Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Water Sample Processing
2.3. Sediment Sample Processing
2.4. Statistical Analysis
3. Results
3.1. Environmental Variables and Nutrient Concentrations
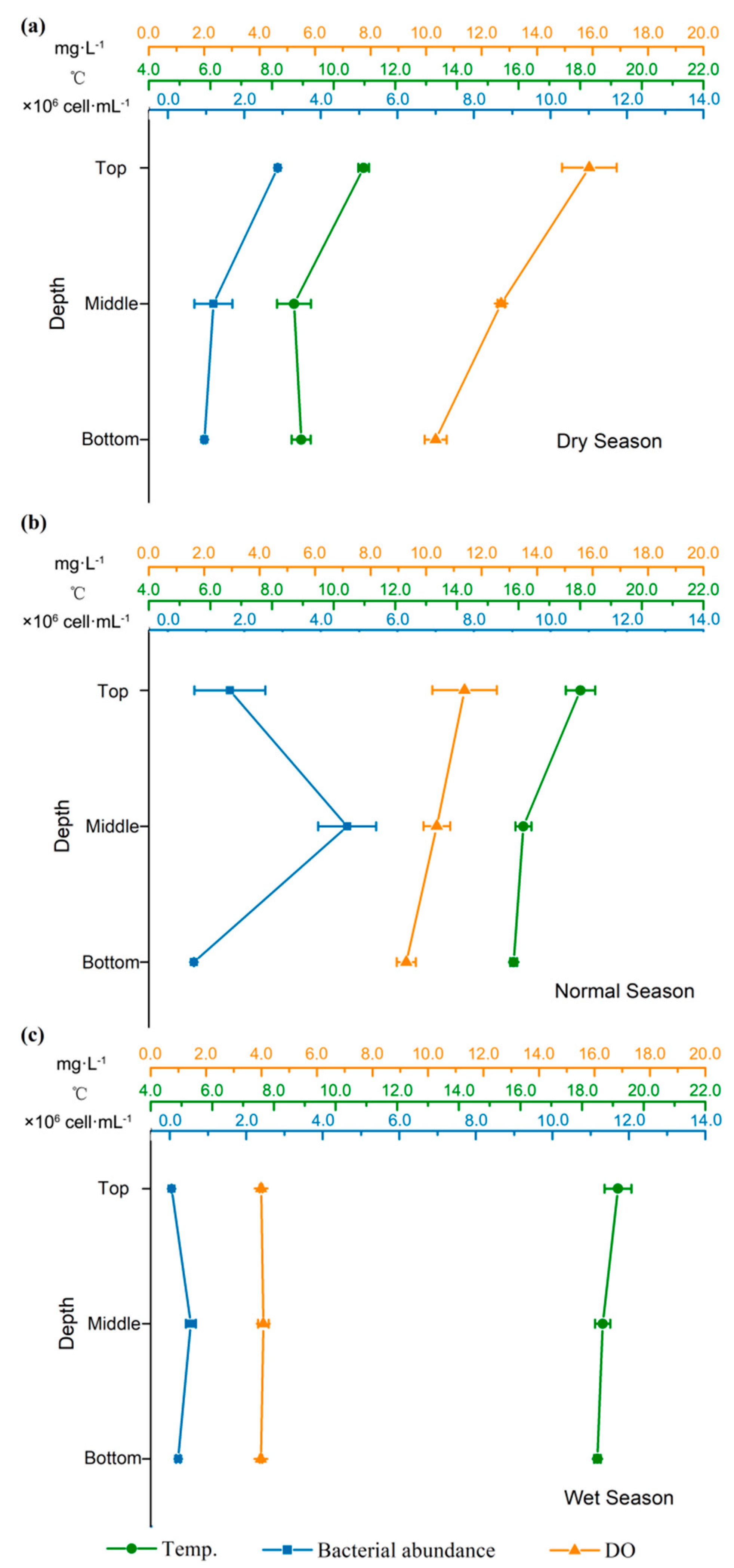
3.1.1. Changes of General Environmental Parameters
3.1.2. Changes of TN Concentrations
3.1.3. Changes of TP Concentrations
3.2. Bacterial Abundance and Algae Biomass
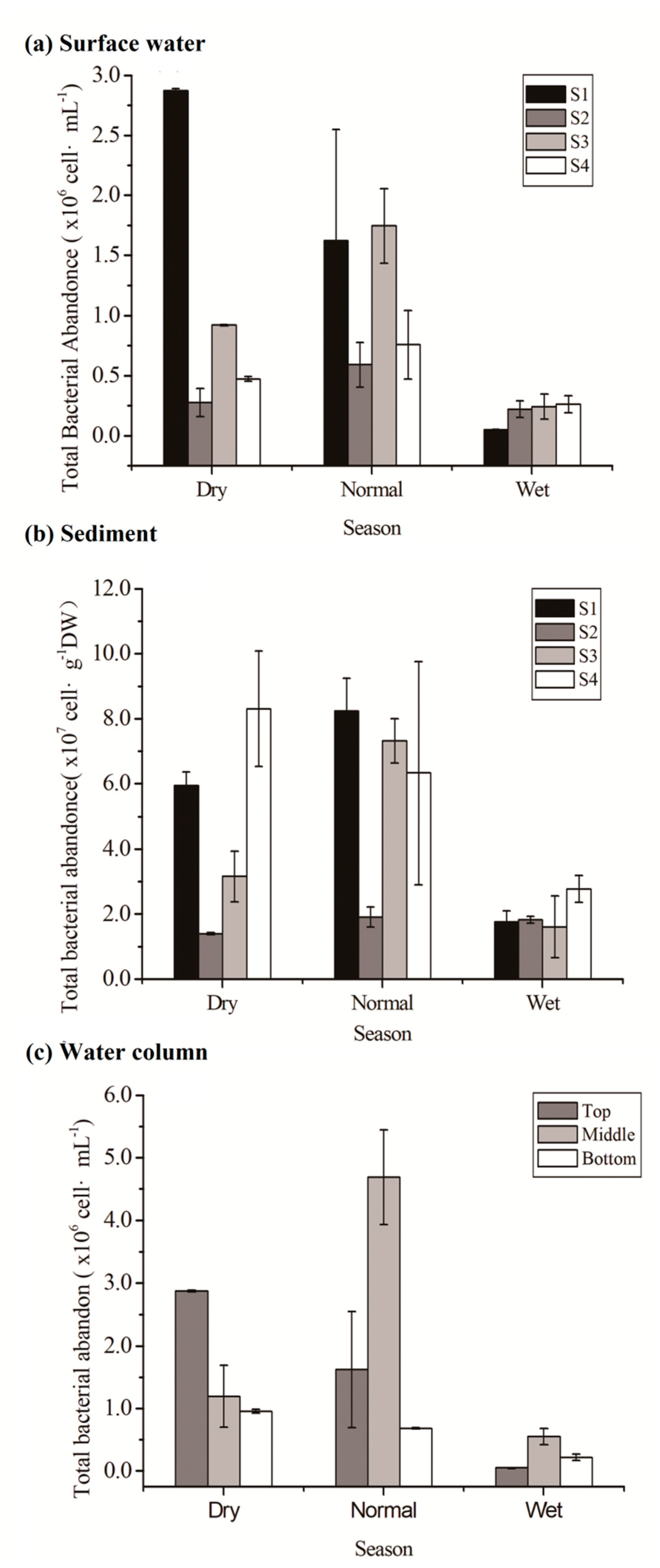
3.2.1. Bacterial Abundances of Water and Sediments
3.2.2. Algae biomass (Chl a)
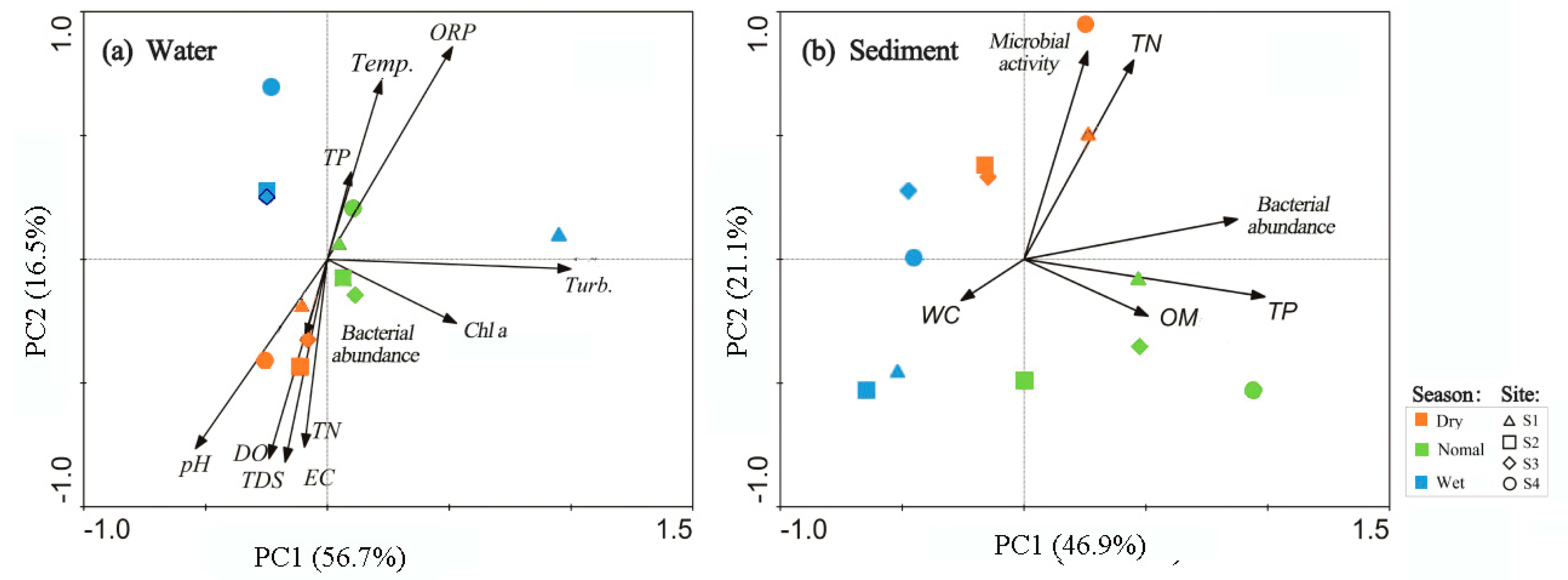
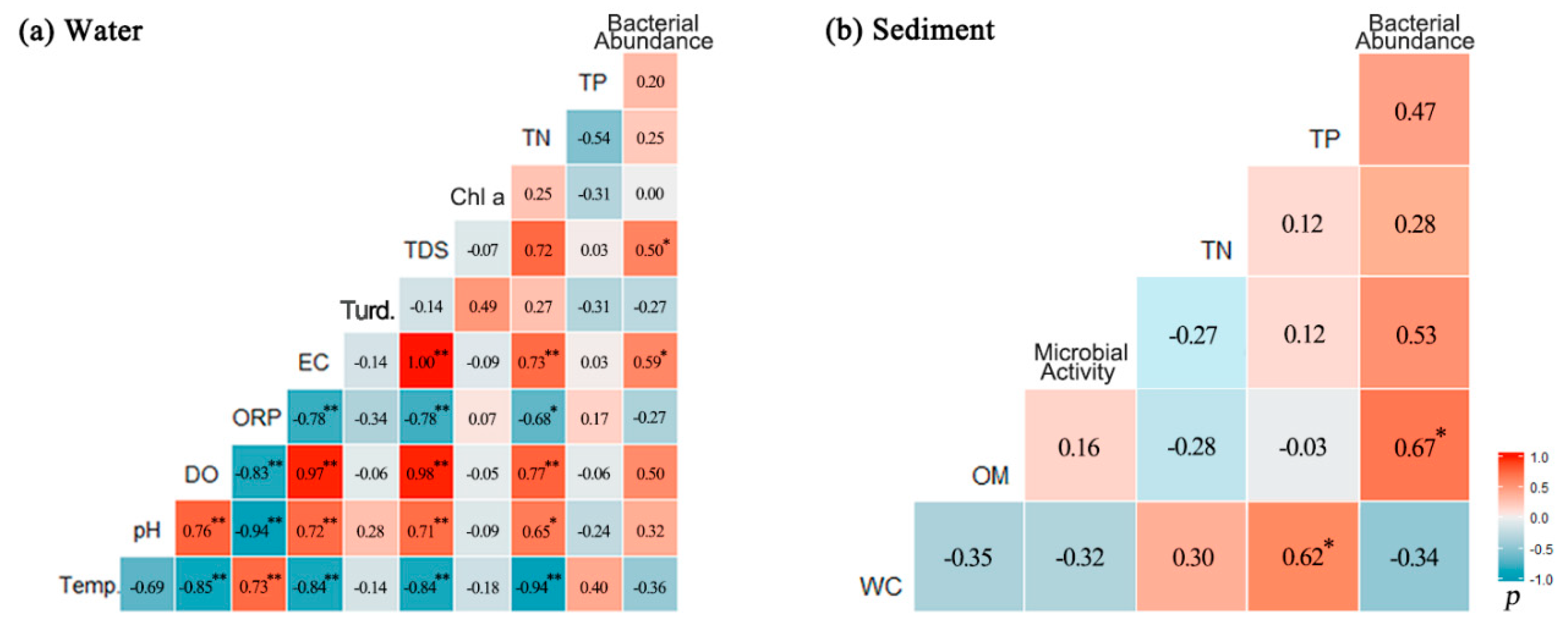
3.3. Relations Between Bacterial Abundance and Environmental Parameters
4. Discussion
5. Conclusions
- (1).
- The presence of dam greatly modified important habitat conditions such as DO, EC, and turbidity in water, as well as WC, TN, and TP in sediment.
- (2).
- Although the retention of P and N in the reservoir led to the nutrient reduction in the downstream of Lancang River, bacterial density continued to grow as anthropogenic sources delivered more nutrients to the downstream river than natural sources.
- (3).
- The effects of hydropower discharge and strong precipitation would be major causes for seasonal variations in bacterial abundance and physicochemical characteristics. Water discharge variations and the enhanced precipitation in the wet season also promoted significant differences in the conditions of the river below the dam, such as the concentration of DO, ORP, EC, turbidity, and TDSs in water and concentrations of microbial activity in sediment.
- (4).
- Bacterial abundance was highest in the reservoir during the dry season and decreased during normal and wet seasons. Physicochemical characteristics of the water, such as TDSs and EC, explained a greater proportion of the variations in bacterial abundance in water. In contrast, bacterial density in sediment was related with hydropower-related discharge, particle size, and type of sediments.
- (5).
