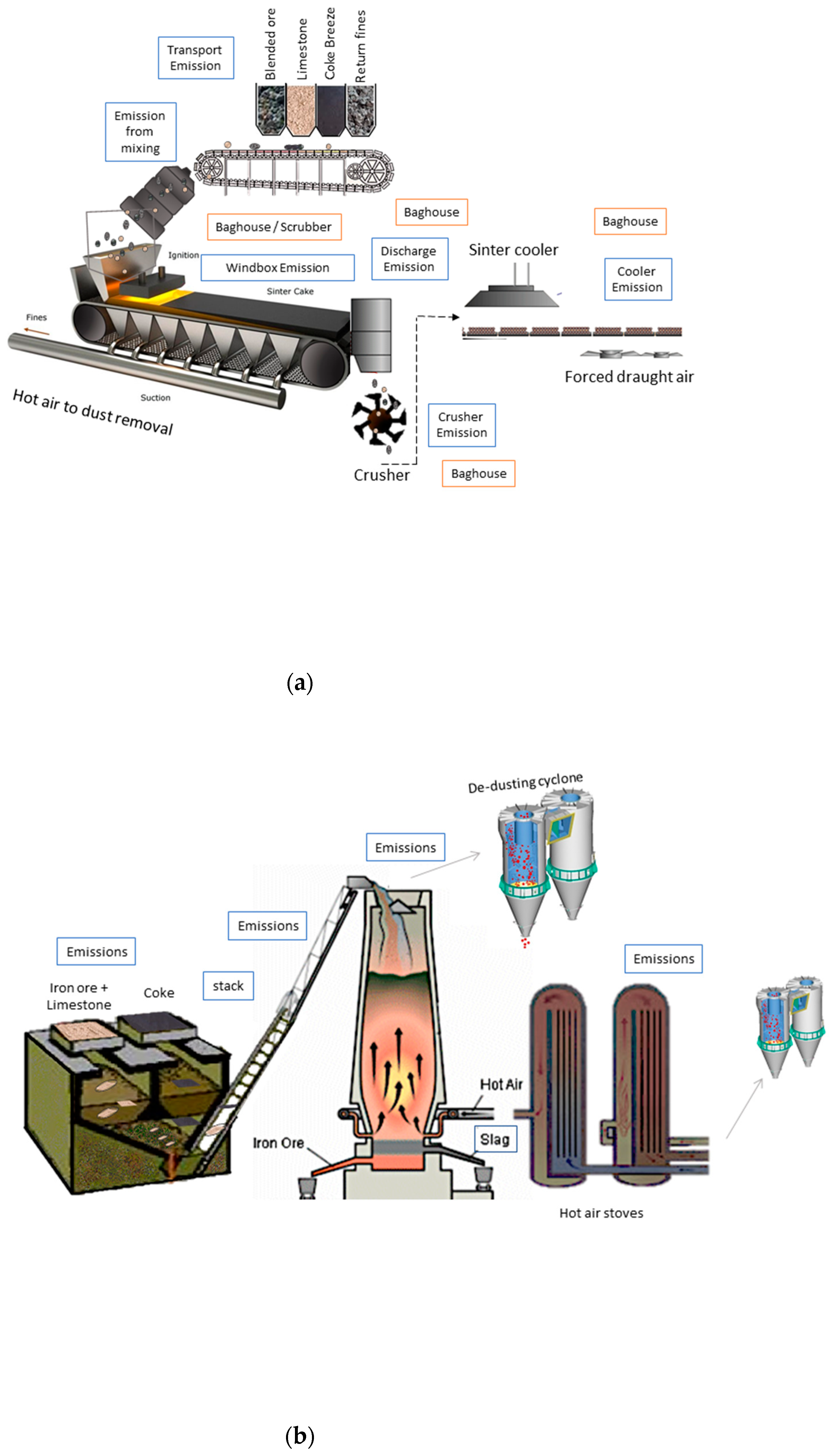
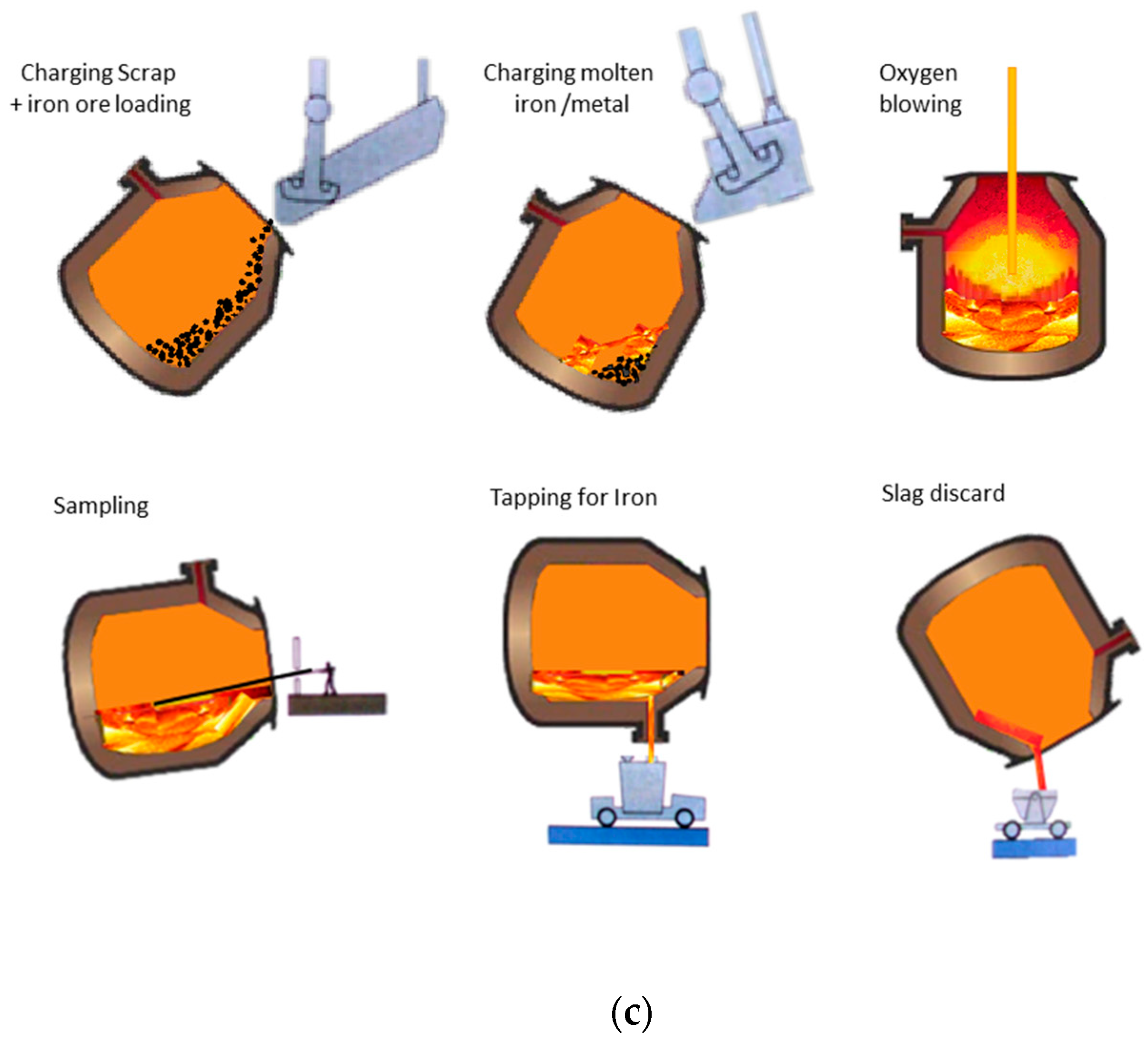
1. Introduction
In 2017 approximately 1.69 billion tonnes of crude steel was produced worldwide [
1] with as much as 400 kg of solid waste being generated per tonne of steel and requiring disposal [
2]. In the last three decades a number of methods for the recovery and reuse of waste by-products (powdered wastes, flue dusts, slag, and sludge) have been developed for application within the steel manufacture industrial life cycle, however, as a result of the pressure from continuous reduction in regulatory classification thresholds worldwide, standard cleaning operations are struggling to meet the legal requirements [
3,
4]. Thresholds from compliance leaching tests across Europe: EN 12457 [
5], and UK BS EN 12457 [
6] known as Waste Acceptance Criteria (WAC) testing has forced the need for investment in alterations to and/or additional steps in the steel production process [
7,
8]. The major contaminants of concern are cadmium (Cd), copper (Cu), chromium (Cr), lead (Pb), nickel (Ni), potassium (K) and zinc [
9,
10,
11,
12]. This research focuses on K, Pb and Zn.
