Evaluation of Microbiological and Free-Living Protozoa Contamination in Dental Unit Waterlines
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Location
2.2. Water Sampling and Microbiological Analysis
2.3. Statistical Analysis
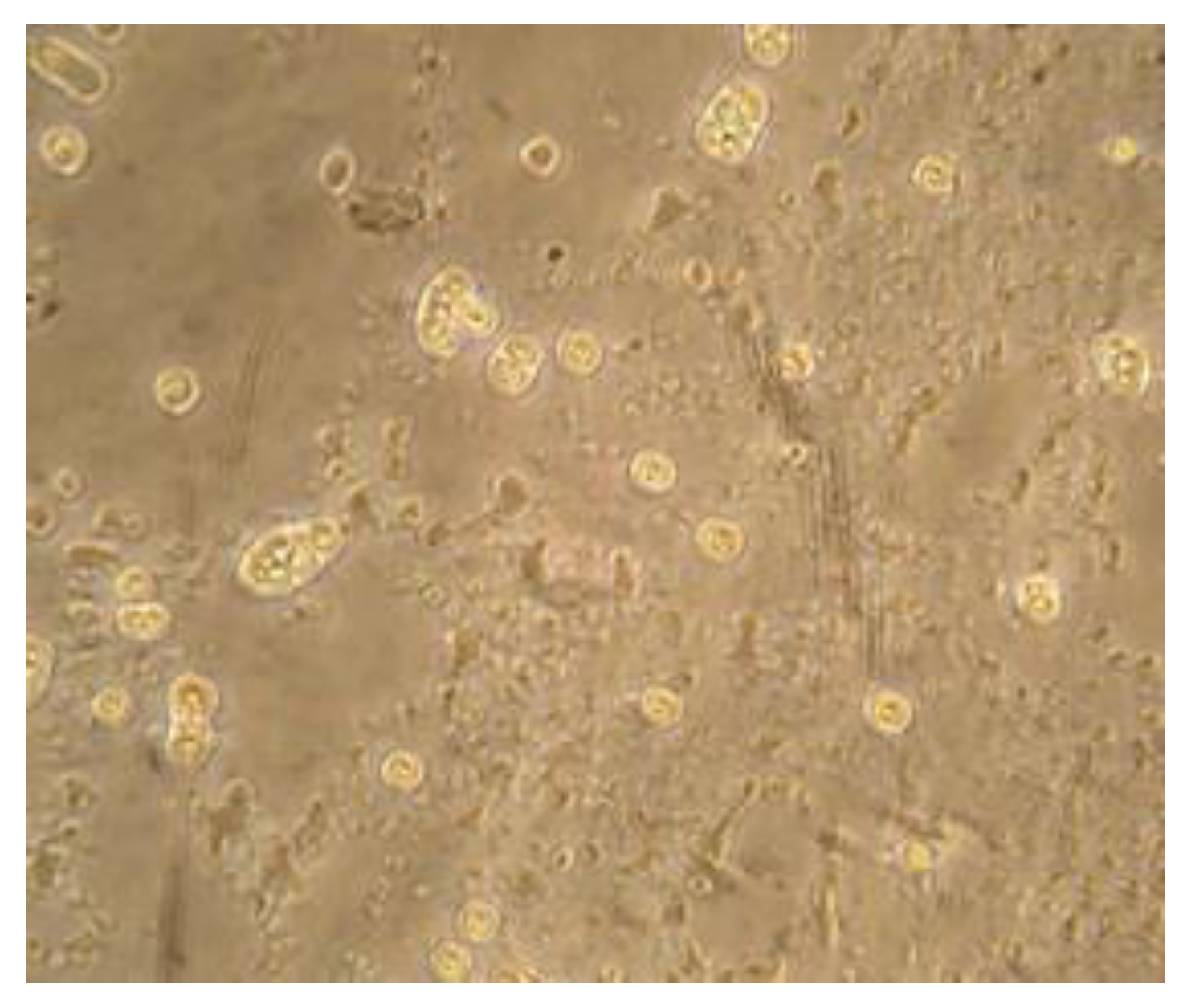
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujita, M.; Mashima, I.; Nakazawa, F. Monitoring the decontamination efficacy of the novel Poseidon-S disinfectant system in dental unit water lines. J. Microbiol. Immunol. Infect. 2015, 50, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.G.; Harte, J.A.; Malvitz, D.M.; Collins, A.S.; Cleveland, J.L.; Eklund, K.J.; Centers for Disease Control and Prevention. Guidelines for infection control in dental health care settings-2003. J. Am. Dent. Assoc. 2004, 135, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Dallera, M.; Ottria, G.; Perdelli, F.; Orlando, P. Investigation of organizational and hygiene features in dentistry: A pilot study. J. Prev. Med. Hyg. 2009, 50, 175–180. [Google Scholar] [PubMed]
- Costa, D.; Mercier, A.; Gravouil, K.; Lesobre, J.; Delafont, V.; Bousseau, A.; Verdon, J.; Imbert, C. Pyrosequencing analysis of bacterial diversity in dental unit waterlines. Water Res. 2015, 81, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.T.; Bradshaw, D.J.; Bennett, A.M.; Fulford, M.R.; Martin, M.V.; Marsh, P.D. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 2000, 66, 3363–3367. [Google Scholar] [CrossRef]
- Leoni, E.; Dallolio, L.; Stagni, F.; Sanna, T.; D’Alessandro, G.; Piana, G. Impact of a risk management plan on Legionella contamination of dental unit water. Int. J. Environ. Res. Public Health 2015, 12, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.T.; Marsh, P.D. Microbial biofilm formation in DUWS and their control using disinfectants. J. Dent. 2007, 35, 721–730. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Boyle, M.A.; Russell, R.J.; Coleman, D.C. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011, 6, 1209–1226. [Google Scholar] [CrossRef]
- Lizzadro, J.; Mazzotta, M.; Girolamini, L.; Dormi, A.; Pellati, T.; Cristino, S. Comparison between Two Types of Dental Unit Waterlines: How Evaluation of Microbiological Contamination Can Support Risk Containment. Int. J. Environ. Res. Public Health 2019, 16, 328. [Google Scholar] [CrossRef]
- Costa, D.; Bossard, V.; Brunet, K.; Fradin, B.; Imbert, C. Planktonic free-living amoebae susceptibility to dental unit waterlines disinfectants. Pathog. Dis. 2017, 75, 75. [Google Scholar] [CrossRef]
- Lal, S.; Singhrao, S.K.; Achilles-Day, U.E.; Morton, L.H.; Pearce, M.; Crean, S. Risk Assessment for the Spread of Serratia marcescens Within Dental-Unit Waterline Systems Using Vermamoeba vermiformis. Curr. Microbiol. 2015, 71, 434–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vanessa, B.; Virginie, M.; Nathalie, Q.; Marie-Hélène, R.; Christine, I. Hartmannella vermiformis can promote proliferation of Candida spp. in tap-water. Water Res. 2012, 46, 5707–5714. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, J.; Buhler, T. Biofilms augment the number of free-living amoebae in dental unit waterlines. Res. Microbiol. 2001, 152, 753–760. [Google Scholar] [CrossRef]
