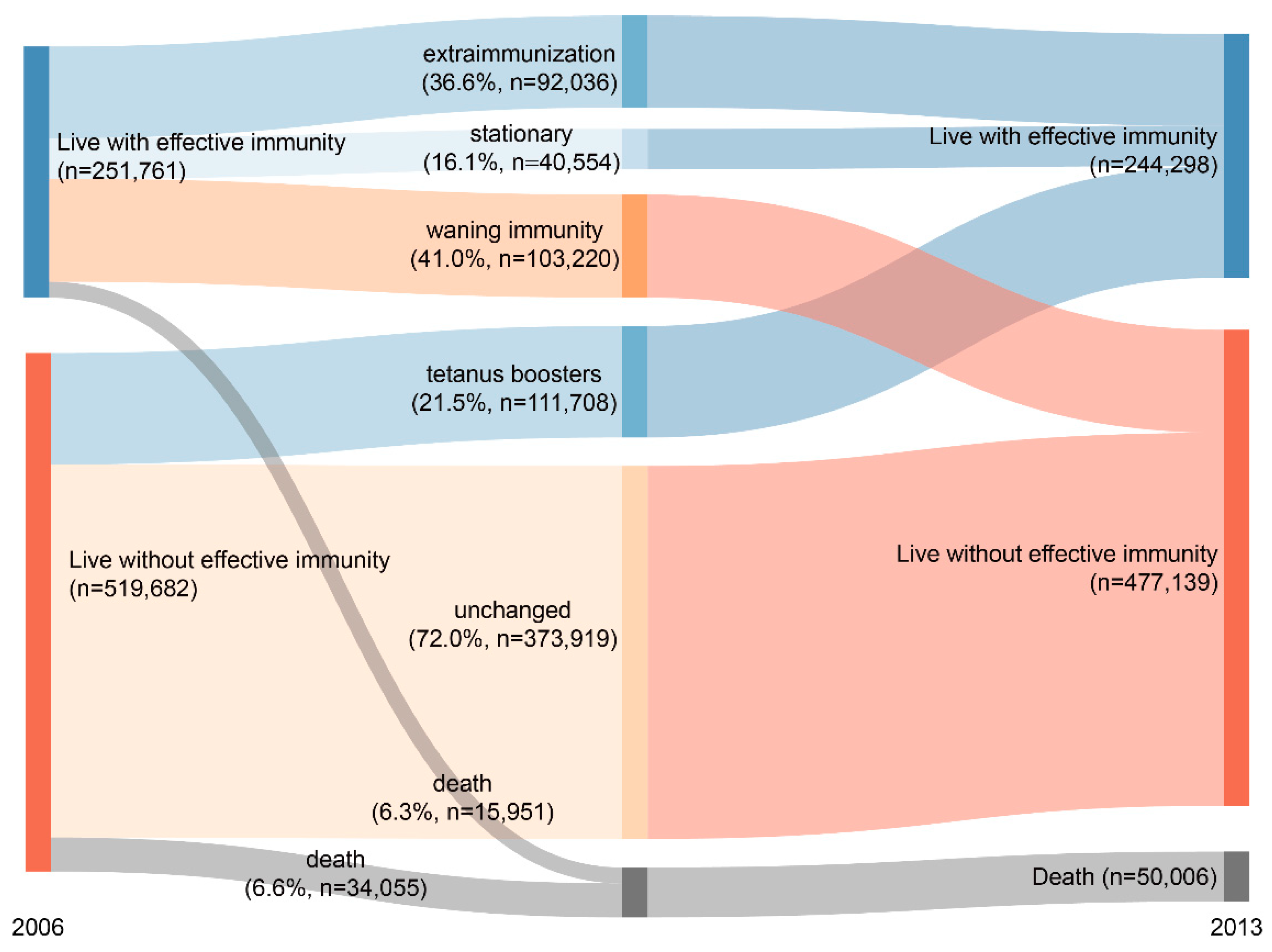
3. Results
3.1. Annual Administration Rates, Extra-Immunization Rates, and Vaccination Coverage
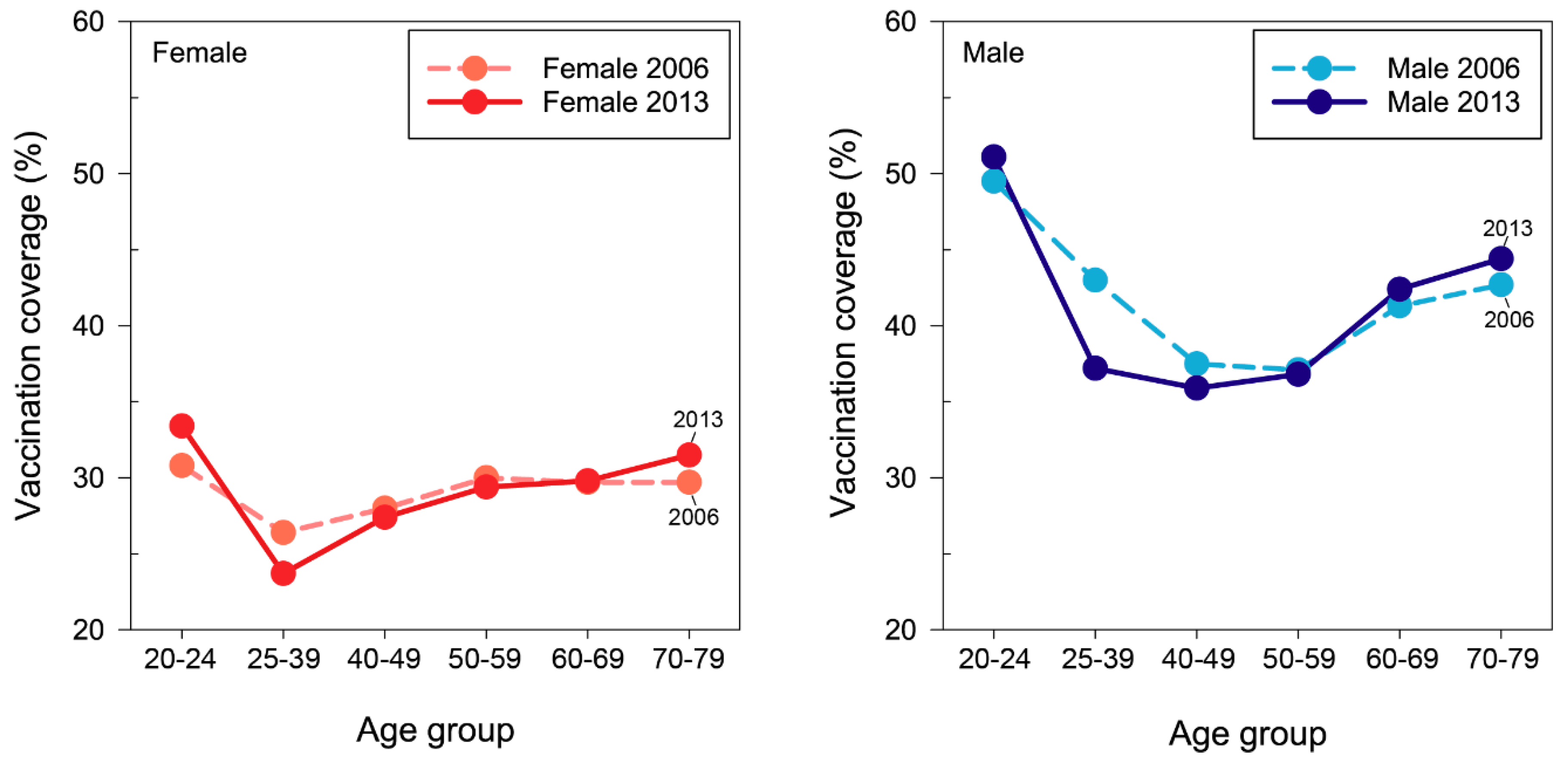
3.2. Change in Age-Specific Vaccination Coverage
3.3. Risk Factors for Extra-Immunization Boosters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bridges, C.B.; Hurley, L.P.; Williams, W.W.; Ramakrishnan, A.; Dean, A.K.; Groom, A.V. Meeting the challenges of immunizing adults. Am. J. Prev. Med. 2015, 49, S455–S464. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.; Rowe, T. Vaccinations in older adults. Clin. Geriatr. Med. 2018, 34, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Mumford, J.E.; Stanaway, J.D.; Barber, R.M.; Hancock, J.R.; Vos, T.; Murray, C.J.L.; Naghavi, M. Mortality from tetanus between 1990 and 2015: Findings from the global burden of disease study 2015. BMC Public Health 2017, 17, 179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, J.H. Tetanus-diphtheria-acellular pertussis vaccination for adults: An update. Clin. Exp. Vaccine Res. 2017, 6, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Akmatov, M.K.; Rübsamen, N.; Deyneko, I.V.; Karch, A.; Mikolajczyk, R.T. Poor knowledge of vaccination recommendations and negative attitudes towards vaccinations are independently associated with poor vaccination uptake among adults—Findings of a population-based panel study in Lower Saxony, Germany. Vaccine 2018, 36, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Halperin, B.A.; MacDougall, D.; MacKinnon-Cameron, D.; Li, L.; McNeil, S.A.; Langley, J.M.; Halperin, S.A. Universal tetanus, diphtheria, acellular pertussis (Tdap) vaccination of adults: What the Canadian public knows and wants to know. Vaccine 2015, 33, 6840–6848. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L.; Kretsinger, K.; Euler, G.L.; Lu, P.J.; Ahmed, F. Barriers to early uptake of tetanus, diphtheria and acellular pertussis vaccine (Tdap) among adults—United States, 2005–2007. Vaccine 2011, 29, 3850–3856. [Google Scholar] [CrossRef] [PubMed]