The modern production of steel is a complex process even when processing is simplified. It includes a diverse range of emission and abatement systems (
Figure 1), and although cleaning treatments have previously been applied [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27], there is still a lack of information on their impact on waste processing. Established treatment techniques are based on pyrometallurgy, hydrometallurgy or a combination of both methods, with the latter offering processes favoured as a treatment which offers the best economic return with a higher degree recovery for valuable metals [
28,
29]. However, the processes have recognised limitations and struggle to digest zinc ferrites, which is dominant form of zinc in baghouse dusts [
30]. Zinc is both of value as a process alloying material e.g., brass [
31], as a by-product recycled back into sinter [
32,
33], but can also have a deleterious effect on steel production if built up through excessive use of recycled metals e.g., from a process feed of galvanised product wastes, or in the feed itself. Furthermore, it can cause structural damage to the plants themselves [
34,
35,
36]. Since emission controls can only be applied by altering the production process or removing the cause during production, coupled with the 365-day operational approach for competitive steel manufacturing, the options for emission reduction are through suppression, extraction, and/or abatement [
37].
With these restrictions the ability to treat industrial wastes by immobilisation or removal of hazardous components in order to alter their disposal classification i.e., hazardous, non-hazardous, inert, can offer great potential to reduce industrial costs and address environmental impact. Immobilisation can take place by applying techniques such as solidification or stabilization (S/S) that use binding reagents to immobilize the hazardous constituents. The approach for S/S can often be coupled together as one can occur as a result of the other which and the reason for S/S being frequently identified together. These approaches are widely used for treatment of hazardous wastes that are mostly inorganic (aqueous wastes, sludge, slags, dust, and ashes containing hazardous metals) and contaminated soils before final disposal [
38].
By utilising experience from previous studies of solidification/stabilisation (S/S) [
9,
39,
40,
41] using binding/bulking agents [
9,
42,
43,
44,
45,
46,
47,
48,
49], or organic additives [
50,
51,
52,
53,
54,
55,
56,
57,
58,
59] we can evaluate the potential of alternative processes to develop more sustainable production strategies. Thus focusing on reducing production costs and impacts on direct negative environmental and human health risk whilst increasing production efficiency, resource utilisation and a reduction in the amount of waste generated [
22]. This will not only reduce emission thresholds to meet legal requirements, but also help minimise any future risk to human and wider environmental impact these contaminants have [
60].
This research focuses on a number of elemental groups: key potentially toxic elements (PTEs) of interest (K, Pb, Zn), associated elements (Al, Ca, Fe and Mn) and additional elements of interest (Cr, Cu, Mn and Ni). The associated elements Al, Ca, Fe and Mn are elements that can have a strong influence upon the liberation of the priority constituents of interest (K, Pb and Zn). The Al and Si content dictates the acidity or buffering capacity of solid samples. Here only Al is reported because Si could not be measured due to instrumental limitations. The elements Ca, Mn and Fe are identified because the acidic conditions created by the citrate solution can lead to the dissolution of iron/manganese hydroxides, which releases through desorption of the associated PTEs [
50]. Additional elements Cr, Cu, Mn and Ni are often reported as components of steel waste and are known problematic PTEs environmentally [
10,
11,
61,
62].
Furthermore, citrate can be highly effective in enhancing the solubilisation of sparingly soluble inorganic iron hydroxides (Fe) in soils. Interestingly, this creates an enhanced sorption system for alternative and potentially more strongly complexed metallic components consequently enhancing their [
51]. The calcium ion binds in one of two ways; a single citrate species can bind one calcium ion or two citrate molecules to one calcium ion causing a collision-induced dissociation of precursor ions, metal:citrate stoichiometry is favoured [
63].
We report here on an assessment of the potential benefits from treatment strategies for steel wastes through reduction of hazard potential and the implications for wider management processes in the steel life cycle.
4. Discussion
4.1. Citric Acid Stabilisation
The main elements of interest (K, Pb and Zn) show varying results from the leaching approach. These can be explained in part by the co-liberation or immobilisation of other elements e.g., Fe, Mn, Ca, Al, Cu, Ni and Cr. Lead has been successfully leached into solution from BF sludge, BOF sludge and ESP dusts using 0.1 M citrate solutions. This indicates that a lower concentration of Pb remained in the solid sample, resulting in a lower environmental threat. Similar results were obtained for zinc in BF sludge and BOF sludge. However, in sinter ESP dusts it appears that zinc is immobilised by the citrate solution due to ionic potential and exchange into a solid or non-soluble complex.
The liberation of potassium by 0.1 M-citrate solution was successful for BF sludge, but the results proved inconclusive for Sinter ESP dust due to the variability of results, which showed both immobilisation and liberation in solution. Leaching of K from BOF fine sludge was less effective in citrate than in water indicating that immobilisation of K occurs with citrate solution.
Iron and manganese were, as predicted, leached out in greater quantities with citrate solution than with water, probably due to dissolution of their associated hydroxides and strong soluble citrate complex formation. This may explain the enhanced concentrations of Pb, Zn and some other elements in citrate leachates; as such species can be desorbed from hydroxides at low pH. Additionally, as Fe and Mn ions compete to occupy available sorption sites.
