The Study of an Ultraviolet Radiation Technique for Removal of the Indoor Air Volatile Organic Compounds and Bioaerosol
Abstract
:1. Introduction
2. Materials and Methods
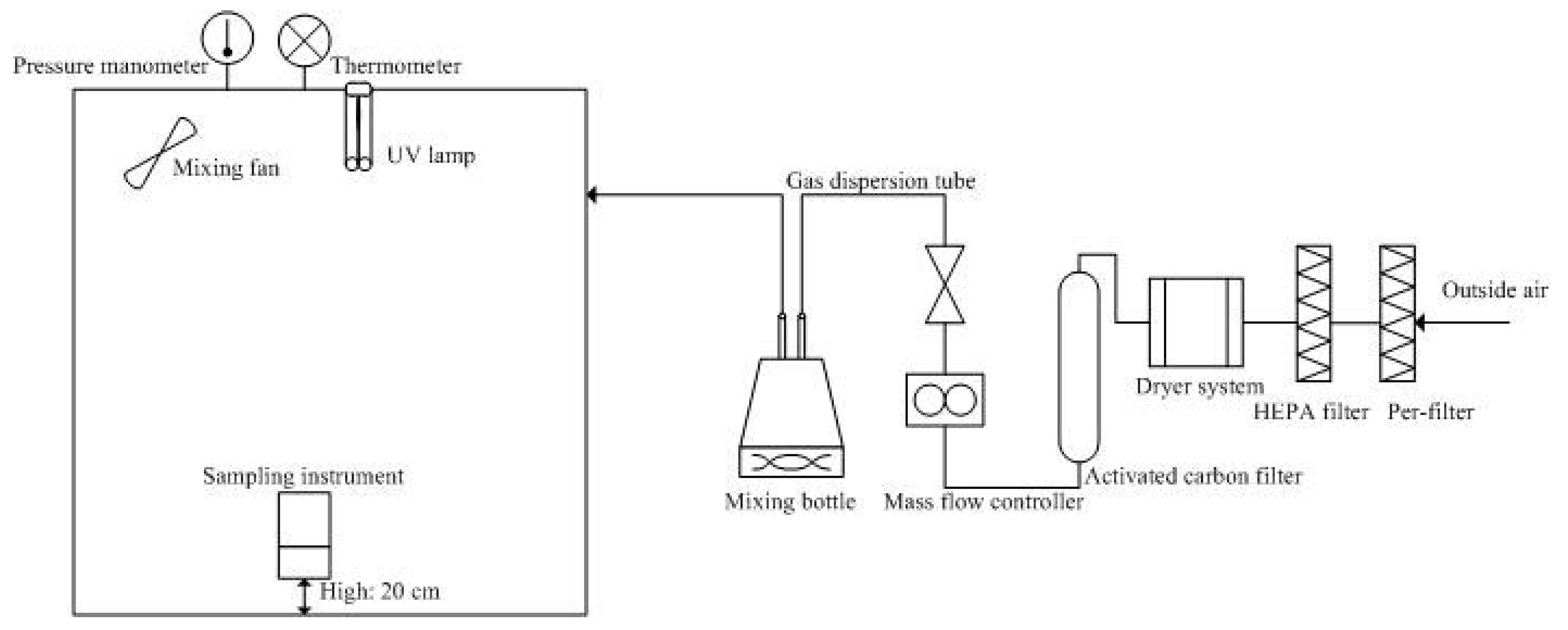
2.1. UVGI Experiments in Environment Chambers
2.2. UVGI Experimental in Field Studies
2.3. UVGI Removal Efficiency of Air Pollutants
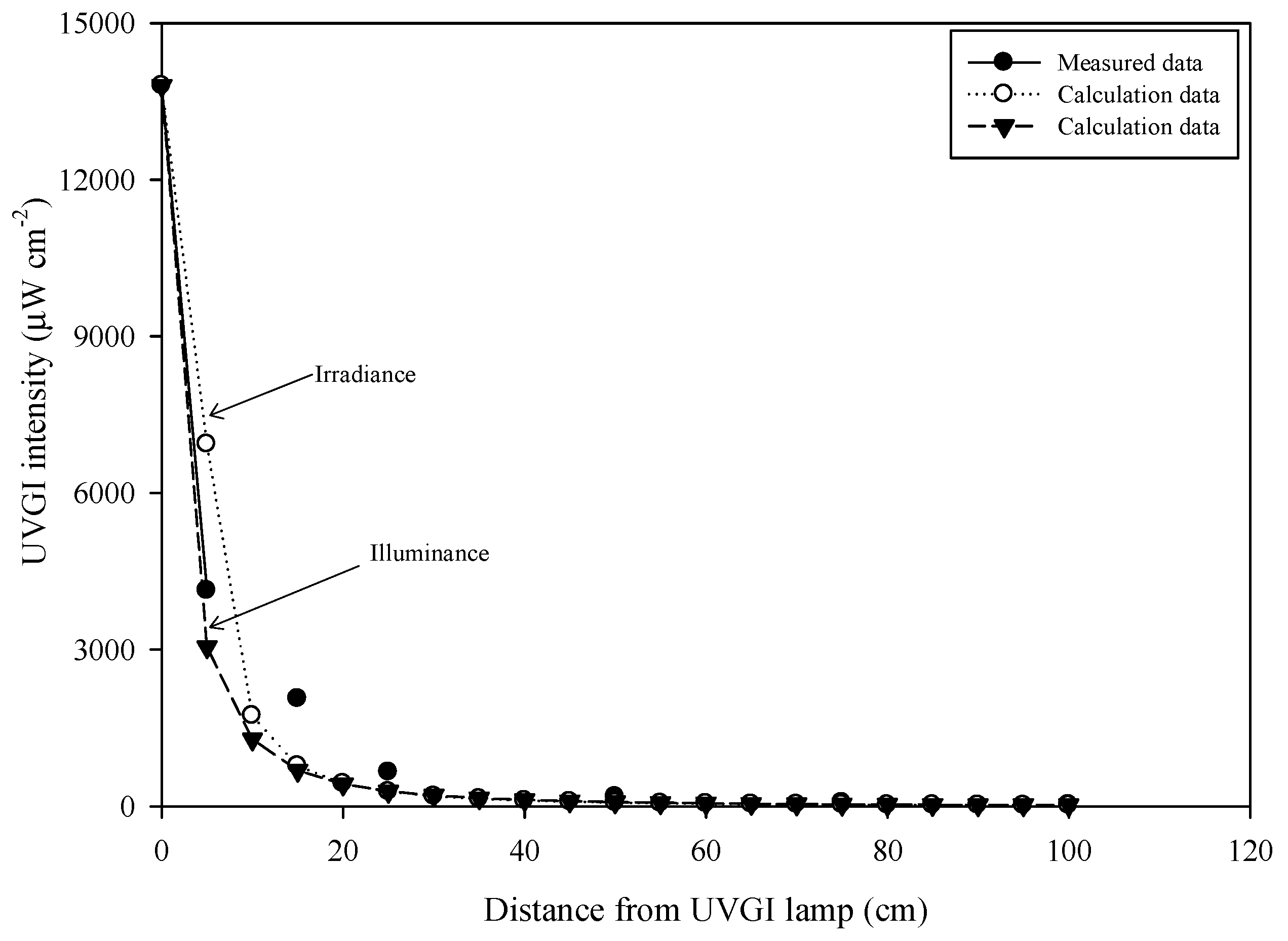
2.4. Measurement of UV Irradiance and Calculation of Dosages
3. Results and Discussion
3.1. Efficiency of Air Pollutant Removal Under Various Levels of Relative Humidity and Various Initial Concentrations of Pollutants