- Flow release did influence the stratification in the dammed reservoir so that stratified conditions were not observed in normal and wet seasons. Higher turbidity and a decrease in water residence time during normal and wet seasons resulted in the buildup of bacterial cells in the middle layer of the reservoir, whereas during the dry season, bacterial density in the reservoir was affected by water temperature.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tamaki, H.; Sekiguchi, Y.; Hanada, S.; Nakamura, K.; Nomura, N.; Matsumura, M.; Kamagata, Y. Comparative analysis of bacterial diversity in freshwater sediment of a shallow eutrophic lake by molecular and improved cultivation—Based techniques. Appl. Environ. Microbiol. 2005, 71, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, M.; Marrale, D.; Misic, C. Bacteria and organic matter dynamics during a bioremediation treatment of organic—Rich harbour sediments. Mar. Pollut. Bull. 2003, 46, 1164–1173. [Google Scholar] [CrossRef]
- Schmidt, J.L.; Deming, J.W.; Jumars, P.A.; Keil, R.G. Constancy of bacterial abundance in surficial marine sediments. Limnol. Oceanogr. 1998, 43, 976–982. [Google Scholar] [CrossRef]
- Bird, D.F.; Kalff, J. Empirical relationships between bacterial abundance and chlorophyll concentration in fresh and marine waters. Can. J. Fish Aquat. Sci. 1984, 41, 1015–1023. [Google Scholar] [CrossRef]
- Vrede, K.; Heldal, M.; Norland, S.; Bratbak, G. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl. Environ. Microbiol. 2002, 68, 2965–2971. [Google Scholar] [CrossRef]
- Steger, K.; Premke, K.; Gudasz, C.; Sundh, I.; Tranvik, L.J. Microbial biomass and community composition in boreal lake sediments. Limnol. Oceanogr. 2011, 56, 725–733. [Google Scholar] [CrossRef]
- Shiah, F.K.; Ducklow, H.W. Temperature Regulation of Heterotrophic Bacterioplankton Abundance, Production, and Specific Growth Rate in Chesapeake Bay. Limnol. Oceanogr. 1994, 39, 1243–1258. [Google Scholar]
- Apple, J.K.; del Giorgi, P.A.; Kemp, W.M. Temperature regulation of bacterial production, respiration, and growth efficiency in a temperate salt-marsh estuary. Aquat. Microb. Ecol. 2006, 43, 243–254. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E. Bacterial consumption of DOC during transport through a temperate estuary. Aquat. Microb. Ecol. 2000, 22, 1–12. [Google Scholar] [CrossRef]
- White, P.A.; Kalff, J.; Rasmussen, J.B.; Gasol, J.M. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb. Ecol. 1991, 21, 99–118. [Google Scholar] [CrossRef]
- Sander, B.C.; Kalff, J. Factors controlling bacterial production in marine and freshwater sediments. Microb. Ecol. 1993, 26, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Timmers, P.H.A.; Suarez-Zuluaga, D.A.; van Rossem, M.; Diender, M.; Stams, A.J.M.; Plugge, C.M. Anaerobic oxidation of methane associated with sulfate reduction in a natural freshwater gas source. ISME J. 2016, 10, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Goldhammer, T.; Bruchert, V.; Ferdelman, T.G.; Zabel, M. Microbial sequestration of phosphorus in anoxic upwelling sediments. Nat. Geosci. 2010, 3, 557–561. [Google Scholar] [CrossRef]
- Sinkko, H.; Lukkari, K.; Sihvonen, L.M.; Sivonen, K.; Leivuori, M.; Rantanen, M.; Paulin, L.; Lyra, C. Bacteria contribute to sediment nutrient release and reflect progressed eutrophication-driven hypoxia in an organic-rich continental sea. PLoS ONE 2013, 8, e67061. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.; Bryant, L.D.; Matzinger, A.; Wueest, A. Hypolimnetic oxygen depletion in eutrophic lakes. Environ. Sci. Technol. 2012, 46, 9964–9971. [Google Scholar] [CrossRef] [PubMed]
- Maavara, T.; Parsons, C.T.; Ridenour, C.; Stojanovic, S.; Duerr, H.H.; Powley, H.R.; Van Cappellen, P. Global phosphorus retention by river damming. Proc. Natl. Acad. Sci. USA 2015, 112, 15603–15608. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, A.J.; Franca, M.J.; Juez, C.; De Cesare, G. Reservoir sedimentation. J. Hydraul. Res. 2016, 54, 595–614. [Google Scholar] [CrossRef]
- Helland-Hansen, E.; Holtedahl, T.; Lye, K.A.; Helland-Hansen, E.; Holtedahl, T.; Ka, L. Dictionary Geotechnical Engineering/wörterbuch Geotechnik; Springer Berlin Heidelberg: Berlin, Germany, 1995. [Google Scholar]