- Ricco, M.; Cattani, S.; Veronesi, L.; Colucci, M.E. Knowledge, attitudes, beliefs and practices of construction workers towards tetanus vaccine in Northern Italy. Ind. Health 2016, 54, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.; Murray, E.; Zipprich, J.; Winter, K.; Harriman, K. Missed opportunities for tetanus postexposure Prophylaxis—California, January 2008–March 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 243–246. [Google Scholar] [PubMed]
- Rodgers, L.; Shaw, L.; Strikas, R.; Hibbs, B.; Wolicki, J.; Cardemil, C.V.; Weinbaum, C. Frequency and cost of vaccinations administered outside minimum and maximum recommended ages—2014 data from 6 sentinel sites of immunization information systems. J. Pediatr. 2018, 193, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Darden, P.M.; Gustafson, K.K.; Nietert, P.J.; Jacobson, R.M. Extra-immunization as a clinical indicator for fragmentation of care. Public Health Rep. 2011, 126, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Feikema, S.M.; Klevens, R.M.; Washington, M.L.; Barker, L. Extraimmunization among US children. JAMA 2000, 283, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Cavenaile, J.C.; Gerard, P.; Duchateau, J. Evaluation of a rapid immunochromatographic test as an aid to tetanus prophylaxis in the Emergency Department. Immuno-Anal. Biol. Spec. 2012, 27, 185–190. [Google Scholar]
- Miller, T.; Olivieri, P.; Singer, E. The utility of Tdap in the Emergency Department. Am. J. Emerg. Med. 2017, 35, 1348–1349. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.W. Are current UK tetanus prophylaxis procedures for wound management optimal? Emerg. Med. J. 2009, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.S.; Groleau, G. Tetanus in the emergency department: A current review. J. Emerg. Med. 2001, 20, 357–365. [Google Scholar] [CrossRef]
- Simsek, G.; Armagan, E.; Koksal, O.; Heper, Y.; Pozam, S.E.; Durak, V.A. Analysis of appropriate tetanus prophylaxis in an Emergency Department. Ulus Travma Acil Cerrahi Derg. 2013, 19, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, A.S.; Agusti, M.L.M.; Herrero, J.C.; Vacunes Germiap, G. Tetanus prophylaxis in an emergency medical service: A quality improvement proposal. Med. Clin. 2007, 128, 515–516. [Google Scholar]