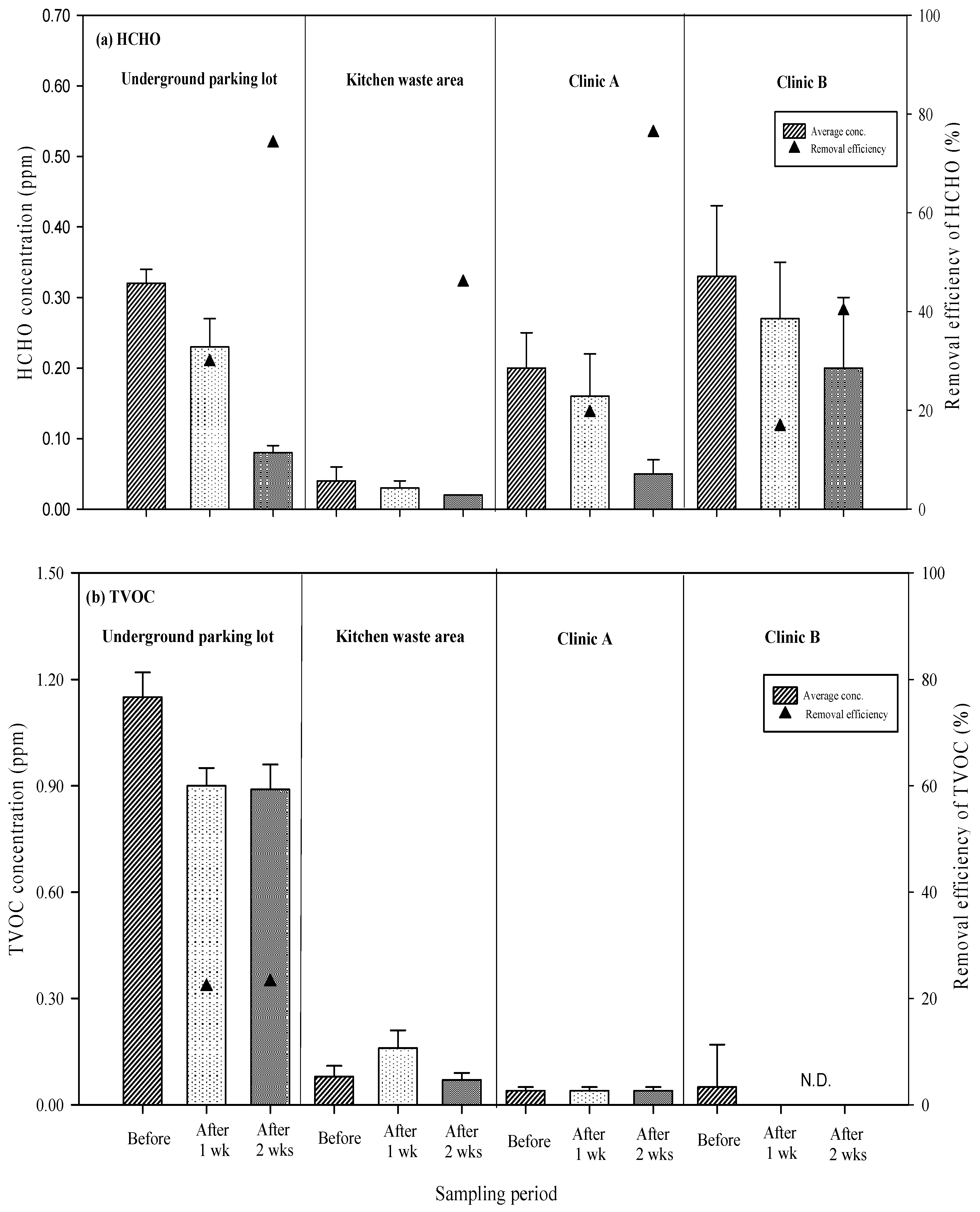
3.2. Efficiency of Air Pollutant Removal Through Long-Term Exposure to UVGI
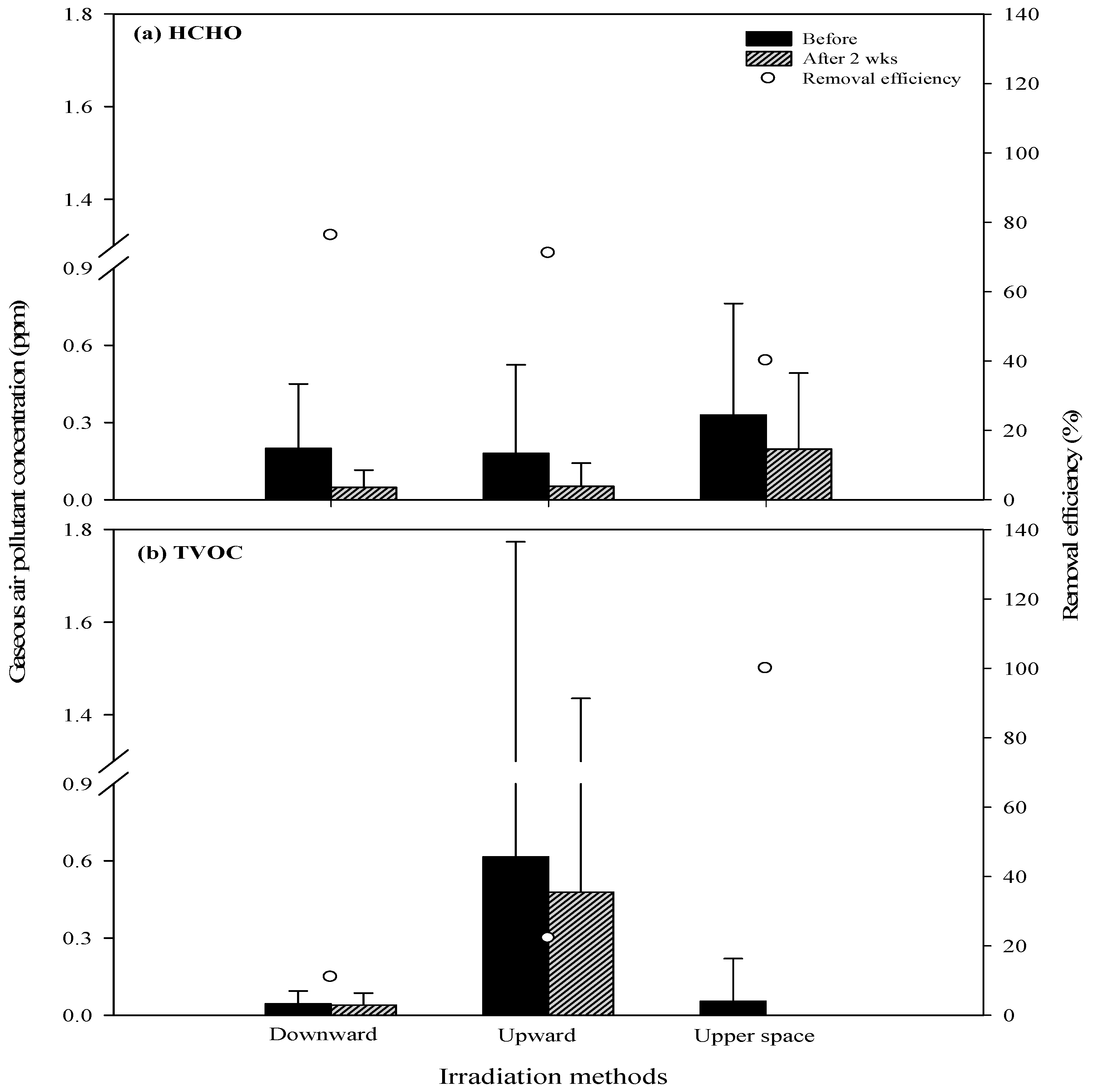
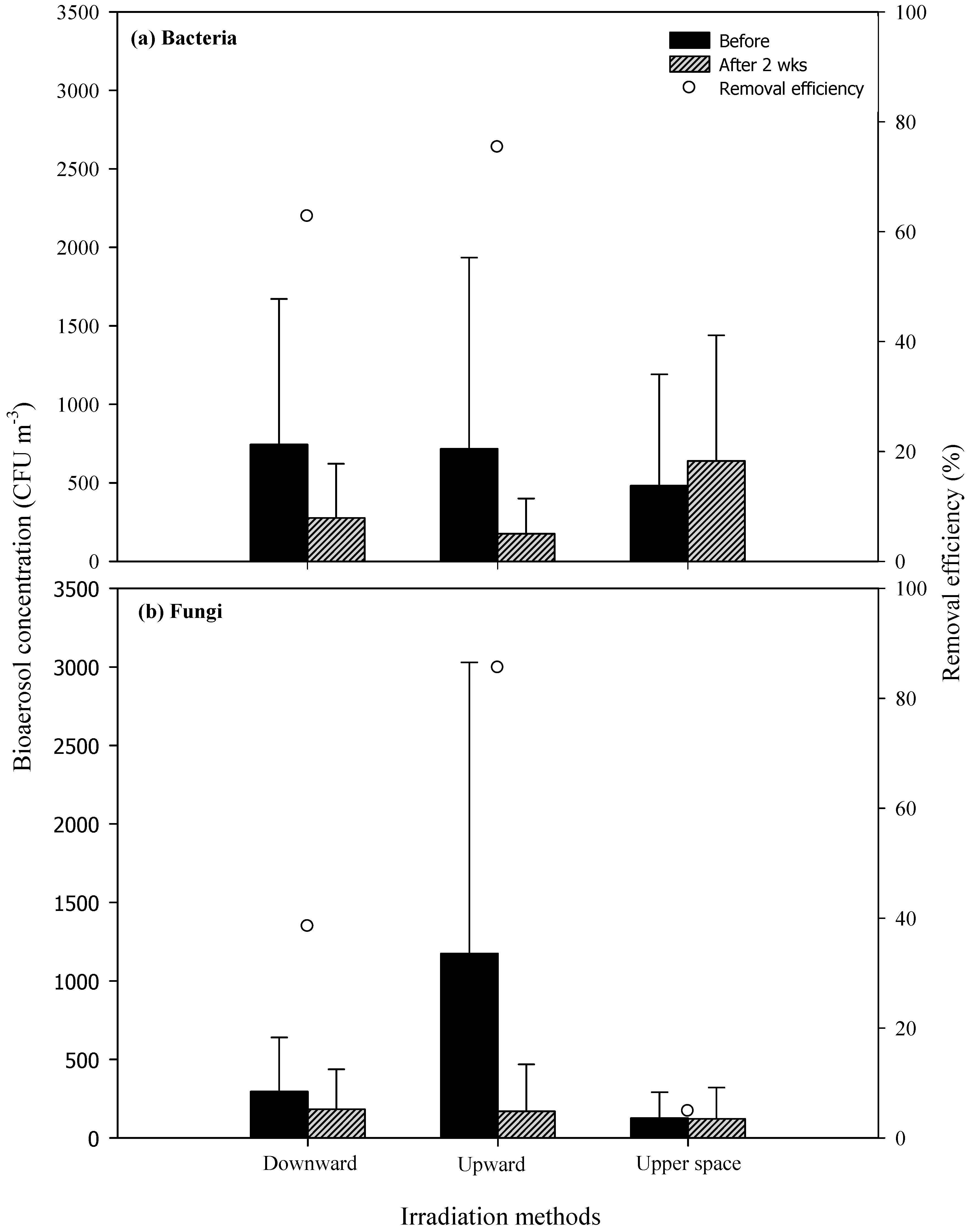
3.3. Efficiency of Air Pollutant Removal Using Various UVGI Irradiation Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, C.F.; Scarpino, P.V. The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria. Environ. Eng. Policy. 2001, 3, 101–107. [Google Scholar] [CrossRef]
- Han, J.; Colditz, G.A.; Samson, L.D.; Hunter, D.J. Polymorphisms in DNA double-strand break repair genes and skin cancer risk. Cancer Res. 2004, 64, 3009–30013. