Chronic Fluoride Exposure and the Risk of Autism Spectrum Disorder
Abstract
:1. Introduction
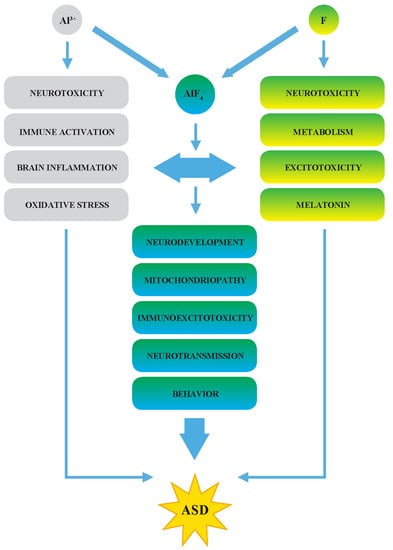
2. Fluoride-Induced ASD Symptoms
2.1. Enzymes and Mitochondrial Disorders
2.2. A Synergy of F and Al3+ in the ASD Etiopathology
2.3. The Effects of F in Oxidative Stress, Inflammation, and Immunoexcitotoxicity
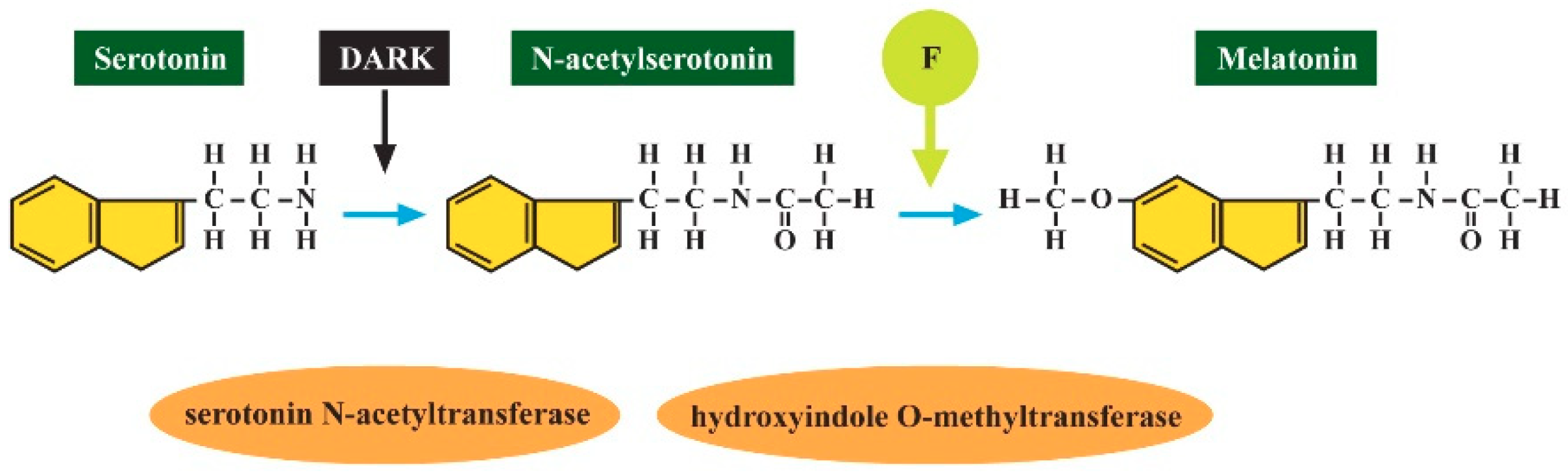
2.4. Decreased Melatonin as the Potential Marker of ASD
3. Fluoride as an Environmental Neurotoxin in the ASD Etiopathogenesis
4. Is There A Link between ASD Prevalence and a Chronic F Exposure?
4.1. The ASD Prevalence in Countries with Fluoridated Water
4.2. The ASD Prevalence in Countries with Endemic Fluorosis
4.3. The ASD Prevalence in the EU Member States
4.4. The Definition of a Safe Concentration of F for Humans
4.5. Socioeconomic Status
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Available online: https://books.google.cz/books?id=-JivBAAAQBAJ (accessed on 23 May 2019).