- Dillon, A.; Achilles-Day, U.E.; Singhrao, S.K.; Pearce, M.; Morton, L.H.G.; Crean, S. Biocide sensitivity of Vermamoeba vermiformis isolated from dental-unit-waterline systems. Int. Biodeterior. Biodegrad. 2014, 88, 97–105. [Google Scholar] [CrossRef]
- Leduc, A.; Gravel, S.; Abikhzer, J.; Roy, S.; Barbeau, J. Polymerase chain reaction detection of potentially pathogenic free-living amoebae in dental units. Can. J. Microbiol. 2012, 58, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Dallera, M.; Ottria, G.; Lombardi, R.; Perdelli, F. Evaluation of the risk of infection through exposure to aerosols and spatters in dentistry. Am. J. Infect. Control. 2008, 36, 304–307. [Google Scholar] [CrossRef]
- Perdelli, F.; Spagnolo, A.M.; Cristina, M.L.; Sartini, M.; Malcontenti, R.; Dallera, M.; Ottria, G.; Lombardi, R.; Orlando, P. Evaluation of contamination by blood aerosols produced during various healthcare procedures. J. Hosp. Infect. 2008, 70, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Hack, A. Microbial contamination of dental unit waterlines in dental practices in Hesse, Germany: A cross-sectional study. Eur. J. Microbiol. Immunol. 2013, 3, 49–52. [Google Scholar] [CrossRef]
- Ricci, M.L.; Fontana, S.; Pinci, F.; Fiumana, E.; Pedna, M.F.; Farolfi, P.; Sabattini, M.A.; Scaturro, M. Pneumonia associated with a dental unit waterline. Lancet 2012, 379, 684. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Health Association Inc.: New York, NY, USA, 2005. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality—Detection and Enumeration of Legionella—Part 2: Direct Membrane Filtration Method for Waters with Low Bacterial Counts; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality: Enumeration of Culturable Micro-Organisms, Colony Count by Inoculation in a Nutrient Agar Culture Medium; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality—Enumeration of Escherichia Coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality—Detection and Enumeration of Pseudomonas Aeruginosa—Method by Membrane Filtration; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Montalbano Di Filippo, M.; Santoro, M.; Lovreglio, P.; Monno, R.; Capolongo, C.; Calia, C.; Fumarola, L.; D’Alfonso, R.; Berrilli, F.; Di Cave, D. Isolation and molecular characterization of free-living amoebae from different water sources in Italy. Int. J. Environ. Res. Public Health 2015, 12, 3417–3427. [Google Scholar] [CrossRef]
- Tsvetkova, N.; Schild, M.; Panaiotov, S.; Kurdova-Mintcheva, R.; Gottstein, B.; Walochnik, J.; Aspock, H.; Lucas, M.S.; Muller, N. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol. Res. 2004, 92, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé e des Solidarités. L’eau Dans les Etablissements de Santé. 2007. Available online: http://nosobase.chu-lyon.fr/Reglementation/2005/guide_eau_etabs.pdf (accessed on 17 June 2016).