- Humborg, C.; Ittekkot, V.; Cociasu, A.; VonBodungen, B. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 1997, 386, 385–388. [Google Scholar] [CrossRef]
- Friedl, G.; Wuest, A. Disrupting biogeochemical cycles-Consequences of damming. Aquatic Sci. 2002, 64, 55–65. [Google Scholar] [CrossRef]
- Sainz-Hernandez, J.C.; Maeda-Martinez, A.N. Sources of Vibrio bacteria in mollusc hatcheries and control methods: a case study. Aquac. Res. 2005, 36, 1611–1618. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Schijven, J.F.; Bonne, P.; Visser, A.; Medema, G.J. Elimination of viruses, bacteria and protozoan oocysts by slow sand filtration. Water Sci. Technol. 2004, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, S.K.; Liu, S.L.; Isange, S.; Li, J.P.; Liu, Q.; Wang, C. Spatial distribution and environmental risk of major elements in surface sediments associated Manwan Dam in Lancang River, China. Eurasian J. Soil Sci. 2015, 4, 22. [Google Scholar] [CrossRef][Green Version]
- Heath, S.K.; Plater, A.J. Records of pan (floodplain wetland) sedimentation as an approach for post-hoc investigation of the hydrological impacts of dam impoundment: The Pongolo river, KwaZulu-Natal. Water Res. 2010, 44, 4226–4240. [Google Scholar] [CrossRef] [PubMed]
- Oyama, Y.; Matsushita, B.; Fukushima, T. Distinguishing surface cyanobacterial blooms and aquatic macrophytes using Landsat/TM and ETM+ shortwave infrared bands. Remote Sens. Environ. 2015, 157, 35–47. [Google Scholar] [CrossRef]
- Luo, X.X.; Yang, S.L.; Zhang, J. The impact of the Three Gorges Dam on the downstream distribution and texture of sediments along the middle and lower Yangtze River (Changjiang) and its estuary, and subsequent sediment dispersal in the East China Sea. Geomorphology 2012, 179, 126–140. [Google Scholar] [CrossRef]
- Francisco, D.E.; Mah, R.A.; Rabin, A.C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans. Am. Microsc. Soc. 1973, 92, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.A.; Colloff, M.J.; Kookana, R.S. Effect of wastewater treatment plant effluent on microbial function and community structure in the sediment of a freshwater stream with variable seasonal flow. Appl. Environ. Microbiol. 2008, 74, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J. Paleolimnol 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, D.L. Joint Determination of Total Nitrogen and Total Phosphor in Sludge Samples. J. Jiangsu Polytech. Univ. 2006, 18, 37. [Google Scholar]
- Ebina, J.; Tsutsui, T.; Shirai, T. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res. 1983, 17, 1721–1726. [Google Scholar] [CrossRef]
- Schnurer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [PubMed]
- Kepner, R.L.; Pratt, J.R. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: Past and present. Microbiol. Rev. 1994, 58, 603–615. [Google Scholar] [PubMed]
- Merovich, G.T.; Stiles, J.M.; Petty, J.T.; Ziemkiewicz, P.F.; Fulton, J.B. Water chemistry-based classification of streams and implications for restoring mined Appalachian watersheds. Environ. Toxicol. Chem. 2007, 26, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Raiber, M.; White, P.A.; Daughney, C.J.; Tschritter, C.; Davidson, P.; Bainbridge, S.E. Three-dimensional geological modelling and multivariate statistical analysis of water chemistry data to analyse and visualise aquifer structure and groundwater composition in the Wairau Plain, Marlborough District, New Zealand. J. Hydrol. 2012, 436-437, 13–34. [Google Scholar] [CrossRef]
- Hair Joseph, F.; Anderson Rolph, E.; Tatham Ronald, L.; Black William, C. Multivariate Data Analysis with Readings; Macmillan Publishing Company: London, UK, 1994. [Google Scholar]
- Remeš, J.; Bílek, L.; Novák, J.; Vacek, Z.; Vacek, S.; Putalová, T.; Koubek, L. Diameter increment of beech in relation to social position of trees, climate characteristics and thinning intensity. J. Forest Sci. 2015, 61, 456–464. [Google Scholar] [CrossRef]
- Kollaus, K.A.; Bonner, T.H. Habitat associations of a semi-arid fish community in a karst spring-fed stream. J. Arid. Environ. 2012, 76, 72–79. [Google Scholar] [CrossRef]