- World Health Organization. Autism Spectrum Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (accessed on 23 May 2019).
- Centers for Disease Control and Prevention (CDC). Data & Statistics on Autism Spectrum Disorder; U.S. Department of Health & Human Services: Atlanta, GA, USA, 2018.
- World Health Organization. ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse11/l-m/en (accessed on 25 May 2019).
- Kogan, M.D.; Vladutiu, C.J.; Schieve, L.A.; Ghandour, R.M.; Blumberg, S.J.; Zablotsky, B.; Perrin, J.M.; Shattuck, P.; Kuhlthau, K.A.; Harwood, R.L.; et al. The prevalence of parent-reported autism spectrum disorder among US children. Pediatrics 2018, 142, e20174161. [Google Scholar] [CrossRef]
- Almandil, N.B.; Alkuroud, D.N.; Abdul Azeez, S.; Al Sulaiman, A.; Elaissari, A.; Borgio, J.F. Environmental and genetic factors in autism spectrum disorders: Special emphasis on data from arabian studies. Int. J. Environ. Res. Public Health 2019, 16, 658. [Google Scholar] [CrossRef]
- Strunecká, A.; Strunecký, O.; Guan, Z. The resemblance of fluorosis pathology to that of autism spectrum disorder: A mini-review. Fluoride 2019, 52, 105–115. [Google Scholar]
- Elsabbagh, M.; Divan, G.; Koh, Y.-J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef]
- Øzerk, K. The issue of prevalence of autism. IEJEE 2017, 9, 263–306. [Google Scholar]
- European Commission Health & Consumer Protection Directorate-General. Some Elements About the Prevalence of Autism Spectrum Disorders (ASD) in the European Union; European Commission: Luxembourg, 2005; 16p. [Google Scholar]
- Strunecka, A.; Blaylock, R.L.; Patocka, J.; Strunecky, O. Immunoexcitotoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: A possible role of fluoride and aluminum. Surg. Neurol. Int. 2018, 9, 74. [Google Scholar] [CrossRef]
- Strunecka, A.; Patocka, J. Pharmacological and toxicological effects of aluminofluoride complexes. Fluoride 1999, 32, 230–242. [Google Scholar]
- Strunecka, A.; Strunecky, O.; Patocka, J. Fluoride plus aluminum: The useful tools in laboratory investigations, but messengers of the false information. Physiol. Res. 2002, 51, 557–564. [Google Scholar]
- Strunecka, A.; Patocka, J.; Blaylock, R.; Chinoy, N. Fluoride interactions: From molecules to disease. Curr. Signal Transduct. Ther. 2007, 2, 190–213. [Google Scholar] [CrossRef]
- European Food Safety Authority. Dietary Reference Values for Nutrients Summary Report; European Food Safety Authority: Parma, Italy, 2017. [Google Scholar] [CrossRef]
- Luke, J. The Effect of Fluoride on the Physiology of the Pineal Gland. Ph.D. Thesis, University of Surrey, Guildford, UK, 1997. [Google Scholar]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 2014, 4, e479. [Google Scholar] [CrossRef]
- Burgstahler, A.W. Paradoxical dose-response effects of fluoride. Fluoride 2002, 35, 143–147. [Google Scholar]
- Strunecka, A.; Blaylock, R.L.; Hyman, M.A.; Paclt, I. Cellular and Molecular Biology of Autism Spectrum Disorders; Bentham e Books Bentham Science: Sharjah, UEA, 2010. [Google Scholar] [CrossRef]
- Hassan, M.H.; Desoky, T.; Sakhr, H.M.; Gabra, R.H.; Bakri, A.H. Possible metabolic alterations among autistic male children: Clinical and biochemical approaches. J. Mol. Neurosci. 2019, 67, 204–216. [Google Scholar] [CrossRef]