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Bahnfleth, W.P. Proposed standards and guidelines for UVGI air disinfection. IUVA News 2004, 6, 20–25. [Google Scholar]
- Miller, S.L.; MacHer, J.M. Evaluation of a methodology for quantifying the effect of room air ultraviolet germicidal irradiation on airborne bacteria. Aerosol. Sci. Tech. 2000, 33, 274–295. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, C.S. Control effectiveness of ultraviolet germicidal irradiation on bioaerosols. Aerosol. Sci. Tech. 2002, 36, 474–478. [Google Scholar] [CrossRef]
- Tseng, C.C.; Li, C.S. Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aerosol. Sci. Tech. 2005, 39, 1136–1142. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Bahnfleth, W.P.; Hernandez, M.T. A Genomic Model for the Prediction of Ultraviolet Inactivation Rate Constants for RNA and DNA Viruses; International Ultraviolet Association: Boston, MA, USA, 2009; pp. 4–10. [Google Scholar]
- Xu, P.; Peccia, J.; Fabian, P.; Martyny, J.W.; Fennelly, K.P.; Hernandez, M.; Miller, S.L. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmos. Environ. 2003, 37, 405–419. [Google Scholar] [CrossRef]
- Lau, J.; Bahnfleth, W.; Freihaut, J. Estimating the effects of ambient conditions on the performance of UVGI air cleaners. Build. Environ. 2009, 44, 1362–1370. [Google Scholar] [CrossRef]
- Begum, M.; Hocking, A.D.; Miskelly, D. Inactivation of food spoilage fungi by ultra violet (UVC) irradiation. Int. J. Food Microbiol. 2009, 129, 74–77. [Google Scholar] [CrossRef]
- Sung, M.; Kato, S. Method to evaluate UV dose of upper-room UVGI system using the concept of ventilation efficiency. Build. Environ. 2010, 45, 1626–1631. [Google Scholar] [CrossRef]
- TLVs and BEIs; American Conference of Governmental Industrial Hygienists (ACGIH): Cincinnati, OH, USA, 2004.
