Variations in Dissolved Nitrate, Chloride, and Sulfate in Precipitation, Reservoir, and Tap Waters, Columbus, Ohio
Abstract
:1. Introduction
2. Materials and Methods
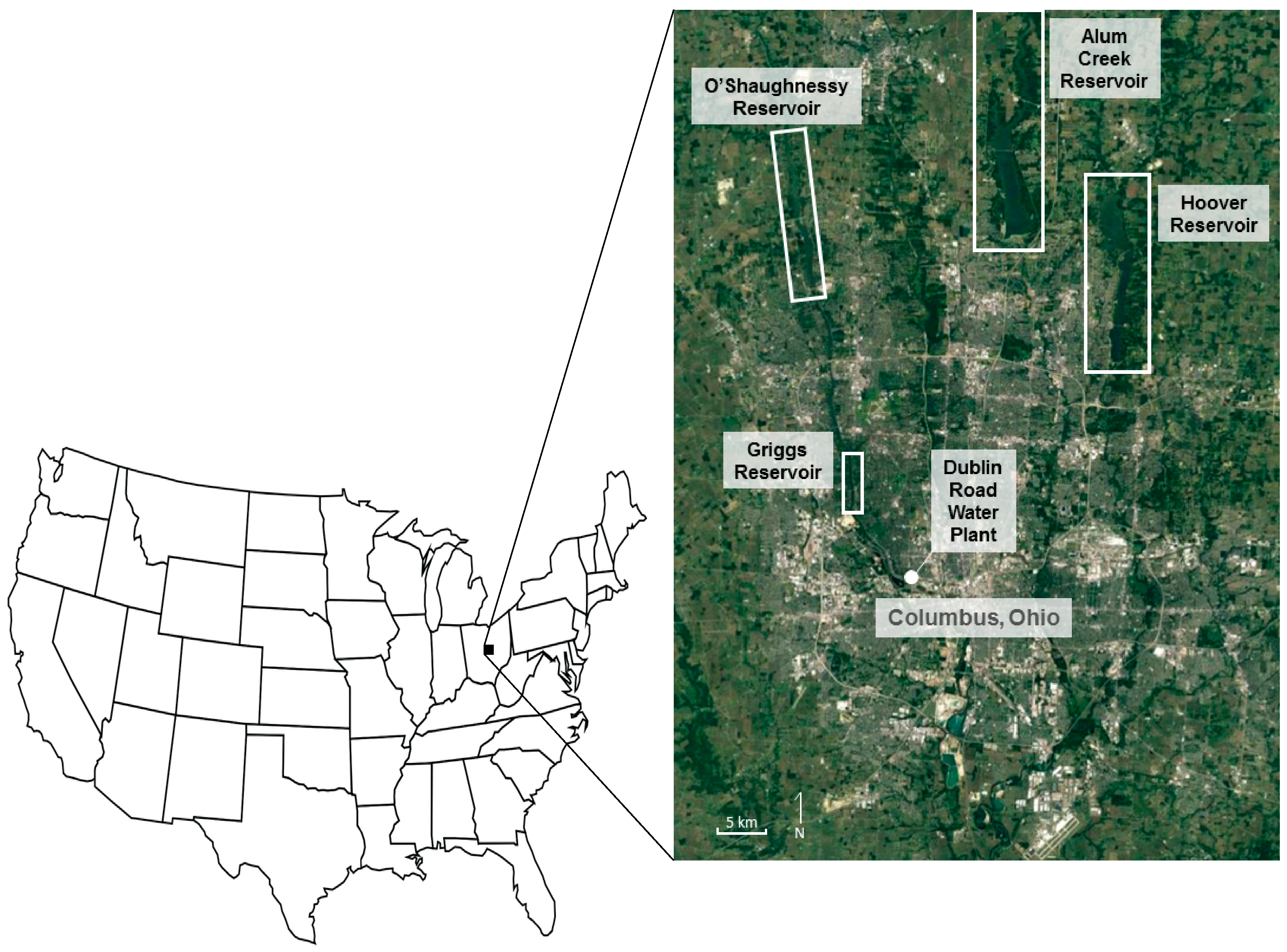
2.1. Columbus, Ohio, Water Supply
2.2. Site Description—Reservoirs and Water Supply Distribution
2.3. Collection, Storage, and Analyses of Water Samples
3. Results and Discussion
3.1. Meteorology
3.2. Precipitation Anion Chemistry
3.3. Reservoir Water Anion Chemistry
3.4. Residential Tap Waters
3.5. Anion Comparison of Precipitation to Reservoir To Tap
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsen, T.A.; Hoffman, S.; Lüthi, C.; Truffer, B.; Maurer, M. Emerging solutions to the water challenges of an urbanizing world. Science 2016, 352, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Gächter, R. Increasing chloride concentrations in Lake Constance: Characterization of sources and estimation of loads. Aquat. Sci. 2012, 74, 101–112. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Groffman, P.M.; Likens, G.E.; Belt, K.T.; Stack, W.P.; Kelly, V.R.; Band, L.E.; Fisher, G.T. Increased salinization of fresh water in the northeastern United States. Proc. Natl. Acad. Sci. USA 2005, 102, 13517–13520. [Google Scholar] [CrossRef] [PubMed]
- Dugan, H.A.; Bartlett, S.L.; Burke, S.M.; Doubek, J.P.; Krivak-Tetley, F.E.; Skaff, N.K.; Summers, J.C.; Farrell, K.J.; McCullough, I.M.; Morales-Williams, A.M.; et al. Salting our freshwater lakes. Proc. Natl. Acad. Sci. USA 2017, 114, 4453–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailey, K.R.; Welch, K.A.; Lyons, W.B. Evaluating the influence on road salt on water quality of Ohio rivers over time. Appl. Geochem. 2017, 47, 25–35. [Google Scholar] [CrossRef]
- Esteban, E.; Rubin, C.H.; McGeehin, M.A.; Flanders, W.D.; Baker, M.J.; Sinks, T.H. Evaluation of infant diarrhea association with elevated levels of sulfate in drinking water: A case control investigation in South Dakota. Int. J. Occup. Environ. Health 1997, 19, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C. Assessing the Acute Gastrointestinal Effects of Ingesting Naturally Occurring High Levels of Sulfate in Drinking Water. In Critical Reviews in Clinical Laboratory Sciences; Taylor & Francis: Milton Park, UK, 2008; Volume 37, pp. 389–400. [Google Scholar]
- Nolan, B.T.; Ruddy, B.C.; Hitt, K.J.; Helsel, D.R. Risk of Nitrate in Groundwaters of the United States—A National Perspective. Environ. Sci. Technol. 1997, 31, 2229–2236. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Drinking Water Regulations and Health Advisories; Office of Water: Washington, DC, USA, 1995.
- National Academy of Sciences (NAS). Board on Environmental Studies and Toxicology. In Nitrate and Nitrite in Drinking Water; National Academy Press: Washington, DC, USA, 1995. [Google Scholar]