- Delhey, L.; Kilinc, E.N.; Yin, L.; Slattery, J.; Tippett, M.; Wynne, R.; Rose, S.; Kahler, S.; Damle, S.; Legido, A.; et al. Bioenergetic variation is related to autism symptomatology. Metab. Brain Dis. 2017, 32, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef] [Green Version]
- Bennuri, S.C.; Rose, S.; Frye, R.E. Mitochondrial dysfunction is inducible in lymphoblastoid cell lines from children with autism and may involve the TORC1 pathway. Front. Psychiatry 2019, 10, 269. [Google Scholar] [CrossRef]
- Sternweis, P.C.; Gilman, A.G. Aluminum: A requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc. Natl. Acad. Sci. USA 1982, 79, 4888–4891. [Google Scholar] [CrossRef]
- Chabre, M. Aluminofluoride and beryllofluoride complexes: New phosphate analogues in enzymology. Trends Biochem. Sci. 1990, 15, 6–10. [Google Scholar] [CrossRef]
- Wittinghofer, A. Aluminum fluoride for molecule of the year. Curr. Biol. 1997, 7, 682–690. [Google Scholar] [CrossRef]
- Tesmer, J.J.; Berman, D.M.; Gilman, A.G.; Sprang, S.R. Structure of RGS4 bound to AlF4- activated Gi1: stabilization of the transition state for GTP hydrolysis. Cell 1997, 89, 251–261. [Google Scholar] [CrossRef]
- Schlichting, I.; Reinstein, J. pH influences fluoride coordination number of the AlFx phosphoryl transfer transition state analog. Nat. Struct. Biol. 1999, 8, 721–723. [Google Scholar] [CrossRef]
- Sondek, J.; Lambright, D.G.; Noel, J.P.; Hamm, H.E.; Sigler, P.B. GTPase mechanism of G proteins from the 1.7-Åcrystal structure of transducing α-GDP·AlF4−. Nature 1994, 372, 276–279. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef]
- Frye, R.E.; James, S.J. Metabolic pathology of autism in relation to redox metabolism. Biomark. Med. 2014, 8, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Strunecka, A. (Ed.) Biochemical Changes in ASD. In Cellular and Molecular Biology of Autism Spectrum Disorders; Bentham e Books Bentham Science: Sharjah, UEA, 2010; pp. 100–120. [Google Scholar] [CrossRef]
- Guan, Z.; Yang, P.; Su, Y.; Wang, Y. Changed levels of lipid peroxidation and anti-oxidation in blood of children in the area of fluoride and aluminium toxication in Shuichen County of Guizhou. J. Guiyang Med. Coll. 1991, 16, 198–200. (In Chinese) [Google Scholar]
- Belardo, A.; Gevi, F.; Zolla, L. The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children. J. Nutr. Biochem. 2019, 70, 38–46. [Google Scholar] [CrossRef]
- Nardone, S.; Sams, D.S.; Reuveni, E.; Getselter, D.; Oron, O.; Karpuj, M.; Elliott, E. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl. Psychiatry 2014, 4, e433. [Google Scholar] [CrossRef]
- Blaylock, R.L. The Cerebellum in Autism Spectrum Disorders. In Cellular and Molecular Biology of Autism Spectrum Disorders; Strunecka, A., Ed.; Bentham e Books Bentham Science: Sharjah, UEA, 2010; pp. 17–31. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Blaylock, R.L. Excitotoxicity: A possible central mechanism in fluoride neurotoxicity. Fluoride 2004, 37, 301–314. [Google Scholar]
