The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Antibodies
2.2. Animals
2.3. Experimental Groups
2.4. Determinations in Blood and Ventricular Tissue
2.5. Measurement of Nitric Oxide
2.6. Measurement of Total Antioxidant Capacity
2.7. Measurement by Capillary Zone Electrophoresis
2.8. Isolated Heart Perfused by the Langendorff’s Method
2.9. Western Blotting of GTPCH-1, eNOS, AKT, and TRPV1
2.10. Statistical Analysis
3. Results
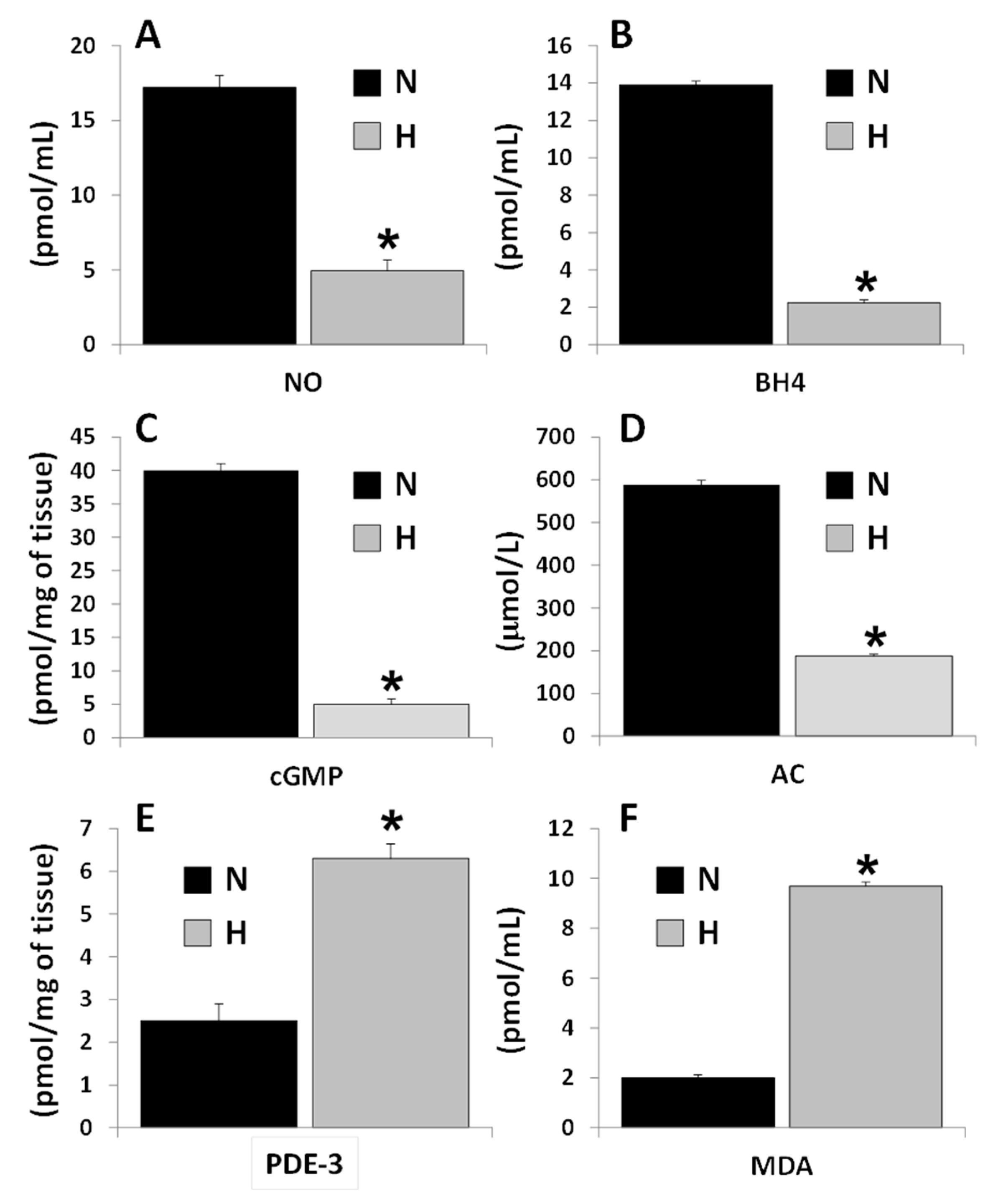
3.1. Cardioprotective Biomarker Concentrations in Serum and in Tissue. Differences between Control and Hypertensive Rats
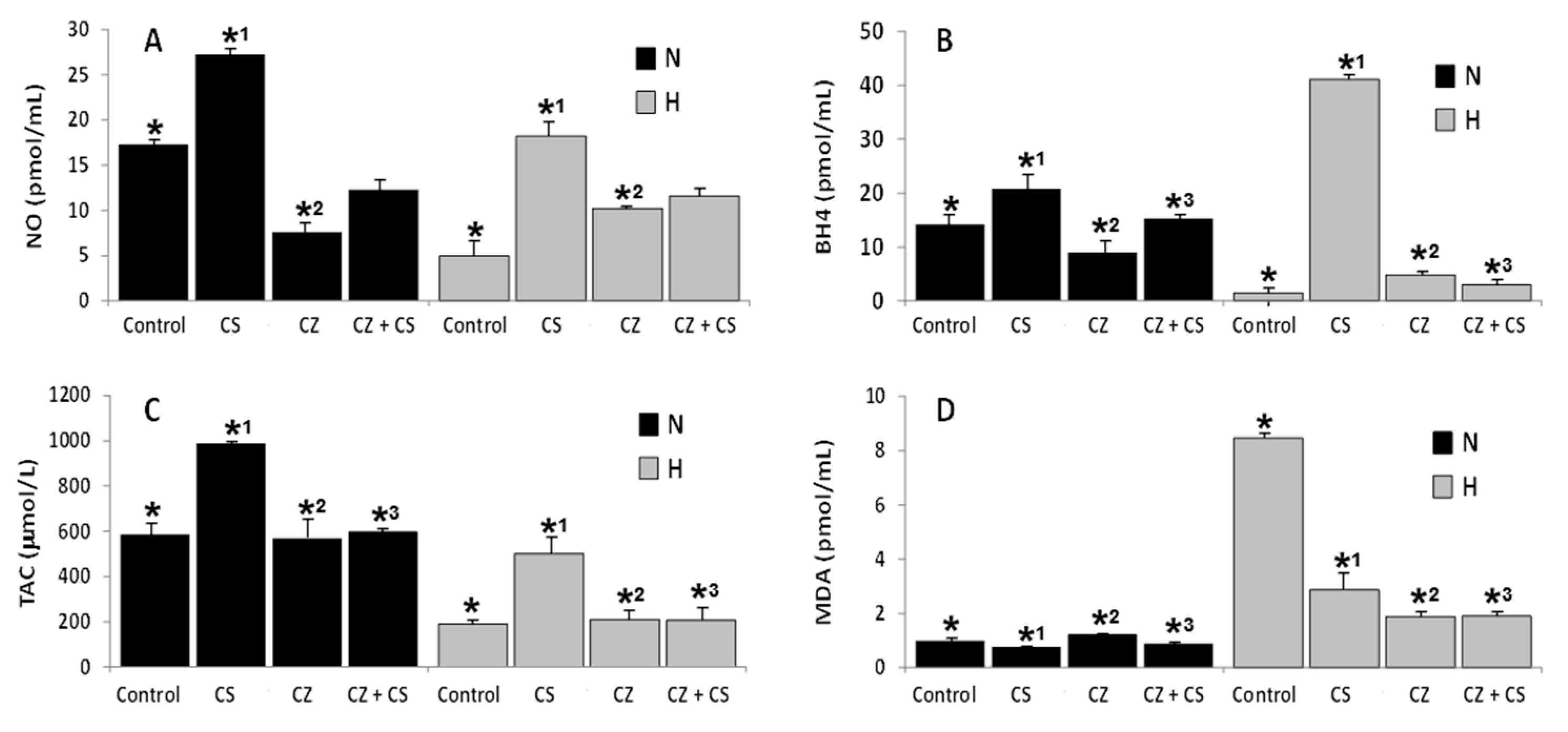