- Chen, Y.C.; Yeh, H.Y.; Wu, J.C.; Haschler, I.; Chen, T.J.; Wetter, T. Taiwan’s National Health Insurance Research Database: Administrative health care database as study object in bibliometrics. Scientometrics 2011, 86, 365–380. [Google Scholar] [CrossRef]
- Grasse, M.; Meryk, A.; Schirmer, M.; Grubeck-Loebenstein, B.; Weinberger, B. Booster vaccination against tetanus and diphtheria: Insufficient protection against diphtheria in young and elderly adults. Immun. Ageing 2016, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.W.; Lu, P.J.; O’Halloran, A.; Kim, D.K.; Grohskopf, L.A.; Pilishvili, T.; Skoff, T.H.; Nelson, N.P.; Harpaz, R.; Markowitz, L.E.; et al. Surveillance of vaccination coverage among adult populations—United States, 2015. Mmwr Surveill. Summ. 2017, 66, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Southern, D.A.; Quan, H.; Ghali, W.A. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med. Care 2004, 42, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, M.S.; Natarajan, K.; Ramakrishnan, R.; Holleran, S.; Forney, K.; Aponte, A.; Vawdrey, D.K. Immunization data exchange with electronic health records. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed]

| Year | # of Cohort | Tetanus Boosters | Extra-Immunized Tetanus Boosters 2 | Vaccination Coverage (VC, %) 3 | ||
|---|---|---|---|---|---|---|
| # of Cose | Annual Administration Rate (%) 1 | # of Doses | % of Boosters | |||
| 2006 | 771,443 | 41,913 | 5.4 | 23,072 | 55.0 | 35.1 |
| 2007 | 763,951 | 40,598 | 5.3 | 22,793 | 56.1 | 35.4 |
| 2008 | 757,278 | 39,014 | 5.2 | 21,624 | 55.4 | 35.3 |
| 2009 | 750,850 | 38,238 | 5.1 | 21,309 | 55.7 | 34.9 |
| 2010 | 745,270 | 37,712 | 5.1 | 21,095 | 55.9 | 34.7 |
| 2011 | 739,592 | 35,971 | 4.9 | 20,021 | 55.7 | 34.4 |
| 2012 | 733,353 | 35,365 | 4.8 | 19,681 | 55.7 | 34.1 |
| 2013 | 727,048 | 34,669 | 4.8 | 18,818 | 54.3 | 33.9 |
| Total | 303,480 | 168,413 | ||||
| Age | VC at the Beginning (1st Year, 2006) of Follow Up (%) | VC at the End (8th Year, 2013) of Follow Up (%) | % Change of VC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | Total | Female | Male | Total | |