- Blaylock, R.L. A possible central mechanism in autism spectrum disorders, part 1. Altern. Ther. Health M. 2008, 14, 46–53. [Google Scholar]
- Strunecka, A.; Blaylock, R.L.; Strunecky, O. Fluoride, aluminum, and aluminofluoride complexes in pathogenesis of the autism spectrum disorders: A possible role of immunoexcitotoxicity. J. Appl. Biomed. 2016, 14, 171–176. [Google Scholar] [CrossRef]
- Luke, J. Fluoride deposition in the aged human pineal gland. Caries Res. 2001, 35, 125–128. [Google Scholar] [CrossRef]
- Veatch, O.J.; Goldman, S.E.; Adkins, K.W.; Malow, B.A. Melatonin in children with autism spectrum disorders: How does the evidence fit together? J. Nat. Sci. 2015, 1, e125. [Google Scholar]
- Pagan, C.; Goubran-Botros, H.; Delorme, R.; Benabou, M.; Lemiere, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017, 7, 2096. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, Y.; Trivedi, A.; Jiang, X.; Chandra, D.; Zheng, J.; Nakano, Y.; Abduweli Uyghurturk, D.; Jalai, R.; Onur, S.G.; et al. Fluoride related changes in behavioral outcomes may relate to increased serotonin. Physiol. Behav. 2019, 206, 76–83. [Google Scholar] [CrossRef]
- Ho, B.T.; McIsaac, W.M.; Tansey, L.W. Hydroxyindole-O-methyltransferase III: Influence of the phenyl moiety on the inhibitory activities of some n-acyltryptamines. J. Pharm. Sci. 1969, 58, 563–566. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef]
- Johansson, A.E.E.; Dorman, J.S.; Chasens, E.R.; Feeley, C.A.; Devlin, B. Variations in genes related to sleep patterns in children with autism spectrum disorder. Biol. Res. Nurs. 2019, 21, 335–342. [Google Scholar] [CrossRef]
- UNICEF. Fluoride in water: An overview. WATERfront 1999, 13, 11–13. [Google Scholar]
- Spittle, B. Fluoride Fatigue: Fluoride Poisoning: Is Fluoride in your Drinking Water, and from Other Sources, Making you Sick? Paua Press Limited: Dunedin, New Zealand, 2008; p. 78. [Google Scholar]
- McClure, F.J. A review of fluorine and its physiological effects. Physiol. Rev. 1933, 13, 277–300. [Google Scholar] [CrossRef]
- Waldbott, G.; Burgstahler, A.; McKinney, H. Fluoridation: The great dilemma. Ann. Intern. Med. 1979, 90, 291. [Google Scholar] [CrossRef]
- Carlsson, A. Current problems of the pharmacology and toxicology of fluorides. Lakartidningen 1978, 75, 1388–1392. [Google Scholar]
- Mullenix, P.J.; Denbesten, P.K.; Schunior, A.; Kernan, W.J. Neurotoxicity of sodium fluoride in rats. Neurotoxicol. Teratol. 1995, 17, 169–177. [Google Scholar] [CrossRef]
- Du, L. The effect of fluorine on the developing human brain. Chin. J. Pathol. 1992, 21, 218–220. [Google Scholar]
- Tang, Q.Q.; Du, J.; Ma, H.H.; Jiang, S.J.; Zhou, X.J. Fluoride and children’s intelligence: A meta-analysis. Biol. Trace. Elem. Res. 2008, 126, 115–120. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Amador, D.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderon, J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad. Saude Publ. 2007, 23 (Suppl. 4), S579–S587. [Google Scholar] [CrossRef]
- Seraj, B.; Shahrabi, M.; Shadfar, M.; Ahmadi, R.; Fallahzadeh, M.; Eslamlu, H.F.; Kharazifard, M.J. Effect of high water fluoride concentration on the intellectual development of children in Makoo-Iran. J. Dent. (Tehran) 2012, 9, 221–229. [Google Scholar]