- Sonya, M.; Stephen, R.; James, M.; Edward, N. Occupant UV exposure measurements for upper-room ultraviolet germicidal irradiation. J. Photochem. Photobiol. B 2016, 159, 88–92. [Google Scholar] [Green Version]
- Kujundzic, E.; Matalkah, F.; Howard, C.J.; Hernandez, M.; Miller, S.L. UV air cleaners and upper-room air ultraviolet germicidal irradiation for controlling airborne bacteria and fungal spores. J. Occup. Environ. Hyg. 2006, 3, 536–546. [Google Scholar] [CrossRef]
- Escombe, A.R.; Moore, D.A.J.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Mitchell, B.; Noakes, C.; Martínez, C.; Sheen, P.; Ramirez, R. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009, 6, e1000043. [Google Scholar] [CrossRef]
- Lee, C.H. Treatment of Volatile Organic Compound from Indoor Pollution Source Using Modified Photocatalyst with LED; Institute of Environmental Engineering, National Taiwan University: Taipei, Taiwan, 2006; pp. 120–124. [Google Scholar]
- Wang, S.C. Determine the Formation of Hydroxyl Radicals in Liquid-Phase Photocatalytic Reaction by Using Methanol as the Radical Capture; Department of Safety, Health, and Environmental Engineering, National Kaohsiung First University of Science and Technology: Kaohsiung, Taiwan, 2006. [Google Scholar]
- Burrows, J.P.; Moortgat, G.K.; Tyndall, G.S.; Cox, R.A.; Jenkin, M.E.; Hayman, G.D.; Veyret, B. Kinetics and mechanism of the photooxidation of formaldehyde. 2. Molecular modulation studies. J. Phys. Chem. 1897, 93, 2375–2382. [Google Scholar] [CrossRef]
- Lin, C.K. Photodissociation and Photoisomerization of Toluene under Ultraviolet Irradiation; Department of Chemistry, National Taiwan University: Taipei, Taiwan, 2001; pp. 47–66. [Google Scholar]
- Ao, C.H.; Lee, S.C.; Yu, J.Z.; Xu, J.H. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B Environ. 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Hsu, H.I. Photocatalytic Degradation of Aqueous 4-Nitrophenol Uesing Couple Semiconductor System; Institute of Safety Health and Environmental Engineering, National Yunlin University of Science & Technology: Yunlin, Taiwan, 2005; pp. 6033–6035. [Google Scholar]
- Shie, J.L.; Lee, C.H.; Chiou, C.S.; Chang, C.T.; Chang, C.C.; Chang, C.Y. Photodegradation kinetics of formaldehyde using light sources of UVA, UVC and UVLED in the presence of composed silver titanium oxide photocatalyst. J. Hazard. Mater. 2008, 155, 164–172. [Google Scholar] [CrossRef]
- Ko, G.; First, M.W.; Burge, H.A. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airborne microorganisms. Environ. Health. Perspect. 2002, 110, 95–101. [Google Scholar] [CrossRef]
- Brickner, P.W.; Vincent, R.L.; First, M.; Nardell, E.; Murray, M.; Kaufman, W. The application of ultraviolet germicidal irradiation to control transmission of airborne disease: Bioterrorism countermeasure. Publ. health rep. 2003, 118, 99. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Blondeau, P.; Kozinski, J. Modeling and physical interpretation of photocatalytic oxidation efficiency in indoor air applications. Build. Environ. 2010, 45, 2689–2697. [Google Scholar] [CrossRef]
- Taiwan, EPA. (2012a). Standard Method of Total Bacteria Count of Indoor Air; Laboratory of Environmental Analysis, Environmental Protection Administration: Taiwan, China, 2012. [Google Scholar]
