CO2 and N2O Emissions from Spring Maize Soil under Alternate Irrigation between Saline Water and Groundwater in Hetao Irrigation District of Inner Mongolia, China
Abstract
:1. Introduction
2. Materials and Methods
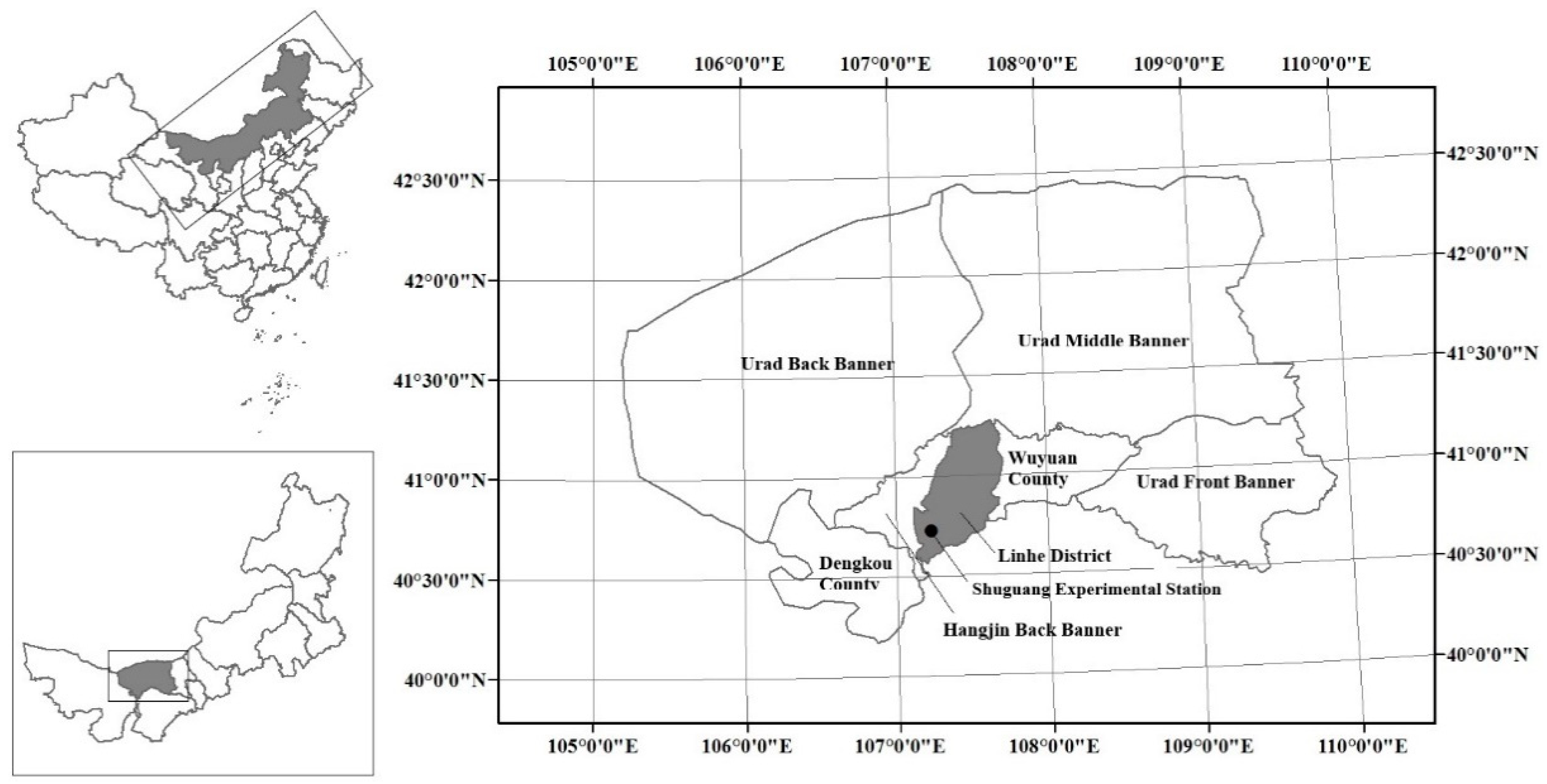
2.1. Study Site
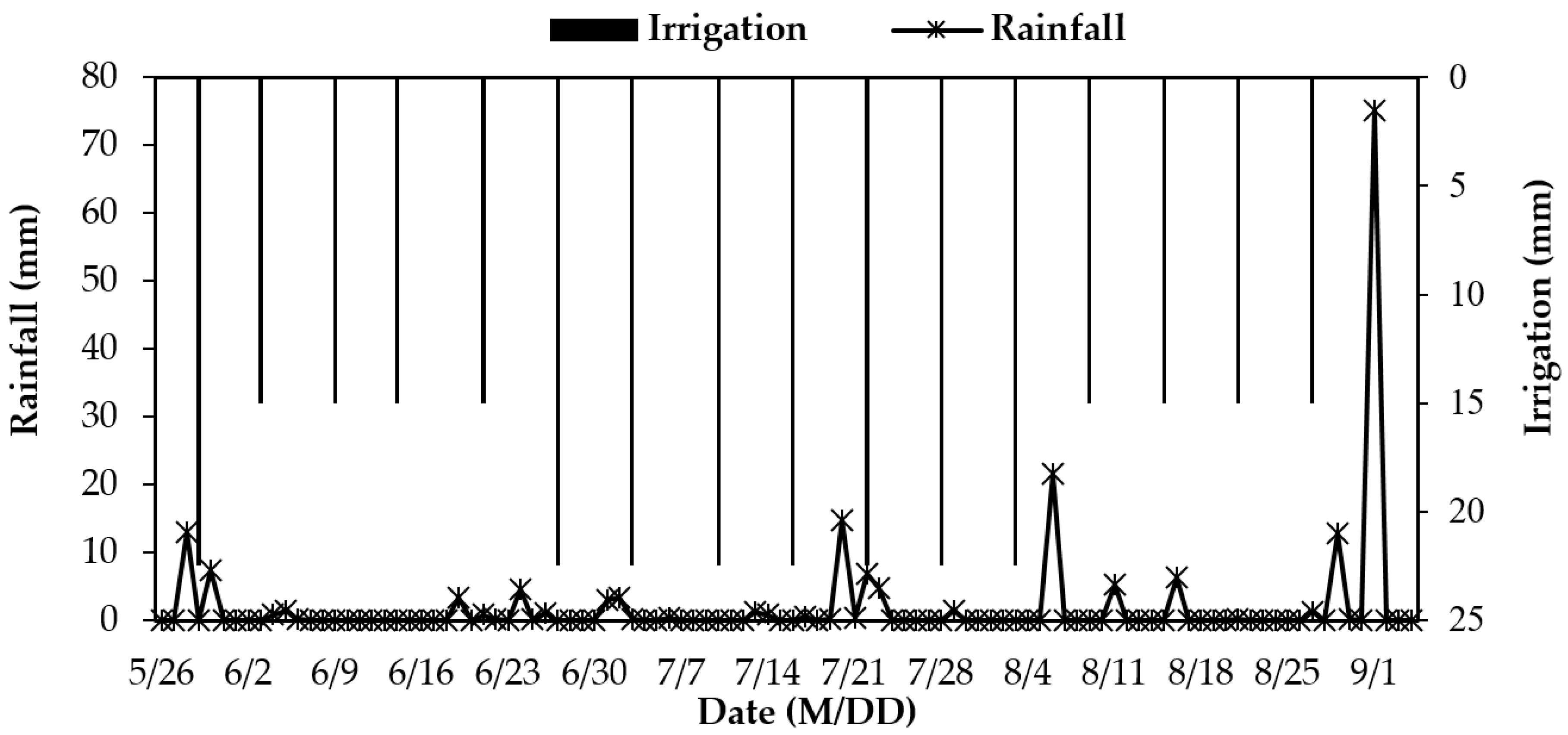
2.2. Experimental Design
2.3. Sample Collection and Measurement
2.4. Calculation Method of Gas Emission