- Aravind, A.; Dhanya, R.S.; Narayan, A.; Sam, G.; Adarsh, V.J.; Kiran, M. Effect of fluoridated water on intelligence in 10–12-year-old school children. J. Int. Soc. Prev. Community Dent. 2016, 6, S237–S242. [Google Scholar] [CrossRef]
- Yu, X.; Chen, J.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Cui, Y.; Zhao, L.; Li, P.; Zhou, Z.; et al. Threshold effects of moderately excessive fluoride exposure on children’s health: A potential association between dental fluorosis and loss of excellent intelligence. Environ. Int. 2018, 118, 116–124. [Google Scholar] [CrossRef]
- Razdan, P.; Patthi, B.; Kumar, J.K.; Agnihotri, N.; Chaudhari, P.; Prasad, M. Effect of fluoride concentration in drinking water on intelligence quotient of 12–14-year-old children in Mathura district: A cross-sectional study. J. Int. Soc. Prev. Community Dent. 2017, 7, 252–258. [Google Scholar] [CrossRef]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose-response meta-analysis. Public Health 2018, 154, 87–97. [Google Scholar] [CrossRef]
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. 2019. [Google Scholar] [CrossRef]
- Hirzy, J.W.; Connett, P.; Xiang, Q.; Spittle, B.; Kennedy, D. Developmental Neurotoxicity of Fluoride: A Quantitative Risk Analysis Toward Establishing a Safe Dose for Children. In Neurotoxins; McDuffie, J.E., Ed.; IntechOpen: London, UK, 2017; pp. 115–132. [Google Scholar] [CrossRef]
- Bellinger, D.C. Environmental chemical exposures and neurodevelopmental impairments in children. Ped. Med. 2018, 1, 9. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J. Global Data on Autism Spectrum Disorders Prevalence: A Review of Facts, Fallacies and Limitations. Univ.J. Clin. Med. 2017, 5, 14–23. [Google Scholar] [CrossRef]
- Adak, B.; Halder, S. Systematic review on prevalence for autism spectrum disorder with respect to gender and socio-economic status. J. Ment. Dis. Treat. 2017, 3. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Wiener, R.C.; Shen, C.; Findley, P.; Tan, X.; Sambamoorthi, U. Dental fluorosis over time: A comparison of national health and nutrition examination survey data from 2001–2002 and 2011–2012. J. Dent. Hyg. 2018, 92, 23–29. [Google Scholar]
- Neurath, C.; Limeback, H.; Osmunson, B.; Connett, M.; Kanter, V.; Wells, C.R. Dental fluorosis trends in US oral health surveys: 1986 to 2012. JDR Clin. Trans. Res. 2019. [Google Scholar] [CrossRef]
- Autism Spectrum Disorder Among Children and Youth in Canada. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018.html (accessed on 1 June 2019).
- Prevalence of Autism Spectrum Disorder Among Children in Select Countries Worldwide as of 2018 (per 10,000 Children). Available online: https://www.statista.com/statistics/676354/autism-rate-among-children-select-countries-worldwide/ (accessed on 9 August 2019).
- Australian Institute of Health and Welfare. Autism in Australia. Available online: https://www.aihw.gov.au/reports/disability/autism-in-australia/contents/autism (accessed on 25 June 2019).
- Ministries of Health and Education. New Zealand Autism Spectrum Disorder Guideline; Ministry of Health: Wellington, New Zealand, 2016; 343p.