- Peruzzo, P.J.; Porta, A.A.; Ronco, A.E. Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ. Pollut. 2008, 156, 61–66. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Wilby, R.L.; Battarbee, R.W.; Kernan, M.; Wade, A.J. A review of the potential impacts of climate change on surface water quality. Hydrol. Sci. J. 2009, 54, 101–123. [Google Scholar] [CrossRef]
- Gurung, T.B.; Kagami, M.; Yoshida, T.; Urabe, J. Relative importance of biotic and abiotic factors affecting bacterial abundance in Lake Biwa: An empirical analysis. Limnology 2001, 2, 19–28. [Google Scholar] [CrossRef]
- Gurung, T.B.; Urabe, J. Temporal and vertical difference in factors limiting growth rate of heterotrophic bacteria in Lake Biwa. Microb. Ecol. 1999, 38, 136–145. [Google Scholar] [CrossRef]
- Wilkes, G.; Edge, T.; Gannon, V.; Jokinen, C.; Lyautey, E.; Medeiros, D.; Neumann, N.; Ruecker, N.; Topp, E.; Lapen, D.R. Seasonal relationships among indicator bacteria, pathogenic bacteria, Cryptosporidium oocysts, Giardia cysts, and hydrological indices for surface waters within an agricultural landscape. Water Res. 2009, 43, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Tornevi, A.; Bergstedt, O.; Forsberg, B. Precipitation Effects on Microbial Pollution in a River: Lag Structures and Seasonal Effect Modification. PLoS ONE 2014, 9, e98546. [Google Scholar] [CrossRef] [PubMed]
- Gannon, V.P.J.; Duke, G.D.; Thomas, J.E.; VanLeeuwen, J.; Byrne, J.; Johnson, D.; Kienzle, S.W.; Little, J.; Graham, T.; Selinger, B. Use of in-stream reservoirs to reduce bacterial contamination of rural watersheds. Sci. Total Environ. 2005, 348, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, R.C.; Joy, D.M.; Lee, H.; Kostaschuk, R.; Gordon, R.J. Resuspension of sediment-associated Escherichia coli in a natural stream. J. Environ. Qual. 2005, 34, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Buesing, N.; Gessner, M.O. Comparison of detachment procedures for direct counts of bacteria associated with sediment particles, plant litter and epiphytic biofilms. Aquat. Microb. Ecol. 2002, 27, 29–36. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Zhao, H.; Deng, L.; Wang, C.; Zhao, Q.; Dong, S. Longitudinal Variability of Phosphorus Fractions in Sediments of a Canyon Reservoir Due to Cascade Dam Construction: A Case Study in Lancang River, China. PLoS ONE 2013, 8, e83329. [Google Scholar] [CrossRef] [PubMed]
- Juez, C.; Hassan, M.A.; Franca, M.J. The Origin of Fine Sediment Determines the Observations of Suspended Sediment Fluxes Under Unsteady Flow Conditions. Water Resour. Res. 2018, 54, 5654–5669. [Google Scholar] [CrossRef]
- Musslewhite, C.L.; McInerney, M.J.; Dong, H.L.; Onstott, T.C.; Green-Blum, M.; Swift, D.; Macnaughton, S.; White, D.C.; Murray, C.; Chien, Y.J. The factors controlling microbial distribution and activity in the shallow subsurface. Geomicrobiol. J. 2003, 20, 245–261. [Google Scholar] [CrossRef]
- Russo, S.A.; Hunn, J.; Characklis, G.W. Considering Bacteria-Sediment Associations in Microbial Fate and Transport Modeling. J. Environ. Eng. ASCE 2011, 137, 697–706. [Google Scholar] [CrossRef]
- Decamp, O.; Warren, A. Investigation of Escherichia coli removal in various designs of subsurface flow wetlands used for wastewater treatment. Ecol. Eng. 2000, 14, 293–299. [Google Scholar] [CrossRef]
- Davies, C.M.; Bavor, H.J. The fate of stormwater—Associated bacteria in constructed wetland and water pollution control pond systems. J. Appl. Microbiol. 2000, 89, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Garzio-Hadzick, A.; Shelton, D.R.; Hill, R.L.; Pachepsky, Y.A.; Guber, A.K.; Rowland, R. Survival of manure-borne E. coli in streambed sediment: Effects of temperature and sediment properties. Water Res. 2010, 44, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Teodoru, C.; Wehrli, B. Retention of sediments and nutrients in the Iron Gate I Reservoir on the Danube River. Biogeochemistry 2005, 76, 539–565. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Abdoli, L.; Li, H. Mg2+/Ca2+ promotes the adhesion of marine bacteria and algae and enhances following biofilm formation in artificial seawater. Colloids Surf. B Biointerfaces 2016, 146, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.S.; Characklis, G.W.; Noble, R.T. Sediment-water exchange of Vibrio sp. and fecal indicator bacteria: Implications for persistence and transport in the Neuse River Estuary, North Carolina, USA. Water Res. 2008, 42, 941–950. [Google Scholar] [CrossRef] [PubMed]