2.5. Determination of the GWP
2.6. Statistical Analysis
3. Results
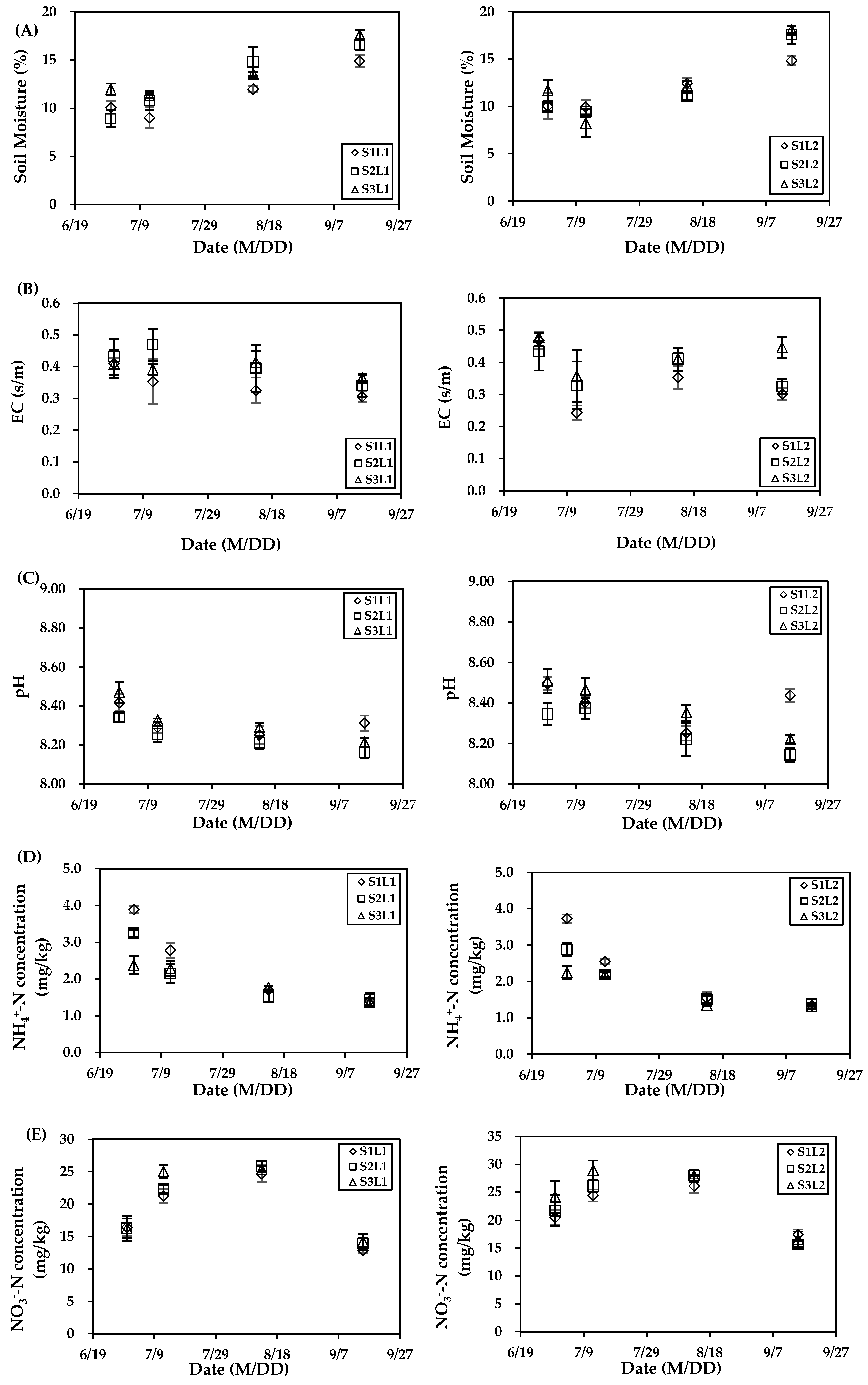
3.1. Soil Properties
3.2. Daily Gas Flux
3.3. Cumulative Greenhouse Gas Emission
3.4. Global Warming Potential
3.5. Maize Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WMO. World Meteorological Organization, Global Ozone Research and Monitoring Project—Report No. 58; World Meteorological Organization: Geneva, Switzerland, 2018; p. 67. [Google Scholar]
- WMO. World Meteorological Organization Global Ozone Research and Monitoring Project—Report No. 55; World Meteorological Organization: Geneva, Switzerland, 2014; p. 416. [Google Scholar]
- WMO Greenhouse Gas Bulletin—No. 14. Available online: https://public.wmo.int/en/resources/library/wmo-greenhouse-gas-bulletin-no-14 (accessed on 3 June 2019).