- Taiwan, EPA. (2012b). Standard Method of Total Fungi Count of Indoor Air; Laboratory of Environmental Analysis, Environmental Protection Administration: China, Taiwan, 2012. [Google Scholar]
- Kowalski, W.J.; Bahnfleth, W.P. Effective UVGI system design through improved modeling. transactions-american society of heating refrigerating and air conditioning engineers. ASHRAE Trans. 2000, 106, 721. [Google Scholar]
- Robinson, S.; Polak, J. Some new perspectives on Urban Link Travel Time Models: Is the k-nearest neighbours approach the solution? In Proceedings of the 36th Annual Conference of the Universities Transport Studies Group, Newcastle, UK, 5–7 January 2004. [Google Scholar]
- Shiue, A.; Hu, S.-C.; Tseng, C.-H.; Kuo, E.-H.; Liu, C.-Y.; Hou, C.-T.; Yu, T. Verification of air cleaner on-site modeling for PM2.5 and TVOC purification in a full-scale indoor air quality laboratory. Atmos. Pollut. Res. 2019, 10, 209–218. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Shi, J.; Zhang, Y.; Hu, H.; Shangguan, W. Degradation of indoor gaseous formaldehyde by hybrid VUV and TiO2/UV processes. Separ. Purif. Tech. 2007, 54, 204–211. [Google Scholar] [CrossRef]
- Kuo, H.W.; Wei, H.C.; Liu, C.S.; Lo, Y.Y.; Wang, W.C.; Lai, J.S.; Chuan Chan, C. Exposure to volatile organic compounds while commuting in Taichung, Taiwan. Atmos. Environ. 2000, 34, 3331–3336. [Google Scholar] [CrossRef]
- Kim, S.R.; Dominici, F.; Buckley, T.J. Concentrations of vehicle-related air pollutants in an urban parking garage. Environ. Res. 2007, 105, 291–299. [Google Scholar] [CrossRef]
- Legan, R.W. Ultraviolet light takes on CPI role. Chem. Eng. 1982, 89, 95–100. [Google Scholar]
- Peccia, J.; Werth, H.M.; Miller, S.; Hernandez, M. Effects of relative humidity on the ultraviolet induced inactivation of airborne bacteria. Aerosol. Sci. Tech. 2001, 35, 728–740. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Olmsted, R.N.; Bartley, J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: Effective adjunct, but not stand-alone technology. Amer. J. Infect. Cont. 2010, 38, S13–S24. [Google Scholar] [CrossRef]
- Kujundzic, E.; Hernandez, M.; Miller, S.L. Ultraviolet germicidal irradiation inactivation of airborne fungal spores and bacteria in upper-room air and HVAC in-duct configurations. J. Environ. Eng. Sci. 2007, 6, 1–9. [Google Scholar] [CrossRef]
- MacHer, J.M.; Alevantis, L.E.; Chang, Y.L.; Liu, K.S. Effect of ultraviolet germicidal lamps on airborne microorganisms in an outpatient waiting room. Appl. Occup. Environ. Hyg. 1992, 7, 505–513. [Google Scholar] [CrossRef]
- First, M.; Rudnick, S.N.; Banahan, K.F.; Vincent, R.L.; Brickner, P.W. Fundamental factors affecting upper-room ultraviolet germicidal irradiation—Part I. Experimental. J. Occup. Environ. Hyg. 2007, 4, 321–331. [Google Scholar] [CrossRef]
- Al Momani, F. Treatment of air containing volatile organic carbon: Elimination and post treatment. Environ. Eng. Sci. 2007, 24, 1038–1047. [Google Scholar] [CrossRef]
- Rudnick, S.N.; First, M.W. Fundamental factors affecting upper-room ultraviolet germicidal irradiation—Part II. Predicting effectiveness. J. Occup. Environ. Hyg. 2007, 4, 352–362. [Google Scholar] [CrossRef]