| 20–24 | 30.8 | 49.5 | 39.6 | 33.4 | 51.1 | 41.7 | 8.6 | 3.2 | 5.4 |
| 25–39 | 26.4 | 43.0 | 34.5 | 23.7 | 37.2 | 30.2 | −10.4 | −13.6 | −12.4 |
| 40–49 | 28.0 | 37.5 | 32.8 | 27.4 | 35.9 | 31.6 | −2.1 | −4.5 | −3.6 |
| 50–59 | 30.0 | 37.1 | 33.5 | 29.4 | 36.8 | 33.0 | −2.0 | −1.0 | −1.7 |
| 60–69 | 29.7 | 41.3 | 35.2 | 29.8 | 42.4 | 35.5 | 0.3 | 2.7 | 0.9 |
| 70–79 | 29.7 | 42.7 | 36.4 | 31.5 | 44.4 | 37.7 | 6.3 | 4.0 | 3.6 |
| All ages | 28.5 | 42.1 | 35.1 | 28.0 | 40.2 | 33.9 | −1.8 | −4.5 | −3.6 |
| Visits for Tetanus Boosters n = 303,480 # of Visits, (% of Visits) | Adjusted Odds Ratio (AOR) for Extra-Immunization Boosters AOR, (95% C.I.) | p-Value Sig. 1 | |||
|---|---|---|---|---|---|
| Individual characteristics | |||||
| Gender | |||||
| Male | 186,684 | (61.5) | 1.96 | (1.93–1.99) | <0.001 *** |
| Female | 116,796 | (38.5) | (ref) | ||
| Age | |||||
| 20–24 | 71,261 | (23.5) | 1.22 | (1.19–1.25) | <0.001 *** |
| 25–39 | 85,663 | (28.2) | 1.06 | (1.04–1.09) | <0.001 *** |
| 40–49 | 58,002 | (19.1) | (ref) | ||
| 50–59 | 41,911 | (13.8) | 1.01 | (0.99–1.04) | 0.28 |
| 60–69 | 28,062 | (9.2) | 1.06 | (1.03–1.09) | <0.001 *** |
| 70–79 | 18,581 | (6.1) | 1.08 | (1.40–1.12) | <0.001 *** |
| Number of comorbidities | |||||
| 0 | 208,814 | (68.8) | (ref) | ||
| 1 | 50,918 | (16.8) | 1.23 | (1.21–1.26) | <0.001 *** |
| 2 | 26,906 | (8.9) | 1.32 | (1.30–1.38) | <0.001 *** |
| 3 | 11,264 | (3.7) | 1.51 | (1.47–1.60) | <0.001 *** |
| ≥4 | 5578 | (1.8) | 1.72 | (1.63–1.82) | <0.001 *** |
| Visit characteristics | |||||
| Place of visit | |||||
| Emergency room | 177,054 | (58.3) | 1.22 | (1.17–1.26) | <0.001 *** |
| Out-patient department | 126,426 | (41.7) | (ref) | ||
| Severity | |||||
| Immediate resuscitation | 3707 | (1.2) | 1.21 | (1.12–1.30) | <0.001 *** |
| Very urgent | 47,177 | (15.5) | 1.09 | (1.05–1.13) | <0.001 *** |
| Urgent | 110,342 | (36.4) | 1.05 | (1.02–1.09) | 0.004 ** |
| Non-urgent | 142,254 | (46.9) | (ref) | ||
| Hospital accreditation levels | |||||
| Academic medical centers | 33,093 | (10.9) | (ref) | ||
| Metropolitan hospitals | 85,806 | (28.3) | 1.07 | (1.04–1.10) | <0.001 *** |