For the effective potential application in industrial processing, it is important to assess whether the addition of a citrate solution has simply resulted in solubilisation of PTE into the leachate or has it successfully significantly reduced the availability of these elements in the residual solid waste, and ultimately reduced its hazard potential. The Waste Acceptance Criteria (WAC) testing indicated that the bulk BF, BOF and ESP dusts were classified as “inert” therefore making standard regulatory reference levels inappropriate for a reactive material. Consequently, we evaluated the effectiveness of the citrate treatments against the percentage difference of the pre and post-treated waste materials to demonstrate the potential this technique to reduce toxicity hazard. Data are presented in
Figure 5, which shows the percentage decrease of the key waste constituents (K, Pb and Zn) relative to the original total elemental concentrations.
From
Figure 11 it can be seen there is a variation in the percentage decrease (or increase) of total PTE concentrations in the residual waste samples (for clarity error bars are removed). Additionally, where K measurements are absent, values are < analytical detection limit after treatment.
Lead and zinc both follow similar trends and show a decrease in residual solid concentrations: Pb (median of 88% ± 20.2) and Zn (61% decrease ± 12.8). The large decrease of Pb (113%) suggests total liberation in BOF fine sludge, however as previously identified [
68], the total content varies in concentration and the median value does not account for fluctuations, which could explain the higher percentage. Potassium however shows an increase in blast furnace sludge (78%) that suggests, as might be anticipated that water was a good leaching agent for K, whereas the citrate is causing a complexing effect holding K in solid complexes. There is no detectable difference in K levels for some samples whereby further analysis would be required for validation. As water washing has been proven an efficient method for potassium removal [
69] meaning citrate may have no conclusive impact.
4.2. BF Stabilisation
There is no definitive trend that can be observed within the different waste types and the elemental concentrations in the varying waste:slag mixes, that can be applied to all of the PTEs measured. The majority of samples show their lowest PTE concentrations to be from the 50/50 mixed, ‘stabilised’ moulds, which were either similar or slightly higher in concentration than the 30:70 mixes. Further comparison of the leached concentrations with their respective raw samples was carried out, which demonstrated that the majority of samples including the 70/30 showed significant reduction levels between 90–100%.
They did however tend to show an increase in Al concentration, for both the 50/50 and 70/30 mixed ratios suggesting release from the stabilising agent. It does seem that optimal use of a 50/50 mixture of the two waste types will create a stabilised material that might be suitable for landfill.
Additionally, it is worth considering that as with other stabilisation materials, e.g., Portland cement, the products may be appropriate for other industrial/commercial uses. Initial tests of larger mould for compressive strength and potential use in construction were found to be too weak after a 28-day curing period.
5. Conclusions
The different approaches evaluated in order to test stabilisation or immobilisation of a number of steel process by-products resulted in a range of outcomes. These are compelling results which demonstrate for the first time potential for active risk reduction of wastes which represent enormous environmental burden worldwide.
The experimental results from stabilisation by mixing the wastes with Portland cements shows that a mixture of 70% waste material and 30% PC offers a 90%+ reduction in leachate concentrations compared to the raw materials themselves. This means that although the key PTEs cannot be recovered for re-use, landfilling with an inert classification would be possible. The same outcome can be observed when using BF slag as a stabilising agent; with the exception of aluminium a significant reduction can be seen with the 50/50 waste:stabiliser mixes with a reduction of over 90%. This may provide direct economic advantage as diversion from hazardous classification and premium landfill tax, once additional processing costs are considered.
The application of citric acid demonstrated an ability to substantially reduce or stabilise PTEs within the various steel production by-products (dust, sludge). For our key PTEs we see a 73% decrease in release from sinter dust i.e., stabilised within its matrix, as opposed to being liberated (78%) from the of BF sludge. Lead and zinc however both showed significant leaching into solution after treatment with citrate solution (averaging 50–80% and 50–60% decrease in total solid concentration). This results in process solids that are greatly reduced in elements of concern (Pb and Zn) and the iron rich solid wastes could be either recycled back into the blast furnace or landfilled as inert wastes.
Both applications show the potential of two treatment approaches in which waste materials can be reclassified from hazardous/non-hazardous to inert. A variety of secondary applications are feasible such as direct recycling back into plant, production of a bulk construction material or even to harbour valuable metals e.g., where Pb and Zn markets are relatively buoyant for additional income. Ultimately treatment allows for better waste management through reduction in negative impacts environmentally and reduction in long term risk for human health.