- Spittle, B. Green light for water fluoridation in New Zealand. Fluoride 2015, 48, 271–273. [Google Scholar]
- Waugh, D.T.; Godfrey, M.; Limeback, H.; Potter, W. Black tea source, production, and consumption: Assessment of health risks of fluoride intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef]
- Sun, D.J.; Gao, Y.H.; Zhao, L.J. Epidemic and control of endemic fluorosis in China. In Proceedings of the XXXIVth conference of the International Society for Fluoride Research, Guiyang, China, 18–20 October 2018. Fluoride2019, 52, 79–80. [Google Scholar]
- Jin, T.X.; Hua, Z.; Guan, Z.Z. The historical review and development strategies on prevention and control of coal-burning type of endemic fluorosis in Liupanshui, Guizhou of China. In Proceedings of the XXXIVth conference of the International Society for Fluoride Research, Guiyang, China, 18–20 October 2018. Fluoride2019, 52, 94–95. [Google Scholar]
- Wang, F.; Lu, L.; Wang, S.-B.; Zhang, L.; Ng, C.H.; Ungvari, G.S.; Cao, X.-L.; Lu, J.-P.; Hou, C.-L.; Jia, F.-J.; et al. The prevalence of autism spectrum disorders in China: A comprehensive meta-analysis. Int. J. Biol. Sci. 2018, 14, 717–725. [Google Scholar] [CrossRef]
- Sun, X.; Allison, C.; Wei, L.; Matthews, F.; Auyeung, B.; Yu Wu, Y.; Griffiths, S.; Zhang, J.; Baron-Cohen, S.; Brayne, C. Autism prevalence in China is comparable to Western prevalence. Mol. Autism 2019, 10. [Google Scholar] [CrossRef]
- Saito, M.; Hirota, T.; Sakamoto, Y.; Adachi, M.; Takahashi, M.; Osato-Kaneda, A.; Kim, Y.S.; Leventhal, B.; Shui, A.; Kato, S.; et al. Prevalence and cumulative incidence of autism spectrum disorders and the patterns of co-occurring neurodevelopmental disorders in a total population sample of 5-years-old children. Lancet 2019, in press. Available online: https://ssrn.com/abstract=3360118 (accessed on 28 March 2019).
- Dean, H.T. Endemic fluorosis and its relation to dental caries, 1938. Public Health Rep. 2006, 121 (Suppl. 1), 213–219; discussion 212. [Google Scholar]
- Komiyama, K.; Kimoto, K.; Taura, K.; Sakai, O. National survey on school-based fluoride mouth-rinsing programme in Japan: Regional spread conditions from preschool to junior high school in 2010. Int. Dent. J. 2014, 64, 127–137. [Google Scholar] [CrossRef]
- Hossain, M.D.; Ahmed, H.U.; Jalal Uddin, M.M.; Chowdhury, W.A.; Iqbal, M.S.; Kabir, R.I.; Chowdhury, I.A.; Aftab, A.; Datta, P.G.; Rabbani, G.; et al. Autism spectrum disorders (ASD) in South Asia: A systematic review. BMC Psychiatry 2017, 17, 281. [Google Scholar] [CrossRef]
- Rudra, A.; Belmonte, M.K.; Soni, P.K.; Banerjee, S.; Mukerji, S.; Chakrabarti, B. Prevalence of autism spectrum disorder and autistic symptoms in a school-based cohort of children in Kolkata, India. Autism Res. 2017, 10, 1597–1605. [Google Scholar] [CrossRef]
- Kim, Y.S.; Leventhal, B.L.; Koh, Y.J.; Fombonne, E.; Laska, E.; Lim, E.C.; Cheon, K.A.; Kim, S.J.; Kim, Y.K.; Lee, H.; et al. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry 2011, 168, 904–912. [Google Scholar] [CrossRef]
- Department of Health. Estimating Prevalence of Autism Spectrum Disorders (ASD) in the Irish Population: A Review of Data Sources and Epidemiological Studies. Available online: https://health.gov.ie/wp-content/uploads/2018/12/ASD-Report-Final-19112018-For-publication.pdf (accessed on 10 June 2019).
- National Autistic Society. Autism Facts and History. Available online: https://www.autism.org.uk/about/what-is/myths-facts-stats.aspx (accessed on 10 May 2019).
- European Commission. Autism Spectrum Disorders in the European Union (ASDEU); European Commission: Luxembourg, 2018; 13p. [Google Scholar]
- Waugh, D.T.; Potter, W.; Limeback, H.; Godfrey, M. Risk assessment of fluoride intake from tea in the republic of Ireland and its implications for public health and water fluoridation. Int. J. Environ. Res. Public Health 2016, 13, 259. [Google Scholar] [CrossRef]
- Aggebornb, L.; Öhmanc, M. The Effects of Fluoride in the Drinking Water; Department of Government at Uppsala University: Upsalla, Sweden, 2017; p. 81. [Google Scholar]
- Campbell, J. Countries with Lowest Autism Rates That May Surprise You. Available online: https://newmiddleclassdad.com/countries-with-lowest-autism-rates/ (accessed on 15 May 2019).