- Nolan, B.T.; Malone, R.W.; Gronberg, J.A.; Thorpl, K.R.; Liwang, M. Verifiable Metamodels for Nitrate Losses to drains and groundwater in the Corn Belt, U.S.A. Environ. Sci. Technol. 2012, 46, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bowen, G.J.; Ehleringer, J.R.; Chesson, L.A.; Stange, E.; Cerling, T.E. Stable isotope ratios of tap water in the contiguous United States. Water Resour. Res. 2007, 43, 1–12. [Google Scholar] [CrossRef]
- Leslie, D.L.; Welch, K.A.; Lyons, W.B. Domestic water supply dynamics using stable isotopes δ18O, δD and d-Excess. J. Water Resour. Prot. 2014, 6, 1517–1532. [Google Scholar] [CrossRef]
- United States Census Bureau. QuickFacts for Columbus City, Ohio. United States Census Bureau, 2016. Available online: https://www.census.gov/quickfacts/fact/table/columbuscityohio/PST045216 (accessed on 6 July 2018).
- City of Columbus. Department of Public Utilities 2005 Annual Report; Municipal Civil Service Commission: Columbus, OH, USA, 2005.
- Allen, G. An Analysis of the fate and transport of nutrients in the Upper and Lower Scioto Watersheds of Ohio. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- McIsaac, G.F.; Hu, X. Net N input and riverine export from Illinois agricultural watersheds with and without extensive tile drainage. Biogeochemistry 2004, 70, 251–271. [Google Scholar] [CrossRef]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of agricultural drainage on aquatic ecosystems: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 909–1001. [Google Scholar] [CrossRef]
- Lyons, W.B.; Fitzgibbon, T.O.; Welch, K.A.; Carey, A.E. Mercury geochemistry of the Scioto River, Ohio: Impact of agriculture and urbanization. Appl. Geochem. 2006, 21, 1880–1888. [Google Scholar] [CrossRef]
- House, P.K.; Riker, K.; Brown, L. Water Resources of Franklin County, Ohio State Extension Fact Sheet AEX-480.25; The Ohio State University Extension: Columbus, OH, USA, 1994. [Google Scholar]
- Ohio Agricultural Research and Development Center (OARDC). 1986–2011 Precipitation Records in Franklin County, Ohio. 1986-Present. 2013. Available online: http://www.oardc.ohio-state.edu/newweather/stationinfo.asp?id=14 (accessed on 6 July 2018).
- Welch, K.A.; Lyons, W.B.; Whisner, C.; Gardner, C.B.; Gooseff, M.N.; McKnight, D.M.; Priscu, J.C. Spatial variations in the geochemistry of glacial meltwater streams in the Taylor Valley, Antarctica. Antarct. Sci. 2010, 22, 662–672. [Google Scholar] [CrossRef] [Green Version]
- National Weather Service/National Oceanic and Atmospheric Administration. 2010–2011 Columbus Ohio Meteorological Data. 2013. Available online: http://www.wpc.ncep.noaa.gov/noaa/noaa_archive.php?reset=yes (accessed on 6 July 2018).