- The World Bank: Agricultural Methane Emissions (% of Total). Available online: https://data.worldbank. org/indicator/EN.ATM.METH.AG.ZS?locations=CN (accessed on 3 June 2019).
- The World Bank: Agricultural Nitrous Oxide Emissions (% of Total). Available online: https://data. worldbank.org/indicator/EN.ATM.NOXE.AG.ZS (accessed on 3 June 2019).
- Zou, X.; Li, Y.E.; Li, K.; Cremades, R.; Gao, Q.; Wan, Y.; Qin, X. Greenhouse gas emissions from agricultural irrigation in China. Mitig. Adapt. Strateg. Glob. Chang. 2013, 20, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Minhas, P.S. Strategies for managing saline/alkali waters for sustainable agricultural production in South Asia. Agric. Water Manag. 2005, 78, 136–151. [Google Scholar] [CrossRef]
- Morales-Garcia, D.; Stewart, K.A.; Seguin, P.; Madramootoo, C. Supplemental saline drip irrigation applied at different growth stages of two bell pepper cultivars grown with or without mulch in non-saline soil. Agric. Water Manag. 2011, 98, 893–898. [Google Scholar] [CrossRef]
- Rameshwaran, P.; Tepe, A.; Yazar, A.; Ragab, R. Effects of drip-irrigation regimes with saline water on pepper productivity and soil salinity under greenhouse conditions. Sci. Hortic. 2016, 199, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, L.; Wang, B.; Shao, H.; Zhang, L.; Qin, X. Co-effects of salinity and moisture on CO2 and N2O emissions of laboratory-incubated salt-affected soils from different vegetation types. Geoderma 2018, 332, 109–120. [Google Scholar] [CrossRef]
- Zhang, L.H.; Song, L.P.; Zhang, L.W.; Shao, H.B. Diurnal dynamics of CH4, CO2 and N2O fluxes in the saline-alkaline soils of the Yellow River Delta, China. Plant Biosyst. 2014, 149, 797–805. [Google Scholar] [CrossRef]
- Nyman, J.A.; DeLaune, R.D. CO2 emission and Eh responses to different hydrological conditions in fresh, brackish, and saline marsh soils. Limnol. Oceanogr. 1991, 36, 1406–1414. [Google Scholar] [CrossRef]
- Yang, W.; Yang, M.; Wen, H.; Jiao, Y. Global Warming Potential of CH4 uptake and N2O emissions in saline–alkaline soils. Atmos. Environ. 2018, 191, 172–180. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Li, Z.; Wang, S.; Qu, Y. Effects of Irrigation Regime and Nitrogen Fertilizer Management on CH4, N2O and CO2 Emissions from Saline–Alkaline Paddy Fields in Northeast China. Sustainability 2018, 10, 475. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, G.; Li, Q.; Liao, N.; Guo, H.; Min, W.; Ma, L.; Ye, J.; Hou, Z. Saline water irrigation stimulate N2O emission from a drip-irrigated cotton field. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2015, 66, 141–152. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Hooijdonk, J.V. Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativus L.). Plant Soil 1999, 215, 57–64. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Zhang, X.; Tian, P.; Li, J.; Tian, Y. Comprehensive comparison of different saline water irrigation strategies for tomato production: Soil properties, plant growth, fruit yield and fruit quality. Agric. Water Manag. 2019, 213, 521–533. [Google Scholar] [CrossRef]