- Van Bakel, M.M.; Delobel-Ayoub, M.; Cans, C.; Assouline, B.; Jouk, P.S.; Raynaud, J.P.; Arnaud, C. Low but increasing prevalence of autism spectrum disorders in a French area from register-based data. J. Autism Dev. Disord. 2015, 45, 3255–3261. [Google Scholar] [CrossRef]
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018, 1–10. [Google Scholar] [CrossRef]
- National Toxicology Program. Systematic Literature Review on the Effects of Fluoride on Learning and Memory in Animal Studies; U.S. Department of Health and Human Services: Triangle Park, NC, USA, 2016.
- U. S. Department of Health, Human Services Federal Panel on Community Water, Fluoridation. U.S. Public health service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public Health Rep. 2015, 130, 318–331. [Google Scholar] [CrossRef]
- Consumerlab.com. Recommended Daily Intakes and Upper Limits for Vitamin and Minerals. Available online: https://www.consumerlab.com/RDAs/Fluoride/#rdatable (accessed on 11 May 2019).
- Department of Health and Ageing. Nutrient Reference Values for Australia and New Zealand; Commonwealth of Australia: Canberra, Australia, 2006; 309p. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, Allergies. Scientific opinion on dietary reference values for fluoride. EFSA J. 2013, 11, 3332. [Google Scholar] [CrossRef]
- European Union. Critical Review of Any New Evidence on the Hazard Profile, Health Effects, and Human Exposure to Fluoride and the Fluoridating Agents of Drinking Water; EC: Brussels, Belgium, 2010; 59p. [Google Scholar]
- Shen, M.D.; Piven, J. Brain and behavior development in autism from birth through infancy. Dialogues Clin. Neurosci. 2017, 19, 325–333. [Google Scholar]
- Leigh, J.P.; Du, J. Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism. Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef]
- Ng, M.; de Montigny, J.G.; Ofner, M.; Do, M.T. Environmental factors associated with autism spectrum disorder: A scoping review for the years 2003–2013. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 1–23. [Google Scholar] [CrossRef]
- Schofield, K. The metal neurotoxins: An important role in current human neural epidemics? Int. J. Environ. Res. Public Health 2017, 14, 1511. [Google Scholar] [CrossRef]
- Bjørklund, G.; Skalny, A.V.; Rahman, M.M.; Dadar, M.; Yassa, H.A.; Aaseth, J.; Chirumbolo, S.; Skalnaya, M.G.; Tinkov, A.A. Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder. Environ. Res. 2018, 166, 234–250. [Google Scholar] [CrossRef]
- Blaylock, R.L. A possible central mechanism in autism spectrum disorders, part 3: The role of excitotoxin food additives and the synergistic effects of other environmental toxins. Altern. Ther. Health. Med. 2009, 15, 56–60. [Google Scholar]
- Blaylock, R.L.; Strunecka, A. Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr. Med. Chem. 2009, 16, 157–170. [Google Scholar] [CrossRef]
- Anderson, G.M. The potential role for emergence in autism. Autism Res. 2008, 1, 18–30. [Google Scholar] [CrossRef]





| Country Year | Prevalence per 10,000 | This is 1 in X Children | Water Fluoridation% of Population | Reference |