- He, L.-M.; Lu, J.; Shi, W. Variability of fecal indicator bacteria in flowing and ponded waters in southern California: Implications for bacterial TMDL development and implementation. Water Res. 2007, 41, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Droppo, I.G.; Liss, S.N.; Williams, D.; Nelson, T.; Jaskot, C.; Trapp, B. Dynamic existence of waterborne pathogens within river sediment compartments. Implications for water quality regulatory affairs. Environ. Sci. Technol. 2009, 43, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Maintinguer, S.I.; Sakamoto, I.K.; Adorno, M.A.T.; Varesche, M.B.A. Diversity of anaerobic bacteria in sediments from a subtropical reservoir. Lakes Reserv. Res. Manag. 2016, 21, 351–361. [Google Scholar] [CrossRef]
- Staley, C.; Gould, T.J.; Wang, P.; Phillips, J.; Cotner, J.B.; Sadowsky, M.J. Sediments and Soils Act as Reservoirs for Taxonomic and Functional Bacterial Diversity in the Upper Mississippi River. Microb. Ecol. 2016, 71, 814–824. [Google Scholar] [CrossRef]
- Manini, E.; Luna, G.M.; Danovaro, R. Benthic bacterial response to variable estuarine water inputs. FEMS Microbiol. Ecol. 2004, 50, 185–194. [Google Scholar] [CrossRef][Green Version]
- Quinn, C.J.; North, R.L.; Dillon, P.J. Year-round patterns in bacterial production and biomass in Lake Simcoe, Ontario, Canada: are heterotrophic bacteria a significant contributor to low hypolimnetic oxygen? Inland Waters 2013, 3, 235–252. [Google Scholar] [CrossRef]
- Chung, S.W.; Hipsey, M.R.; Imberger, J. Modelling the propagation of turbid density inflows into a stratified lake: Daecheong Reservoir, Korea. Environ. Model. Software 2009, 24, 1467–1482. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, T.-L.; Ma, W.-X.; Zhou, Z.-Z.; Li, Y. Water—quality responses of the intrusion of high-turbidity runoff to the thermal stratified Jin-pen Reservoir during flood season. China Environ. Sci. 2015, 35, 2778–2786. [Google Scholar]
- Irvine, K.N.; Pettibone, G.W. Resuspension of sediment-associated Escherichia coli in a natural stream. Environ. Technol. 1993, 14, 531–542. [Google Scholar] [CrossRef]

| Site | Temperature (°C) | pH | DO (mg·L−1) | ORP (mV) | EC (μS·cm−1) | Turbidity (NTU) | Chl a (μg·L−1) | TDS (g·L−1) | TN (mg·L−1) | TP (mg·L−1) | Bacterial Abundance (×106 Cell·mL−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry season | |||||||||||
| S1 | 10.9 ± 0.2 | 8.0 ± 0.2 | 15.9 ± 1.3 | 240.7 ± 5.8 | 48.6 ± 2.5 | 84.9 ± 14.4 | 2.2 1 | 0.3 ± 0.0 | 1.6 ± 0.1 | 0.02 ± 0.00 | 2.87 ± 0.01 |
| S2 | 11.6 ± 1.2 | 8.0 ± 0.1 | 16.9 ± 2.9 | 207.0 ± 11.4 | 49.1 ± 1.2 | 85.5 ± 2.2 | 1.3 1 | 0.3 ± 0.0 | 1.5 ± 0.1 | 0.03 ± 0.01 | 0.28 ± 0.12 |
| S3 | 10.2 ± 0.6 | 8.1 ± 0.0 | 14.2 ± 0.5 | 224.7 ± 2.5 | 51.1 ± 0.6 | 99.4 ± 9.5 | 1.4 1 | 0.3 ± 0.0 | 1.7± 0.0 | 0.03 ± 0.00 | 0.92 ± 0.01 |
| S4 | 13.1 ± 0.1 | 8.2 ± 0.2 | 11.4 ± 1.4 | 197.3 ± 11.6 | 41.8 ± 0.2 | 10,300.0 ± 667.9 | 2.9 1 | 0.3 ± 0.0 | 1.5 ± 0.1 | 0.10 ± 0.02 | 0.47 ± 0.02 |
| Normal season | |||||||||||
| S1 | 18.0 ± 0.5 | 7.6 ± 0.0 | 11.4 ± 1.4 | 284.7 ± 10.2 | 40.5 ± 0.2 | 158.7 ± 0.6 | 1.3 ± 0.1 | 0.3 ± 0.0 | 1.5 ± 0.3 | 0.03 ± 0.06 | 1.62 ± 0.93 |
| S2 | 21.0 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.6 | 267.7 ± 27.0 | 41.9 ± 0.1 | 170.3 ± 3.2 | 1.2 ± 0.0 | 0.3 ± 0.0 | 1.3 ± 0.3 | 0.14 ± 0.08 | 0.59 ± 0.19 |
| S3 | 17.9 ± 0.8 | 7.3 ± 0.0 | 8.2 ± 0.4 | 262.3 ± 37.5 | 41.1 ± 0.3 | 197.3 ± 19.3 | 1.5 ± 0.1 | 0.3 ± 0.0 | 1.4 ± 0.1 | 0.16 ± 0.05 | 1.74 ± 0.31 |
| S4 | 17.4 ± 0.6 | 7.2 ± 0.1 | 8.5 ± 0.2 | 309.3 ± 8.1 | 40.8 ± 0.2 | 185.3 ± 1.5 | 1.3 ± 0.0 | 0.3 ± 0.0 | 1.5 ± 0.2 | 0.85 ± 0.13 | 0.76 ± 0.29 |
| Wet season | |||||||||||
| S1 | 19.1 ± 0.5 | 6.5 ± 0.0 | 4.0 ± 0.1 | 360.0 ± 0.0 | 32.1 ± 0.3 | 625.7 ± 149.6 | 3.3 ± 0.3 | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.10 ± 0.02 | 0.05 ± 0.01 |