- Li, W.; Liu, R.; Zheng, H.; Li, S.; Gao, F.; Li, J. Study on Index System of Optimal Fertilizer Recommendation for Spring Corn in Hetao Irrigation Area of Inner Mongolia. Sci. Agric. Sin. 2012, 45, 93–101. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Su, Y.; Shang, F.; Wei, C.; Ren, S. Effects of alternative irrigation between brackish water and fresh water on CO2 and N2O emission from spring maize soil. J. China Agric. Univ. 2018, 23, 41–48. (In Chinese) [Google Scholar] [CrossRef]
- Mosier, A.R.; Hutchinson, G.L. Nitrous Oxide Emissions from Cropped Fields 1. J. Environ. Qual. 1981, 10, 169–173. [Google Scholar] [CrossRef]
- Ball, B.C.; Horgan, G.W.; Clayton, H.; Parker, J.P. Spatial variability of nitrous oxide fluxes and controlling soil and topographic properties. J. Environ. Qual. 1997, 26, 1399–1409. [Google Scholar] [CrossRef]
- Junna, S.; Bingchen, W.; Gang, X.; Hongbo, S. Effects of wheat straw biochar on carbon mineralization and guidance for large-scale soil quality improvement in the coastal wetland. Ecol. Eng. 2014, 62, 43–47. [Google Scholar] [CrossRef]
- Adviento-Borbe, M.A.A.; Kaye, J.P.; Bruns, M.A.; McDaniel, M.D.; McCoy, M.; Harkcom, S. Soil Greenhouse Gas and Ammonia Emissions in Long-Term Maize-Based Cropping Systems. Soil Sci. Soc. Am. J. 2010, 74. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Cambridge University Press. Available online: http://www.ipcc.ch/report/ar5/wg1/ (accessed on 3 June 2019).
- Robertson, G.P.; Paul, E.A.; Harwood, R.R. Greenhouse Gases in Intensive Agriculture: Contributions of Individual Gases to the Radiative Forcing of the Atmosphere. Science 2000, 289, 1922–1925. [Google Scholar] [CrossRef] [Green Version]
- Ellert, B.; Janzen, H.H. Nitrous oxide, carbon dioxide and methane emissions from irrigated cropping systems as influenced by legumes, manure and fertilizer. Can. J. Soil Sci. 2008, 88, 207–217. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D. Stability of black carbon in soils across a climatic gradient. J. Geophys. Res.-Biogeosci. 2008, 113, n. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Aciego Pietri, J.C.; Brookes, P.C. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D.; Verma, V. Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biol. Biochem. 2011, 43, 667–674. [Google Scholar] [CrossRef]
- Maucieri, C.; Zhang, Y.; McDaniel, M.D.; Borin, M.; Adams, M.A. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 2017, 307, 267–276. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Bengtson, P.; Rousk, J. Comparative Toxicities of Salts on Microbial Processes in Soil. Appl. Environ. Microbiol. 2016, 82, 2012–2020. [Google Scholar] [CrossRef] [Green Version]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D.; Smith, P.; Smith, J. Salinity effects on carbon mineralization in soils of varying texture. Soil Biol. Biochem. 2011, 43, 1908–1916. [Google Scholar] [CrossRef]
