Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Growth Conditions of the Fungal Strains in PDA with Differing Concentrations of NaCl
2.3. Dual Culture Antagonism Assays
2.4. Evaluation of Growth Promotion Effects of Trichoderma Isolates on Melon Seedlings under Salinity Stress
2.5. Effects of Trichoderma Strains on the Severity of Pythium Ultimum in Melon Seedlings under Saline Conditions
2.6. Statistical Analysis
3. Results
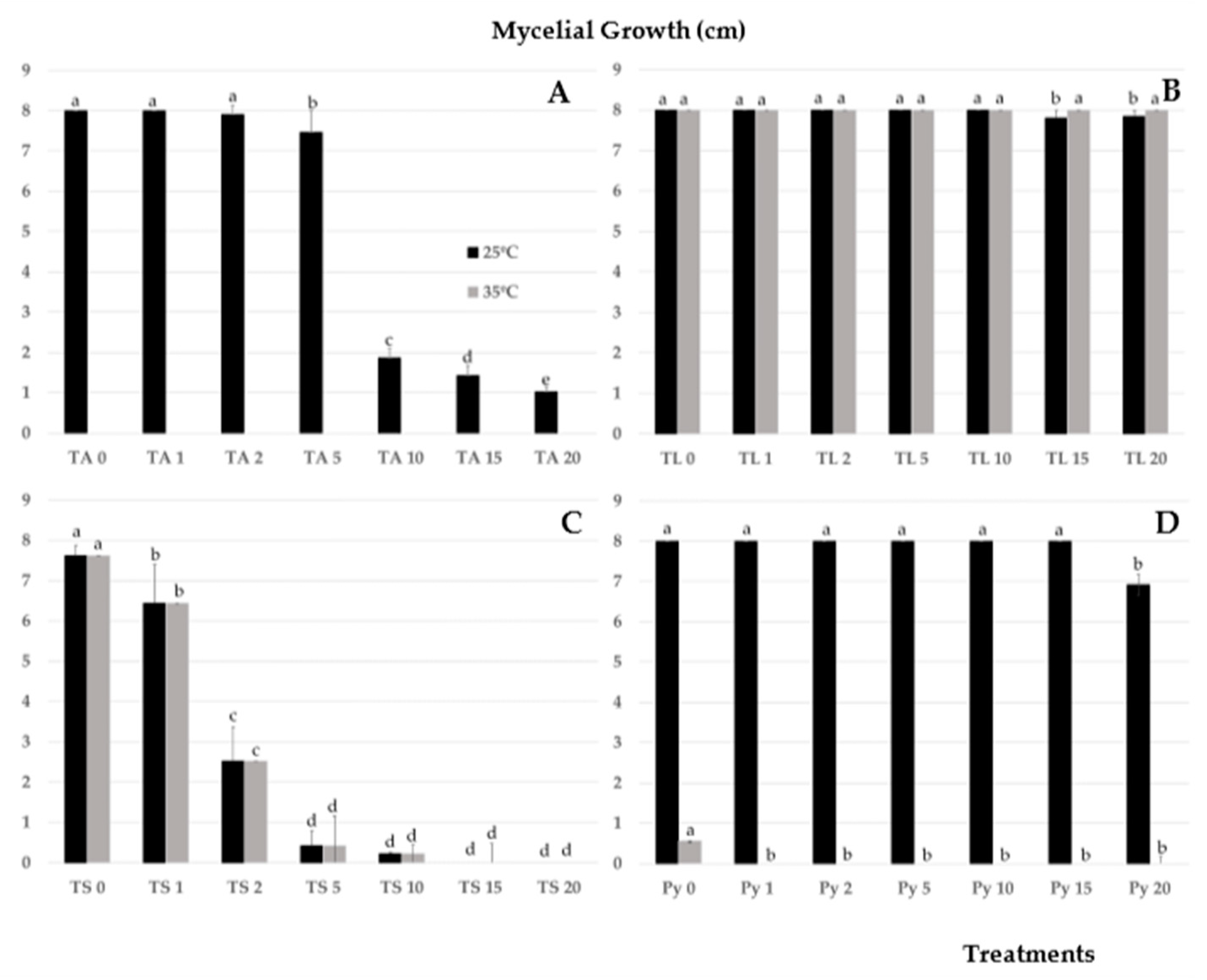
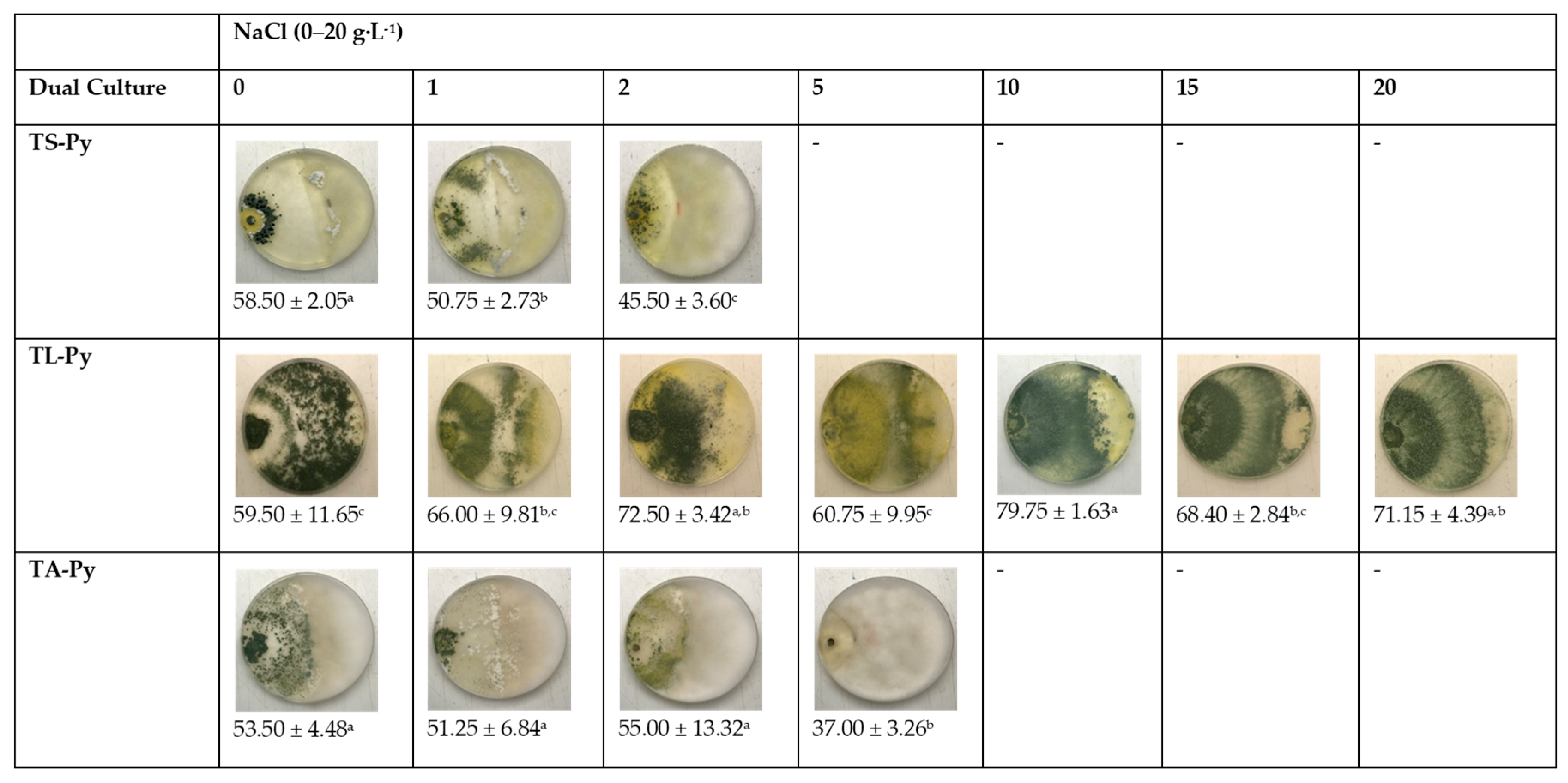
3.1. Effects of Salinity and Temperature on Colony Growth of Trichoderma Isolates
3.2. Effects of Trichoderma Isolates on the Radial Growth of P. ultimum
3.3. Promoter Effects of Trichoderma Isolates on Melon Seedlings and Salinity Treatments
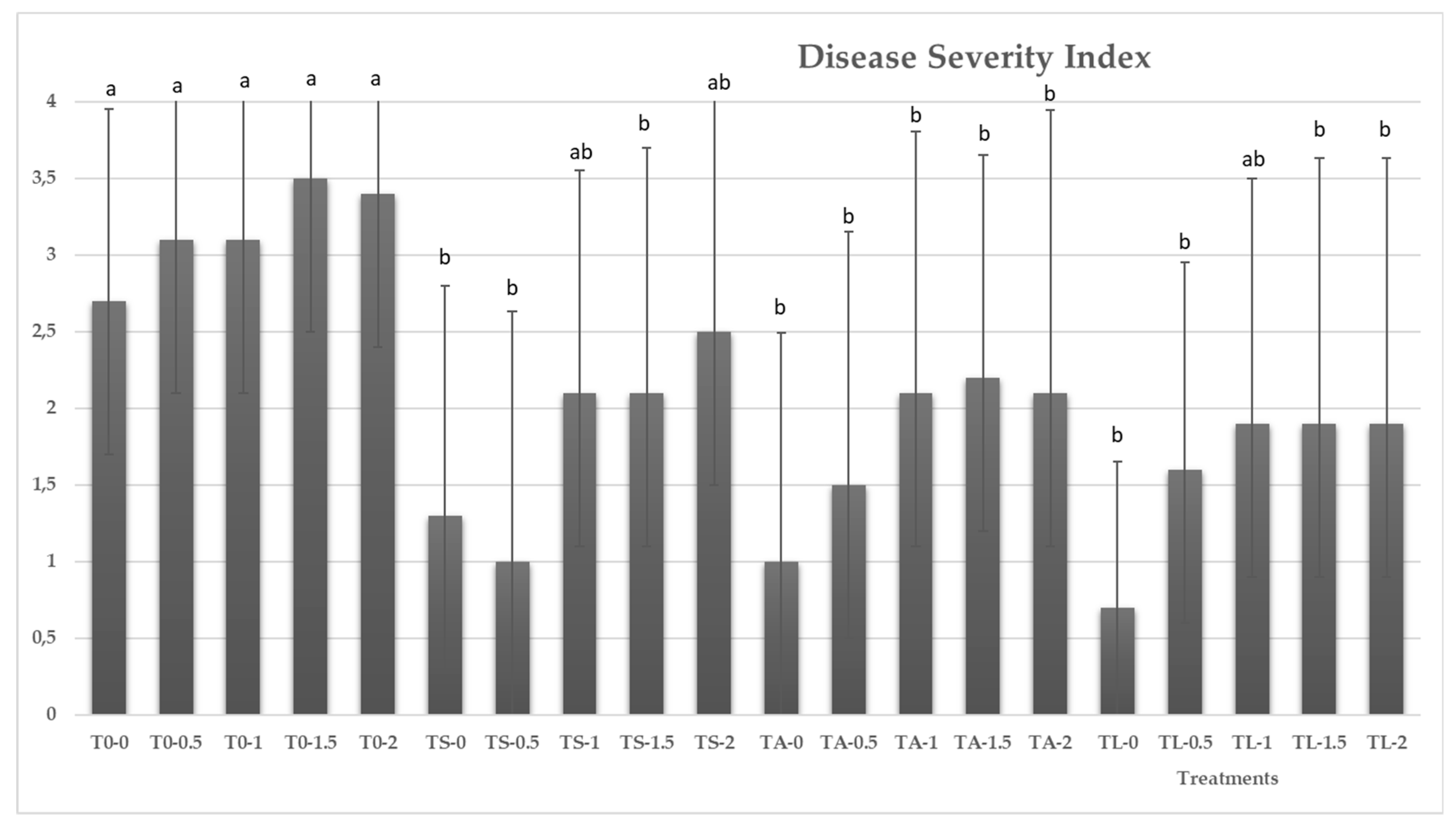
3.4. Effects of Trichoderma Strains on the Severity of Pythium ultimum in Melon Seedlings under Saline Conditions
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riesgos Asociados a la Presencia Simultánea de Plaguicidas en Alimentos: La Importancia de Los Análisis Multi-Residuo. Available online: https://www.ainia.es/tecnoalimentalia/tecnologia/riesgos-asociados-a-la-presencia-simultanea-de-plaguicidas-en-alimentos/ (accessed on 12 May 2019).