- Madhavan, N.; Subramanian, V. Fluoride concentration in river waters of South Asia. Curr. Sci. 2001, 80, 1312–1319. [Google Scholar]
- Neal, C.; Christophersen, N.; Neale, R.; Smith, C.J.; Whitehead, P.G.; Reynolds, B. Chloride in precipitation and streamwater for the upland catchment of the River Severn, Mid-Wales; some consequences for hydrochemical models. Hydrol. Process. 1988, 2, 155–165. [Google Scholar] [CrossRef]
- Camp, M.J. Roadside Geology of Ohio; Mountain Press Publishing Company: Missoula, MT, USA, 2006. [Google Scholar]
- Berner, E.K.; Berner, R.A. Global Environment: Water, Air and Geochemical Cycles, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Gardner, C.B.; Carey, A.E. Trace metal and major ion inputs into the Olentangy River from an urban storm sewer. Environ. Sci. Technol. 2004, 38, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, A.J.; Nawaz, N.R.; Montaseri, M. Climate Change Water Resources planning impacts incorporating reservoir surface net evaporation fluxes: A case study. Water Resour. Dev. 1999, 15, 561–581. [Google Scholar] [CrossRef]
- City of Columbus. Department of Public Utilities. Consumer Confidence Report (CCR); Municipal Civil Service Commission: Columbus, OH, USA, 2011.






| 2010–2011 Precipitation | ||||||
|---|---|---|---|---|---|---|
| n = 119 | Day of Year | Amount | Temp | Cl− | NO3− | SO42− |
| Day of Year | 1 | |||||
| Amount | 1 | |||||
| Temp | 0.18 | 1 | ||||
| Cl− | −0.25 | 1 | ||||
| NO3− | −0.32 | 0.46 | 1 | |||
| SO42− | −0.20 | 0.68 | 0.46 | 1 |
| Griggs Reservoir | |||||
|---|---|---|---|---|---|
| n = 31 | Day of Year | Temp | Cl− | NO3− | SO42− |
| Day of Year | 1 | ||||
| Temp | −0.47 | 1 | |||
| Cl− | 1 | ||||
| NO3− | −0.42 | −0.41 | 1 | ||
| SO42− | 0.93 | 1 |
| O’Shaughnessy Reservoir | |||||
|---|---|---|---|---|---|
| n = 16 | Day of Year | Temp | Cl− | NO3− | SO42− |
| Day of Year | 1 | ||||
| Temp | 1 | ||||
| Cl− | 0.54 | 1 | |||
| NO3− | 1 | ||||
| SO42− | 0.59 | 0.96 | 1 |
| Hoover Reservoir | |||||
|---|---|---|---|---|---|
| n = 17 | Day of Year | Temp | Cl− | NO3− | SO42− |
| Day of Year | 1 | ||||
| Temp | 1 | ||||
| Cl− | 1 | ||||
| NO3− | 1 | ||||
| SO42− | −0.56 | 0.91 | 1 |
| Alum Creek Reservoir | |||||
|---|---|---|---|---|---|
| n = 16 | Day of Year | Temp | Cl− | NO3− | SO42− |
| Day of Year | 1 | ||||
| Temp | 1 | ||||
| Cl− | −0.55 | 1 | |||
| NO3− | 0.81 | 1 | |||
| SO42− | 0.92 | 1 |
| Dublin Road Water Plant Tap Water | ||||||
|---|---|---|---|---|---|---|
| n = 54 | Day of Year | Temp | F− | Cl− | NO3− | SO42− |
| Day of Year | 1 | |||||
| Temp | 1 | |||||
| F− | −0.31 | 1 | ||||
| Cl− | −0.28 | −0.51 | 0.35 | 1 | ||
| NO3− | −0.43 | −0.61 | 0.35 | 0.36 | 1 | |
| SO42− | 0.33 | −0.35 | 0.29 | 0.61 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leslie, D.L.; Lyons, W.B. Variations in Dissolved Nitrate, Chloride, and Sulfate in Precipitation, Reservoir, and Tap Waters, Columbus, Ohio. Int. J. Environ. Res. Public Health 2018, 15, 1752. https://doi.org/10.3390/ijerph15081752
Leslie DL, Lyons WB. Variations in Dissolved Nitrate, Chloride, and Sulfate in Precipitation, Reservoir, and Tap Waters, Columbus, Ohio. International Journal of Environmental Research and Public Health. 2018; 15(8):1752. https://doi.org/10.3390/ijerph15081752
Chicago/Turabian StyleLeslie, Deborah L., and W. Berry Lyons. 2018. "Variations in Dissolved Nitrate, Chloride, and Sulfate in Precipitation, Reservoir, and Tap Waters, Columbus, Ohio" International Journal of Environmental Research and Public Health 15, no. 8: 1752. https://doi.org/10.3390/ijerph15081752
APA StyleLeslie, D. L., & Lyons, W. B. (2018). Variations in Dissolved Nitrate, Chloride, and Sulfate in Precipitation, Reservoir, and Tap Waters, Columbus, Ohio. International Journal of Environmental Research and Public Health, 15(8), 1752. https://doi.org/10.3390/ijerph15081752