| Local community hospitals | 98,814 | (32.6) | 1.27 | (1.24–1.31) | <0.001 *** |
| Physician clinics | 85,767 | (28.3) | 1.31 | (1.26–1.35) | <0.001 *** |
| Hospital urbanization levels | |||||
| Most urbanization | 67,889 | (22.4) | (ref) | ||
| More | 106,891 | (35.2) | 1.11 | (1.09–1.13) | <0.001 *** |
| Moderate | 49,643 | (16.4) | 1.18 | (1.15–1.21) | <0.001 *** |
| Less | 58,073 | (19.1) | 1.25 | (1.21–1.28) | <0.001 *** |
| Least urbanization | 20,984 | (6.9) | 1.31 | (1.26–1.35) | <0.001 *** |
| Hospital geolocations | |||||
| Northern area | 131,803 | (43.4) | 1.03 | (1.01–1.05) | 0.004 ** |
| Middle area | 73,905 | (24.4) | 1.28 | (1.26–1.31) | <0.001 *** |
| South area | 89,188 | (29.4) | (ref) | ||
| East area | 8584 | (2.8) | 1.10 | (1.05–1.15) | <0.001 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-W.; Huang, L.-C.; Chung, W.-F.; Wu, J.; Chen, L.-F.; Chen, Y.-C. Tetanus Vaccination and Extra-Immunization among Adult Populations: Eight-Year Follow Up Cohort Study of 771,443 Adults in Taiwan, 2006–2013. Int. J. Environ. Res. Public Health 2018, 15, 1622. https://doi.org/10.3390/ijerph15081622
Liu S-W, Huang L-C, Chung W-F, Wu J, Chen L-F, Chen Y-C. Tetanus Vaccination and Extra-Immunization among Adult Populations: Eight-Year Follow Up Cohort Study of 771,443 Adults in Taiwan, 2006–2013. International Journal of Environmental Research and Public Health. 2018; 15(8):1622. https://doi.org/10.3390/ijerph15081622
Chicago/Turabian StyleLiu, Shih-Wei, Liang-Chung Huang, Wu-Fu Chung, Jauching Wu, Li-Fu Chen, and Yu-Chun Chen. 2018. "Tetanus Vaccination and Extra-Immunization among Adult Populations: Eight-Year Follow Up Cohort Study of 771,443 Adults in Taiwan, 2006–2013" International Journal of Environmental Research and Public Health 15, no. 8: 1622. https://doi.org/10.3390/ijerph15081622
APA StyleLiu, S. -W., Huang, L. -C., Chung, W. -F., Wu, J., Chen, L. -F., & Chen, Y. -C. (2018). Tetanus Vaccination and Extra-Immunization among Adult Populations: Eight-Year Follow Up Cohort Study of 771,443 Adults in Taiwan, 2006–2013. International Journal of Environmental Research and Public Health, 15(8), 1622. https://doi.org/10.3390/ijerph15081622