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Paul, D. Osmotic stress adaptations in rhizobacteria. J. Basic Microbiol. 2012, 53, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wanga, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Yanga, T.; Ma, K.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- Gaind, S. Phosphate dissolving fungi: Mechanism and application in alleviation of salt stress in wheat. Microbiol. Res. 2016, 193, 94–102. [Google Scholar] [CrossRef]
- Yasmeen, R.; Siddiqui, Z.S. Ameliorative effects of Trichoderma harzianum on monocot crops under hydroponic saline environment. Acta Physiol. Plant. 2018, 40, 4. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Apostolakis, A.; Wagner, K.; Deligianni, A.; Koutskoudis, D.; Stamatakis, A.; Tsanis, I.K. Effectiveness of Trichoderma harzianum in soil and yield conservation of tomato crops under saline irrigation. Catena 2019, 175, 144–153. [Google Scholar] [CrossRef]
- Mohamed, H.; Haggag, W. Biocontrol potential of salinity tolerant mutants of Trichoderma harzianum against Fusarium oxysporum. Braz. J. Microbiol. 2006, 37, 181–191. [Google Scholar] [CrossRef]
- Bheemaraya, P.M.B.; Ramesh, Y.S.T.; Amaresh, Y.S.; Naik, M.K. Salinity stress tolerance in native Trichoderma isolates. Environ. Ecol. 2013, 31, 727–729. [Google Scholar]
- Gal-Hemed, I.; Atanasova, L.; Komon-Zelazowska, M.; Druzhinina, I.S.; Viterbo, A.; Yarden, O. Marine isolates of Trichoderma spp. as potential halotolerant agents of biological control for arid-Zone agriculture. Appl. Environ. Microbiol. 2011, 77, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Wang, B.G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016, 80, 301–342. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diánez, F.; Santos, M.; Carretero, F.; Marín, F. Trichoderma saturnisporum, a new biological control agent. J. Sci. Food Agric. 2016, 96, 1934–1944. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Carretero, F.; Marín, F. Biostimulant activity of Trichoderma saturnisporum in melon (Cucumis melo). Hortscience 2018, 53, 810–815. [Google Scholar]
- Vohník, M.; Borovec, O.; Župan, I.; Vondrášek, D.; Petrtýl, M.; Sudová, R. Anatomically and morphologically unique dark septate endophytic association in the roots of the Mediterranean endemic seagrass Posidonia oceanica. Mycorrhiza 2015, 25, 663–672. [Google Scholar] [CrossRef]
- Santos, M.; Diánez, F. Los Antagonistas Microbianos en el Manejo de Micosis de la Parte Aérea de la Planta. In Organismos Para el Control de Patógenos en los Cultivos Protegidos. Prácticas Culturales Para una Agricultura Sostenible; Tello, J.C., Camacho, F., Eds.; Fundacion Cajamar: Almeria, Spain, 2010; pp. 523–528. [Google Scholar]
- Marín, F.; Diánez, F.; Santos, M.; Carretero, F.; Gea, F.J.; Castañeda, C.; Navarro, M.J.; Yau, J.A. Control of Phytophthora capsici and Phytophthora parasitica on pepper (Capsicum annuum L.) with compost teas from different sources, and their effects on plant growth promotion. Phytopathol. Med. 2014, 53, 216–228. [Google Scholar]