|---|---|---|---|---|
| US 2014 | 169 | 1:59 | 70% for 70 y | [72] |
| US 2016 | 250 | 1:40 | 70% for 70 y | [5] |
| Canada 2018 | 152 | 1:66 | 45% for 12 y | [75,76] |
| New Zealand 2016 | 152 | 1:66 | 62% for 50 y | [78,79] |
| Australia 2015 | 144 | 1:150 | 80% for 35 y | [77] |
| Country Year | Prevalence per 10,000 | This is 1 in X Children | Reference |
|---|---|---|---|
| Bangladesh 2016 | 15; 80 | 1:666; 1:125 | [88] |
| Dhaka 2016 | 300 | 1:33 | [88] |
| China 2013–2016 | 19; 42 | 1:526; 1:238 | [9,83] |
| China 2016 | 429; 530 | 1:23; 1:19 | [83] |
| China 2013 Jilin | 108 | 1:92.5 | [84] |
| Japan | 161 | 1:62 | [76] |
| Japan 2018 | 322 | 1:31 | [85] |
| India 2016 | 9 | 1:1111 | [88] |
| India 2017 | 23 | 1:435 | [89] |
| South Korea 2011 | 220 | 1:45 | [90] |
| Sri Lanka 2016 | 93 | 1:107 | [88] |
| Country | Prevalence per 10,000 | This is 1 in X Children | Reference |
|---|---|---|---|
| EU total 2015–2018 | 44–197, average 122 | 1:82 | [93] |
| Belgium | 60 | 1:167 | [96] |
| Czech Republic | 12 | 1:833 | (pers. comm.) |
| Denmark | 34; 68 | 1:294; 1:147 | [76,96] |
| Finland | 77 | 1:130 | [91] |
| France | 27; 36 | 1:370; 1:277 | [97] |
| Germany | 38 | 1:263 | [96] |
| Ireland | 150 | 1:66 | [91] |
| Italy Pisa | 86 | 1:116 | [91,98] |
| Netherland | 57, 84 | 1:175; 1:119 | [9,71] |
| Northern Ireland | 290 | 1:35 | [91] |
| Norway | 12; 70 | 1:833; 1:142 | [9,91,96] |
| Poland | 3 | 1:3333 | [96] |
| Portugal | 9.2 | 1:1086 | [9,71,76] |
| Spain | 13; 100 | 1:769; 1:100 | [9,71,76] |
| Sweden | 71; 115 | 1:141; 1:87 | [9,71,91] |
| UK | 100 | 1:100 | [92] |
| Age | US | Australia, NZ | EU | |||
|---|---|---|---|---|---|---|
| AI | UL | AI | UL | Age | AI | |
| 0–6 m | 0.01 | 0.7 | – | 1.2 | 0–6 m | – |
| 7–12 m | 0.5 | 0.9 | 0.5 | 1.8 | 7–11 m | 0.4 |
| 1–3 y | 0.7 | 1.3 | 0.6 | 2.4 | 1–3 y | 0.6 |
| 4–8 y | 1.0 | 2.2 | 1.1 | 4.4 | 4–6 y | 1.0 |
| 9–13 y | 2.0 | 10 | 2.0 *; 3.0 + | 10 | 7–10 y | 1.5 |
| 14–18 y | 3.0 | 10 | 2.0 *; 3.0 + | 10 | 11–14 y | 2.2 |
| Males | 4.0 | 10 | 4.0 | 10 | 15–17 y | 3.2 |
| Adult females | 3.0 | 10 | 3.0 | 10 | Adults | 3.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strunecka, A.; Strunecky, O. Chronic Fluoride Exposure and the Risk of Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2019, 16, 3431. https://doi.org/10.3390/ijerph16183431
Strunecka A, Strunecky O. Chronic Fluoride Exposure and the Risk of Autism Spectrum Disorder. International Journal of Environmental Research and Public Health. 2019; 16(18):3431. https://doi.org/10.3390/ijerph16183431
Chicago/Turabian StyleStrunecka, Anna, and Otakar Strunecky. 2019. "Chronic Fluoride Exposure and the Risk of Autism Spectrum Disorder" International Journal of Environmental Research and Public Health 16, no. 18: 3431. https://doi.org/10.3390/ijerph16183431
APA StyleStrunecka, A., & Strunecky, O. (2019). Chronic Fluoride Exposure and the Risk of Autism Spectrum Disorder. International Journal of Environmental Research and Public Health, 16(18), 3431. https://doi.org/10.3390/ijerph16183431