- Wong, V.N.L.; Dalal, R.C.; Greene, R.S.B. Carbon dynamics of sodic and saline soils following gypsum and organic material additions: A laboratory incubation. Appl. Soil Ecol. 2009, 41, 29–40. [Google Scholar] [CrossRef]
- Marton, J.M.; Herbert, E.R.; Craft, C.B. Effects of Salinity on Denitrification and Greenhouse Gas Production from Laboratory-incubated Tidal Forest Soils. Wetlands 2012, 32, 347–357. [Google Scholar] [CrossRef]
- McClung, G.; Frankenberger, W.T.J. Nitrogen mineralization rates in saline vs. salt-amended soils. Plant Soil 1987, 104, 13–21. [Google Scholar] [CrossRef]
- Mkhabela, M.S.; Gordon, R.; Burton, D.; Madani, A.; Hart, W.; Elmi, A. Ammonia and nitrous oxide emissions from two acidic soils of Nova Scotia fertilised with liquid hog manure mixed with or without dicyandiamide. Chemosphere 2006, 65, 1381–1387. [Google Scholar] [CrossRef]
- Zheng, X.; Han, S.; Huang, Y.; Wang, Y.; Wang, M. Re-quantifying the emission factors based on field measurements and estimating the direct N2O emission from Chinese croplands. Glob. Biogeochem. Cycle 2004, 18. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Silva, C.C.; Guido, M.L.; Ceballos, J.M.; Marsch, R.; Dendooven, L. Production of carbon dioxide and nitrous oxide in alkaline saline soil of Texcoco at different water contents amended with urea: A laboratory study. Soil Biol. Biochem. 2008, 40, 1813–1822. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sanchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef]
- Šimek, M.; Jıšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Thapa, R.; Chatterjee, A.; Wick, A.; Butcher, K. Carbon Dioxide and Nitrous Oxide Emissions from Naturally Occurring Sulfate-Based Saline Soils at Different Moisture Contents. Pedosphere 2017, 27, 868–876. [Google Scholar] [CrossRef]
- Inubushi, K.; Barahona, M.; Yamakawa, K.J.B.; Soils, F.O. Effects of salts and moisture content on N2O emission and nitrogen dynamics in Yellow soil and Andosol in model experiments. Biol. Fertil. Soils 1999, 29, 401–407. [Google Scholar] [CrossRef]
- Kanako, K.; Ronggui, H.; Sawamoto, T.; Hatano, R. Three years of nitrous oxide and nitric oxide emissions from silandic andosols cultivated with maize in Hokkaido, Japan. Soil Sci. Plant Nutr. 2006, 52, 103–113. [Google Scholar] [CrossRef]
- Setschenow, J. Über die konstitution der salzlösungen auf grund ihres verhaltens zu kohlensäure. Zu Kohlensäure. Z. Phys. Chem. 1889, 4, 117–125. [Google Scholar] [CrossRef]



| Moisture | EC | pH | NH4+-N | NO3−-N | CO2 | N2O | |
|---|---|---|---|---|---|---|---|
| Moisture | 1 | - | - | - | - | - | - |
| EC | 0.448 | 1 | - | - | - | - | - |
| pH | −0.128 | −0.048 | 1 | - | - | - | - |
| NH4+-N | −0.587 * | −0.473 * | 0.019 | 1 | - | - | - |
| NO3−-N | −0.110 | 0.317 | 0.399 | −0.523 * | 1 | - | - |
| CO2 | −0.121 | −0.372 | 0.347 | - | - | 1 | - |
| N2O | 0.594 ** | 0.539 * | 0.319 | −0.758 ** | 0.503 * | - | 1 |
| Treatments | Gases | |||
|---|---|---|---|---|