- Santos, M.; Diánez, F.; González, M.; Tello, J.C. Grape marc compost: Microbial studies and suppression of soilborne mycosis in vegetable seedlings. World J. Microbiol. Biotechnol. 2008, 24, 1493–1505. [Google Scholar] [CrossRef]
- Sobieralski, K.; Siwulski, M.; Frużyńska-Jóźwiak, D. Growth of aggressive isolates of Trichoderma aggressivum f. europaeum in dependence on temperature and medium. Phytopathologia 2009, 53, 11–18. [Google Scholar]
- Santos, M.; Diánez, F.; Carretero, F.; Gea, F.J. Evaluation of selected soils for suppression of Fusarium diseases. Phytopathol. Med. 2017, 56, 278–378. [Google Scholar]
- Dela Cruz, T.E.; Wagner, S.; Schulz, B. Physiological responses of marine Dendryphiella species from different geographical locations. Mycol. Prog. 2006, 5, 108–119. [Google Scholar] [CrossRef]
- Migheli, Q.; González-Candelas, L.; Dealessi, L.; Camponogara, A.; Ramón-Vidal, D. Transformants of Trichoderma longibrachiatum overexpressing the β-1,4-Endoglucanase gene egl1 show enhanced biocontrol of Pythium ultimum on cucumber. Biol. Control 1998, 88, 673–677. [Google Scholar]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144–157. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; D’Agostino, N.; Proietti, S.; Bertini, L.; Lorito, M.; Ruocco, M.; Caruso, C.; Chiusano, M.L.; Tucci, M. Suppression subtractive hybridization analysis provides new insights into the tomato (Solanum lycopersicum L.) response to the plant probiotic microorganism Trichoderma longibrachiatum MK1. J. Plant. Physiol. 2016, 190, 79–94. [Google Scholar] [CrossRef]
- Porto de Souza, L.; Blandon, L.M.; Rodrigues, C.; Cândido, M.; de Melo, G.V.; Pereira, G.; de Oliveira, J.; Soccol, C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef]
- Sharma, V.; Shanmugam, V. Unraveling the multilevel aspects of least explored plant beneficial Trichoderma saturnisporum isolate GITX-Panog. Eur. J. Plant Pathol. 2018, 152, 169–183. [Google Scholar] [CrossRef]
- Meng, J.; Cheng, W.; Heydari, H.; Wang, B.; Zhu, K.; Konuklugil, B.; Lin, W. Sorbicillinoid-based metabolites from a sponge-derived fungus Trichoderma saturnisporum. Mar. Drugs 2018, 16, 226–241. [Google Scholar] [CrossRef]
- Ospina-Giraldo, M.D.; Royse, D.M.; Thon, M.R.; Chen, X.; Romaine, C.P. Phylogenetic relationships of Trichoderma harzianum causing mushroom green mold in Europe and North America to other species of Trichoderma from world-wide sources. Mycologia 1998, 90, 76–81. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system—A review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Khan, M.R.; Mohiddin, F.A. Trichoderma: Its Multifarious Utility in Crop Improvement. In Crop Improvement Through Microbial Biotechnology. New and Future Developments in Microbial Biotechnology and Bioengineering; Aligarh Muslim University: Aligarh, India; Srinagar, India, 2018; Chapter 13; pp. 263–291. [Google Scholar]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Sustainable Use of Pesticides. Available online: https://ec.europa.eu/food/plant/pesticides/sustainable_use_pesticides (accessed on 12 May 2019).