- Casini, B.; Buzzigoli, A.; Cristina, M.L.; Spagnolo, A.M.; Del Giudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Valentini, P.; Baggiani, A.; et al. Long-term effects of hospital water network disinfection on Legionella and other waterborne bacteria in an Italian university hospital. Infect. Control Hosp. Epidemiol. 2014, 35, 293–299. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association. ADA Statement on Dental unit waterlines. J. Am. Dent. Assoc. 1996, 127, 185–186. [Google Scholar] [CrossRef]
- Orlando, P.; Cristina, M.L.; Dallera, M.; Ottria, G.; Vitale, A.; Badolati, G. Surface disinfection: Evaluation of the efficacy of a nebulization system spraying hydrogen peroxide. J. Prev. Med. Hyg. 2008, 49, 116–119. [Google Scholar] [PubMed]
- Estrich, C.G.; Gruninger, S.E.; Lipman, R.D. Rates and predictors of exposure to Legionella pneumophila in the United States among dental practitioners: 2002 through 2012. J. Am. Dent. Assoc. 2017, 148, 164–171. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Napoli, C.; Diella, G.; Rutigliano, S.; Agodi, A.; Auxilia, F.; Baldovin, T.; Bisetto, F.; Arnoldo, L.; et al. Control and prevention measures for legionellosis in hospitals: A cross-sectional survey in Italy. Environ. Res. 2018, 166, 55–60. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Cristina, M.L.; Napoli, C.; Pacifico, C.; Agodi, A.; Baldovin, T.; Casini, B.; Coniglio, M.A.; D’Errico, M.M.; et al. Evaluation of Legionella Air Contamination in Healthcare Facilities by Different Sampling Methods: An Italian Multicenter Study. Int. J. Environ. Res. Public Health 2017, 14, 670. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Cristina, M.L.; Albertini, R.; Pasquarella, C.; GISIO-SItI Working Group; AIA Working Group; SIMPIOS Working Group; Agodi, A.; Coniglio, M.A. Legionella indoor air contamination in healthcare environments. In SpringerBriefs in Public Health; Springer: Cham, Switzerland, 2017; pp. 63–71. [Google Scholar]
- Montagna, M.T.; De Giglio, O.; Napoli, C.; Cannova, L.; Cristina, M.L.; Deriu, M.G.; Delia, S.A.; Giuliano, A.; Guida, M.; Laganà, P.; et al. Legionella spp. contamination in indoor air: Preliminary results of an Italian multicenter study. Epidemiol. Prev. 2014, 38, 62–65. [Google Scholar]
- Pankhurst, C.L.; Coulter, W.A. Do contaminated dental unit waterlines pose a risk of infection? J. Dent. 2007, 35, 712–720. [Google Scholar] [CrossRef]
- Carinci, F.; Scapoli, L.; Contaldo, M.; Santoro, R.; Palmieri, A.; Pezzetti, F.; Lauritano, D.; Candotto, V.; Mucchi, D.; Baggi, L.; et al. Colonization of Legionella spp. in dental unit waterlines. J. Biol. Regul. Homeost. Agents 2018, 32, 139–142. [Google Scholar]