| CO2 (2017) (t·hm−2) | N2O (2017) (kg·hm 2) | CO2 (2018) (t·hm−2) | N2O (2018) (kg·hm 2) | |
| S1L1 | 15,701.37 ± 1931.76 a | 1.02 ± 0.18 a | 19,206.77 ± 271.04 a | 1.01 ± 0.03 a |
| S2L1 | 12,047.80 ± 985.34 b | 1.06 ± 0.06 a | 16,900.17 ± 363.35 b | 1.09 ± 0.08 bc |
| S3L1 | 11,461.20 ± 1737.17 b | 0.87 ± 0.20 a | 16,587.64 ± 1204.97 bc | 1.28 ± 0.07 b |
| S1L2 | 15,283.79 ± 1022.61 a | 0.99 ± 0.15 a | 19,805.29 ± 518.01 a | 1.04 ± 0.05 c |
| S2L2 | 10,181.29 ± 828.66 b | 0.89 ± 0.09 a | 15,969.49 ± 1011.29 bc | 1.11 ± 0.14 bc |
| S3L2 | 9840.09 ± 1003.86 b | 0.79 ± 0.06 a | 15,057.84 ± 708.78 c | 1.53 ± 0.09 a |
| Year | Treatments | |||||
|---|---|---|---|---|---|---|
| S1L1 | S2L1 | S3L1 | S1L2 | S2L2 | S3L2 | |
| 2017 | 15972.05 ± 1980.45 a | 12328.94 ± 1002.46 b | 11690.74 ± 1789.48 b | 15546.50 ± 1061.35 a | 10416.32 ± 851.45 b | 10048.33 ± 1018.94 b |
| 2018 | 19473.25 ± 280.15 a | 17189.61 ± 385.74 b | 16927.00 ± 1224.56 b | 20081.84 ± 530.17 a | 16263.83 ± 1049.12 b | 15464.18 ± 733.18 b |
| Year | Treatments | |||||
|---|---|---|---|---|---|---|
| S1L1 | S2L1 | S3L1 | S1L2 | S2L2 | S3L2 | |
| 2017 | 15165.66 ± 660.52 a | 13676.09 ± 394.04 bc | 13361.77 ± 734.98 bc | 14376.26 ± 834.68 ab | 14113.59 ± 375.38 abc | 12869.31 ± 222.15 c |
| 2018 | 15418.40 ± 304.02 a | 14354.73 ± 891.39 ab | 14066.00 ± 595.84 b | 14481.80 ± 368.06 ab | 12920.57 ± 471.48 c | 12215.50 ± 163.97 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, P.; Ren, S.; He, X.; Wei, C.; Wang, S.; Xu, Y.; Xu, Z.; Zhang, Y.; Ismail, H. CO2 and N2O Emissions from Spring Maize Soil under Alternate Irrigation between Saline Water and Groundwater in Hetao Irrigation District of Inner Mongolia, China. Int. J. Environ. Res. Public Health 2019, 16, 2669. https://doi.org/10.3390/ijerph16152669
Wang Y, Yang P, Ren S, He X, Wei C, Wang S, Xu Y, Xu Z, Zhang Y, Ismail H. CO2 and N2O Emissions from Spring Maize Soil under Alternate Irrigation between Saline Water and Groundwater in Hetao Irrigation District of Inner Mongolia, China. International Journal of Environmental Research and Public Health. 2019; 16(15):2669. https://doi.org/10.3390/ijerph16152669
Chicago/Turabian StyleWang, Yu, Peiling Yang, Shumei Ren, Xin He, Chenchen Wei, Shuaijie Wang, Yao Xu, Ziang Xu, Yanxia Zhang, and Hassan Ismail. 2019. "CO2 and N2O Emissions from Spring Maize Soil under Alternate Irrigation between Saline Water and Groundwater in Hetao Irrigation District of Inner Mongolia, China" International Journal of Environmental Research and Public Health 16, no. 15: 2669. https://doi.org/10.3390/ijerph16152669
APA StyleWang, Y., Yang, P., Ren, S., He, X., Wei, C., Wang, S., Xu, Y., Xu, Z., Zhang, Y., & Ismail, H. (2019). CO2 and N2O Emissions from Spring Maize Soil under Alternate Irrigation between Saline Water and Groundwater in Hetao Irrigation District of Inner Mongolia, China. International Journal of Environmental Research and Public Health, 16(15), 2669. https://doi.org/10.3390/ijerph16152669