- Ko, G.; Burge, H.A.; Nardell, E.A.; Thompson, K.M. Estimation of tuberculosis risk and incidence under upper room ultraviolet germicidal irradiation in a waiting room in a hypothetical scenario. Risk Analysis 2001, 21, 657–674. [Google Scholar] [CrossRef]


| Case | Parking Lot | Kitchen Waste Area | Clinic A | Clinic B |
|---|---|---|---|---|
| Building age (year) | 3 | 3 | >15 | <6 months |
| Volume (m3) | 322 | 756 | 250 | 80 |
| Number of population | <2 | 0 | 10–15 | 10–15 |
| Air ventilation type | Mechanical ventilation (exhaust fan) | Natural ventilation | Mechanical ventilation (fan coil unit) | |
| UVGI luminaire | Upward irradiation | Direct irradiation over night | Upper space irradiation | |
| Number of UVGI lamp fixture | 8 | 8 | 6 | 6 |
| UVGI lamp intensity | 13,800 μW/cm2 | |||
| Item | Instrument/Model | Principle | Detection Range | Resolution |
|---|---|---|---|---|
| HCHO | PPM Technology/PPM Formaldmeter htv-m | Electrochemical | 0–10 ppm | 0.01 ppm |
| TVOC | RAE/ppbRAE 3000-10.6 eV | Photo-ionization detector | 1ppb–10,000 ppm | 1 ppb |
| Bacteria/ fungi | Thermo/Anderson one-stage sampler | Impacting on agar with incubation (Q: 28.3 LPM) | Stage 0 (8–24 μm) Stage 1 (1–8 μm) | - |
| Item | Test Condition | High Conc./ Low RH | High Conc./ High RH | Low Conc./ High RH | ||||
|---|---|---|---|---|---|---|---|---|
| 1.0 ppm/40% RH 24 ± 1 °C | 1.0 ppm/70% RH 24 ± 1 °C | 0.5 ppm/70% RH 24 ± 1 °C | ||||||
| HCHO | Removal efficiency (%) | #1 | #2 | #1 | #2 | #1 | #2 | |
| 32.54 | 32.66 | 15.95 | 15.99 | 18.39 | 17.88 | |||
| Avg. ± S.D. (%) | 32.6 ± 0.09 | 15.97 ± 0.03 | 18.14 ± 0.36 | |||||
| Removal of percentage error (%) | −0.36 | −0.25 | 2.77 | |||||
| TVOC | Test condition | 3.0 ppm/40%RH | 3.0 ppm/70%RH | 1.4 ppm/70%RH | ||||
| Removal efficiency (%) | 13.61 | 13.5 | 7.24 | 7.00 | 6.10 | 5.75 | ||
| Avg. ± S.D. (%) | 13.56 ± 0.08 | 7.12 ± 0.17 | 5.93 ± 0.25 | |||||
| Removal of percentage error (%) | 4.81 | 3.31 | 5.74 | |||||
| Air Pollutant | HCHO | TVOC | Bacteria | Fungi | |
|---|---|---|---|---|---|
| UVGI Luminaires | |||||
| Upper space irradiation | 0.606 | 0.212 | 0.500 | 0.007 ** | |
| Direct irradiation over night | 0.103 | 0.404 | 0.031 * | 0.035 | |
| Upward irradiation | 0.109 | 0.268 | 0.027 * | 0.244 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-Y.; Tseng, C.-H.; Wang, H.-C.; Dai, C.-F.; Shih, Y.-H. The Study of an Ultraviolet Radiation Technique for Removal of the Indoor Air Volatile Organic Compounds and Bioaerosol. Int. J. Environ. Res. Public Health 2019, 16, 2557. https://doi.org/10.3390/ijerph16142557
Liu C-Y, Tseng C-H, Wang H-C, Dai C-F, Shih Y-H. The Study of an Ultraviolet Radiation Technique for Removal of the Indoor Air Volatile Organic Compounds and Bioaerosol. International Journal of Environmental Research and Public Health. 2019; 16(14):2557. https://doi.org/10.3390/ijerph16142557
Chicago/Turabian StyleLiu, Chao-Yun, Chao-Heng Tseng, Huang-Chin Wang, Chuan-Fa Dai, and Yi-Hsuan Shih. 2019. "The Study of an Ultraviolet Radiation Technique for Removal of the Indoor Air Volatile Organic Compounds and Bioaerosol" International Journal of Environmental Research and Public Health 16, no. 14: 2557. https://doi.org/10.3390/ijerph16142557