| Treatments Isolate/NaCl (g·L−1) | Aereal Fresh Weight (g) | Root Fresh Weight (g) | Aereal Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|
| T0-0 | 6.74 ± 1.93 c | 1.61 ± 0.70 b,c | 0.48 ± 0.15 b | 0.09 ± 0.06 b |
| TS-0 | 7.92 ± 1.79 a | 2.31 ± 0.50 a | 0.78 ± 0.16 a | 0.13 ± 0.02 a |
| TA-0 | 7.01 ± 0.98 b,c | 1.59 ± 0.85 b,c | 0.71 ± 0.12 a | 0.12 ± 0.03 a |
| TL-0 | 7.27 ± 1.71 b | 1.74 ± 0.49 b | 0.76 ± 0.07 a | 0.10 ± 0.04 a,b |
| T0-0.5 | 6.94 ± 1.80 b | 1.42 ± 0.42 b | 0.37 ± 0.09 c | 0.07 ± 0.03 b |
| TS-0.5 | 7.19 ± 1.77 a,b | 1.68 ± 0.54 a | 0.55 ± 0.11 b | 0.08 ± 0.02 a,b |
| TA-0.5 | 7.49 ± 1.34 a | 1.70 ± 0.61 a | 0.66 ± 0.16 a,b | 0.09 ± 0.02 a |
| TL-0.5 | 7.05 ± 1.22 b | 1.39 ± 0.48 a,b | 0.69 ± 0.23 a | 0.08 ± 0.03 a,b |
| T0-1 | 5.32 ± 1.45 b | 0.93 ± 0.55 b | 0.39 ± 0.18 b | 0.07 ± 0.03 a,b |
| TS-1 | 6.01 ± 1.30 a | 1.02 ± 0.35 b | 0.49 ± 0.11 a,b | 0.06 ± 0.02 c |
| TA-1 | 5.90 ± 1.05 a,b | 1.46 ± 0.42 a | 0.76 ± 0.12 a | 0.10 ± 0.01 a |
| TL-1 | 6.30 ± 2.43 a | 1.35 ± 0.46 a | 0.77 ± 0.16 a | 0.07 ± 0.03 a,b |
| T0-1.5 | 5.26 ± 1.40 c | 0.67 ± 0.35 c | 0.39 ± 0.09 c | 0.07 ± 0.04 a,b |
| TS-1.5 | 5.57 ± 1.03 b | 1.02 ± 0.24 b | 0.50 ± 0.15 b | 0.05 ± 0.02 b |
| TA-1.5 | 7.43 ± 1.79 a | 1.27 ± 0.25 a | 0.63 ± 0.18 a | 0.09 ± 0.02 a |
| TL-1.5 | 5.07 ± 0.87 c | 1.04 ± 0.23 b | 0.50 ± 0.11 b | 0.08 ± 0.02 a |
| T0-2 | 5.22 ± 1.12 c | 0.55 ± 0.28 b | 0.44 ± 0.14 b | 0.07 ± 0.03 a |
| TS-2 | 5.82 ± 0.83 b | 0.72 ± 0.28 a | 0.51 ± 0.13 a | 0.05 ± 0.02 b |
| TA-2 | 6.35 ± 1.00 a | 1.08 ± 0.21 a | 0.58 ± 0.08 a | 0.07 ± 0.03 a |
| TL-2 | 5.42 ± 1.18 b,c | 0.82 ± 0.33 a | 0.49 ± 0.12 a,b | 0.07 ± 0.03 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. https://doi.org/10.3390/ijerph16112053
Sánchez-Montesinos B, Diánez F, Moreno-Gavira A, Gea FJ, Santos M. Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. International Journal of Environmental Research and Public Health. 2019; 16(11):2053. https://doi.org/10.3390/ijerph16112053
Chicago/Turabian StyleSánchez-Montesinos, Brenda, Fernando Diánez, Alejandro Moreno-Gavira, Francisco J. Gea, and Mila Santos. 2019. "Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress" International Journal of Environmental Research and Public Health 16, no. 11: 2053. https://doi.org/10.3390/ijerph16112053
APA StyleSánchez-Montesinos, B., Diánez, F., Moreno-Gavira, A., Gea, F. J., & Santos, M. (2019). Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. International Journal of Environmental Research and Public Health, 16(11), 2053. https://doi.org/10.3390/ijerph16112053