- Montagna, M.T.; Cristina, M.L.; De Giglio, O.; Spagnolo, A.M.; Napoli, C.; Cannova, L.; Deriu, M.G.; Delia, S.A.; Giuliano, A.; Guida, M.; et al. Serological and molecular identification of Legionella spp. isolated from water and surrounding air samples in Italian healthcare facilities. Environ. Res. 2016, 146, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Dietersdorfer, E.; Cervero-Aragó, S.; Sommer, R.; Kirschner, AK.; Walochnik, J. Optimized methods for Legionella pneumophila release from its Acanthamoeba hosts. BMC Microbiol. 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Marciano-Cabral, F.; Jamerson, M.; Kaneshiro, E.S. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J. Water Health 2010, 8, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, A.M.; Orlando, P.; Perdelli, F.; Cristina, M.L. Hospital water and prevention of waterborne infections. Rev. Med. Microbiol. 2016, 27, 25–32. [Google Scholar] [CrossRef]
- Balczun, C.; Scheid, P.L. Free-Living Amoebae as Hosts for and Vectors of Intracellular Microorganisms with Public Health Significance. Viruses 2017, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Feldman, H.A. Isolation of Hartmannella species from human throats. N. Engl. J. Med. 1967, 277, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Michel, R.; Just, H.M. Acanthamoebae, Naegleria and other free-living Amoebae in cooling and rinsing water of dental treatment units. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 1984, 79, 56–72. [Google Scholar]
- Rogerson, A.; Berger, J. Effect of crude oil and petroleum-degrading micro-organisms on the growth of freshwater and soil protozoa. Microbiology 1981, 124, 53–59. [Google Scholar] [CrossRef][Green Version]
- Weitere, M.; Bergfeld, T.; Scott, S.A.; Matz, C.; Kjelleberg, S. Grazing resistance of Pseudomonas aeruginosa biofilms depends on type of protective mechanism, developmental stage and protozoan feeding mode. Environ. Microbiol. 2005, 7, 1593–1601. [Google Scholar] [CrossRef]
- Groscop, J.A.; Brent, M.M. The effects of selected strains of pigmented microorganisms on small free-living amoebae. Can. J. Microbiol. 1964, 10, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.N.; Perez, A.A.; Madayag, R.M.; Bottone, E.J. Inhibition of Acanthamoeba species by Pseudomonas aeruginosa: Rationale for their selective exclusion in corneal ulcers and contact lens care systems. J. Clin. Microbiol. 1993, 31, 1908–1910. [Google Scholar] [PubMed]
- Singh, R.N. The selection of bacterial food by soil amoebae and the toxic effects of bacterial pigments and other products on soil protozoa. Br. J. Exp. Pathol. 1945, 26, 316–325. [Google Scholar]
- Delafont, V.; Rodier, M.H.; Maisonneuve, E.; Cateau, E. Vermamoeba vermiformis: A Free-Living Amoeba of Interest. Microb. Ecol. 2018, 76, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, A.M.; Cristina, M.L.; Casini, B.; Perdelli, F. Legionella pneumophila in healthcare facilities. Rev. Med. Microbiol. 2013, 24, 70–80. [Google Scholar] [CrossRef]
- Perdelli, F.; Dallera, M.; Cristina, M.L.; Sartini, M.; Ottria, G.; Spagnolo, A.M.; Orlando, P. A new microbiological problem in intensive care units: Environmental contamination by MRSA with reduced susceptibility to glycopeptides. Int. J. Hyg. Environ. Health 2008, 211, 213–218. [Google Scholar] [CrossRef]