| S2 | 20.3 ± 2.1 | 7.5 ± 0.1 | 4.1 ± 0.4 | 289.0 ± 6.6 | 32.5 ± 0.4 | 1715.0 ± 17.3 | 1.4 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.10 ± 0.08 | 0.22 ± 0.07 |
| S3 | 20.0 ± 1.9 | 7.7 ± 0.0 | 5.4 ± 0.2 | 286.0 ± 0.0 | 31.8 ± 0.8 | 1826.7 ± 86.7 | 1.2 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.2 | 0.06 ± 0.01 | 0.24 ± 0.01 |
| S4 | 19.2 ± 0.6 | 6.4 ± 0.0 | 3.3 ± 0.1 | 347.3 ± 1.5 | 31.1 ± 0.3 | 1815.6 ± 10.2 | 1.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.24 ± 0.01 | 0.26 ± 0.07 |
| Site | WC (%) | OM (%) | TN (mg·g−1 DW) | TP (mg·g−1 DW) | Microbial Activity (µg FDA g−1 DW h−1) | Bacterial Abundance (×107 cell·g−1 DW) |
|---|---|---|---|---|---|---|
| Dry season | ||||||
| S1 | 50.8 ± 4.0 | 2.7 ± 3.5 | 5.4 ± 0.3 | 4.3 ± 1.2 | 2.5 ± 0.0 | 5.94 ± 0.42 |
| S2 | 79.4 ± 4.6 | 0.8 ± 0.2 | 4.8 ± 0.2 | 2.5 ± 0.1 | 2.0 ± 0.1 | 1.40 ± 0.04 |
| S3 | 69.0 ± 3.6 | 1.4 ± 0.3 | 5.0 ± 0.2 | 2.5 ± 0.1 | 1.6 ± 0.3 | 3.16 ± 0.78 |
| S4 | 65.8 ± 0.4 | 3.6 ± 2.0 | 5.7 ± 0.1 | 3.9 ± 0.1 | 3.8 ± 0.9 | 8.30 ± 1.780 |
| Normal season | ||||||
| S1 | 55.2 ± 8.7 | 13.0 ± 2.1 | 4.3 ± 0.1 | 5.77 ± 0.6 | 1.7 ± 0.2 | 8.24 ± 1.00 |
| S2 | 79.2 ± 6.8 | 2.6 ± 0.5 | 3.5 ± 0.1 | 3.8 ± 0.3 | 0.3 ± 0.0 | 1.91 ± 0.31 |
| S3 | 64.9 ± 5.2 | 4.9 ± 0.6 | 4.2 ± 0.2 | 5.9 ± 0.3 | 0.8 ± 0.3 | 7.31 ± 0.68 |
| S4 | 68.0 ± 3.8 | 2.6 ± 0.0 | 4.5 ± 0.4 | 8.2 ± 0.0 | 0.6 ± 0.1 | 6.33 ± 3.43 |
| Wet season | ||||||
| S1 | 56.1 ± 4.0 | 2.2 ± 0.2 | 2.4 ± 3.0 | 0.9 ± 0.0 | 0.6 ± 0.1 | 1.76 ± 0.34 |
| S2 | 79.8 ± 0.9 | 1.2 ± 0.1 | 1.7 ± 1.4 | 0.5 ± 0.0 | 0.8 ± 0.3 | 1.82 ± 0.1 |
| S3 | 76.8 ± 0.1 | 0.8 ± 0.0 | 5.2 ± 0.0 | 0.9± 0.2 | 0.6 ± 0.2 | 1.61 ± 0.95 |
| S4 | 66.9 ± 0.2 | 1.5 ± 0.4 | 4.1 ± 0.5 | 1.2 ± 0.2 | 0.6 ± 0.2 | 2.77 ± 0.41 |
| Water | Sediment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site (S) | Sampling Season (Ss) | S × Ss | Site (S) | Sampling Season (Ss) | S × Ss | ||||||||
| F | p | F | p | F | p | F | p | F | p | F | p | ||
| Temperature (°C) | 5.55 | 0.007 NS | 10.25 | <0.01 | 4.36 | 0.043 | WC (%) | 96.03 | <0.01 | 12.12 | <0.01 | 2.38 | 0.110 NS |
| pH | 2.66 | 0.154 NS | 137.81 | <0.01 | 110.38 | 0.011 | OM (%) | 68.92 | <0.01 | 59.24 | <0.01 | 29.99 | <0.01 |
| DO (mg·L−1) | 20.59 | <0.01 | 96.55 | <0.01 | 16.56 | <0.01 | TN (mg·g−1 DW) | 12.91 | <0.01 | 7.74 | 0.022 | 8.43 | 0.002 |
| ORP (mV) | 15.36 | <0.01 | 178.22 | <0.01 | 7.06 | <0.01 | TP (mg·g−1 DW) | 36.73 | <0.01 | 477.05 | <0.01 | 14.44 | <0.01 |
| EC (μS·cm−1) | 39.67 | <0.01 | 550.78 | <0.01 | 24.67 | <0.01 | Microbial activity (µg FDA·g−1 DW·h−1) | 68.05 | <0.01 | 168.52 | <0.01 | 35.62 | <0.01 |
| Turbidity (NTU) | 675.66 | <0.01 | 494.93 | <0.01 | 657.13 | <0.01 | Bacterial abundance (×107 cell·g−1 DW) | 26.12 | <0.01 | 44.64 | <0.01 | 7.99 | <0.01 |
| Chl a (μg·L−1) | 233.17 | <0.01 | 170.16 | <0.01 | 196.04 | <0.01 | |||||||
| TDS (g·L−1) | 129.27 | <0.01 | 1962.18 | <0.01 | 83.94 | <0.01 | |||||||
| TN (mg·L−1) | 1.60 | <0.01 | 307.07 | <0.01 | 0.57 | <0.01 | |||||||
| TP (mg·L−1) | 84.01 | <0.01 | 85.88 | <0.01 | 28.84 | <0.01 | |||||||
| Bacterial abundance (×106 cell·mL−1) | 21.55 | <0.01 | 90.72 | <0.01 | 14.07 | <0.01 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Xiang, X.; Huang, G.; Song, X.; Wang, P.; Fu, K. Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China. Int. J. Environ. Res. Public Health 2019, 16, 2031. https://doi.org/10.3390/ijerph16112031
Luo X, Xiang X, Huang G, Song X, Wang P, Fu K. Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China. International Journal of Environmental Research and Public Health. 2019; 16(11):2031. https://doi.org/10.3390/ijerph16112031
Chicago/Turabian StyleLuo, Xia, Xinyi Xiang, Guoyi Huang, Xiaorui Song, Peijia Wang, and Kaidao Fu. 2019. "Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China" International Journal of Environmental Research and Public Health 16, no. 11: 2031. https://doi.org/10.3390/ijerph16112031
APA StyleLuo, X., Xiang, X., Huang, G., Song, X., Wang, P., & Fu, K. (2019). Bacterial Abundance and Physicochemical Characteristics of Water and Sediment Associated with Hydroelectric Dam on the Lancang River China. International Journal of Environmental Research and Public Health, 16(11), 2031. https://doi.org/10.3390/ijerph16112031