- Ministero della Salute. Linee Guida Per la Prevenzione ed il Controllo Della Legionellosi; Ministero Della Salute: Rome, Italy, 2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 17 June 2016). (In Italian)
- Montebugnoli, L.; Dolci, G. A new chemical formulation for control of dental unit water line contamination: An in vitro and clinical study. BMC Oral Health 2002, 2, 1. [Google Scholar] [CrossRef]
- Rice, E.W.; Rich, W.K.; Johnson, C.H.; Lye, D.J. The role of flushing dental water lines for the removal of microbial contaminants. Public Health Rep. 2006, 121, 270–274. [Google Scholar] [CrossRef]

| Mean ± SD | Min–Max | Median | Interquartile Range | p Value | ||
|---|---|---|---|---|---|---|
| HPCs at 22 °C | Hand-piece | 1168.53 ± 906.00 | 145–3200 | 881.5 | 548–1710 | <0.001 |
| Tap water | 385.27 ± 836.41 | 2–2880 | 37.5 | 15–200 | ||
| HPCs at 36 °C | Hand-piece | 827.90 ± 746.87 | 27–2116 | 518.5 | 109–1620 | <0.001 |
| Tap water | 242.93 ± 241.64 | 2–1860 | 9.5 | 5–121 | ||
| P. aeruginosa | Hand-piece | 25.13 ± 77.75 | 0–308 | 0 | 0–0 | 0.26 |
| Tap water | 0 | 0–0 | 0 | 0–0 | ||
| L. pneumophila | Hand-piece | 676.67 ± 746.34 | 0–2700 | 350 | 200–1300 | 0.15 |
| Tap water | 343.33 ± 313.69 | 0–900 | 300 | 0–700 |
| Mean ± SD | Min-Max | Median | Interquartile Range | |||
|---|---|---|---|---|---|---|
| L. pneumophila | Hand-pieces | No amoebae | 333.33 ± 405.27 | 0–1400 | 200 | 100–400 |
| Amoebae | 905.56 ± 839.80 | 0–2700 | 550 | 300–1500 | ||
| Tap water | No amoebae | 321.74 ± 293.81 | 0–900 | 300 | 0–500 | |
| Amoebae | 414.29 ± 389.14 | 0–800 | 700 | 0–700 | ||
| P. aeruginosa | Hand-pieces | No amoebae | 46.50 ± 109.30 | 0–308 | 0 | 0–0 |
| Amoebae | 10.89 ± 45.21 | 0–192 | 0 | 0–0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, A.M.; Sartini, M.; Di Cave, D.; Casini, B.; Tuvo, B.; Cristina, M.L. Evaluation of Microbiological and Free-Living Protozoa Contamination in Dental Unit Waterlines. Int. J. Environ. Res. Public Health 2019, 16, 2648. https://doi.org/10.3390/ijerph16152648
Spagnolo AM, Sartini M, Di Cave D, Casini B, Tuvo B, Cristina ML. Evaluation of Microbiological and Free-Living Protozoa Contamination in Dental Unit Waterlines. International Journal of Environmental Research and Public Health. 2019; 16(15):2648. https://doi.org/10.3390/ijerph16152648
Chicago/Turabian StyleSpagnolo, Anna Maria, Marina Sartini, David Di Cave, Beatrice Casini, Benedetta Tuvo, and Maria Luisa Cristina. 2019. "Evaluation of Microbiological and Free-Living Protozoa Contamination in Dental Unit Waterlines" International Journal of Environmental Research and Public Health 16, no. 15: 2648. https://doi.org/10.3390/ijerph16152648
APA StyleSpagnolo, A. M., Sartini, M., Di Cave, D., Casini, B., Tuvo, B., & Cristina, M. L. (2019). Evaluation of Microbiological and Free-Living Protozoa Contamination in Dental Unit Waterlines. International Journal of Environmental Research and Public Health, 16(15), 2648. https://doi.org/10.3390/ijerph16152648