3.2. Effects of CS and CZ on Cardioprotective Biomarkers in Sera of N and H Rats
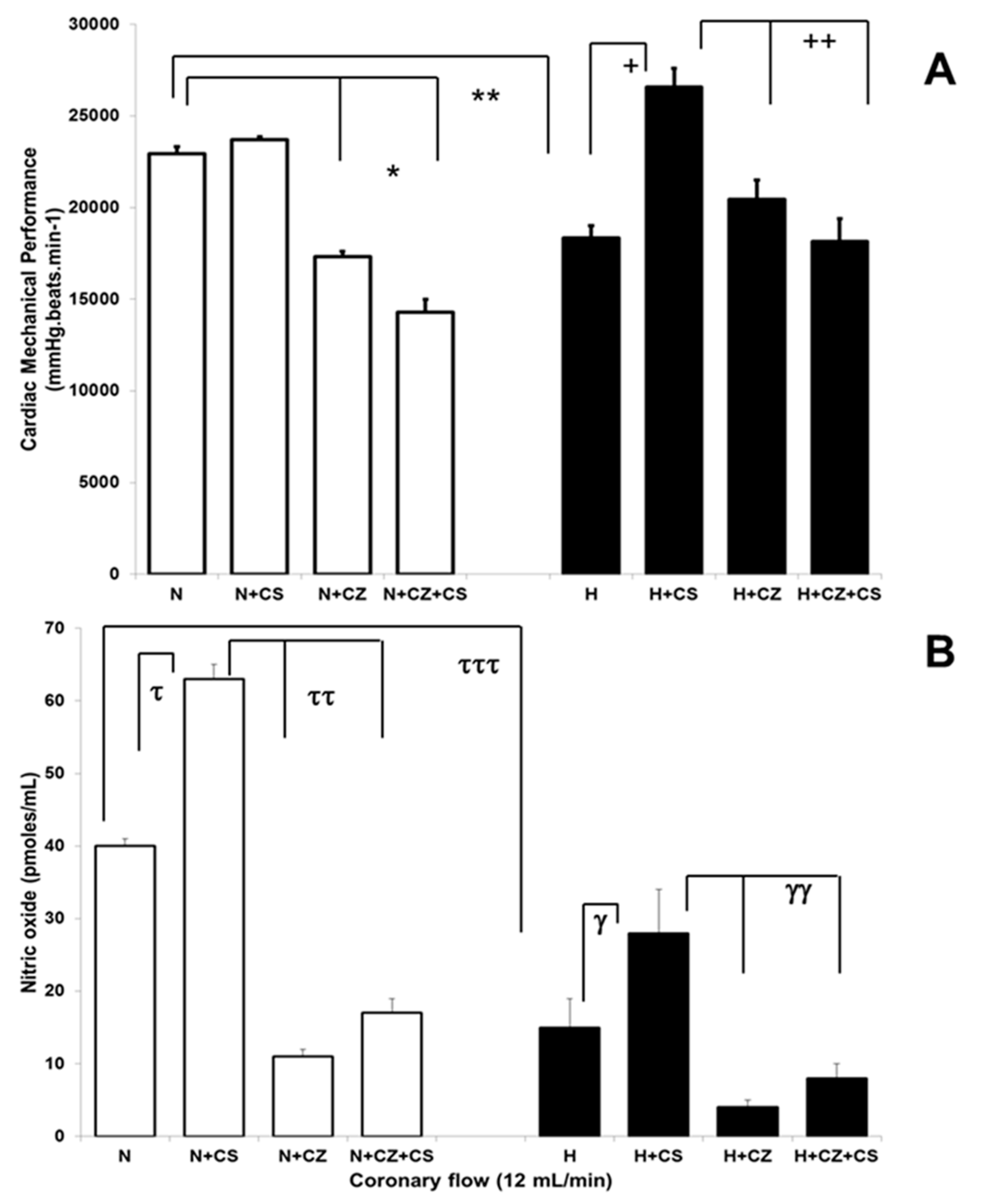
3.3. Cardiac Mechanical Performance in Normotensive and Hypertensive Rats
3.4. Nitric Oxide Levels in Coronary Effluent of Normotensive and Hypertensive Hearts
3.5. Coronary Vascular Resistance in Normotensive and Hypertensive Hearts
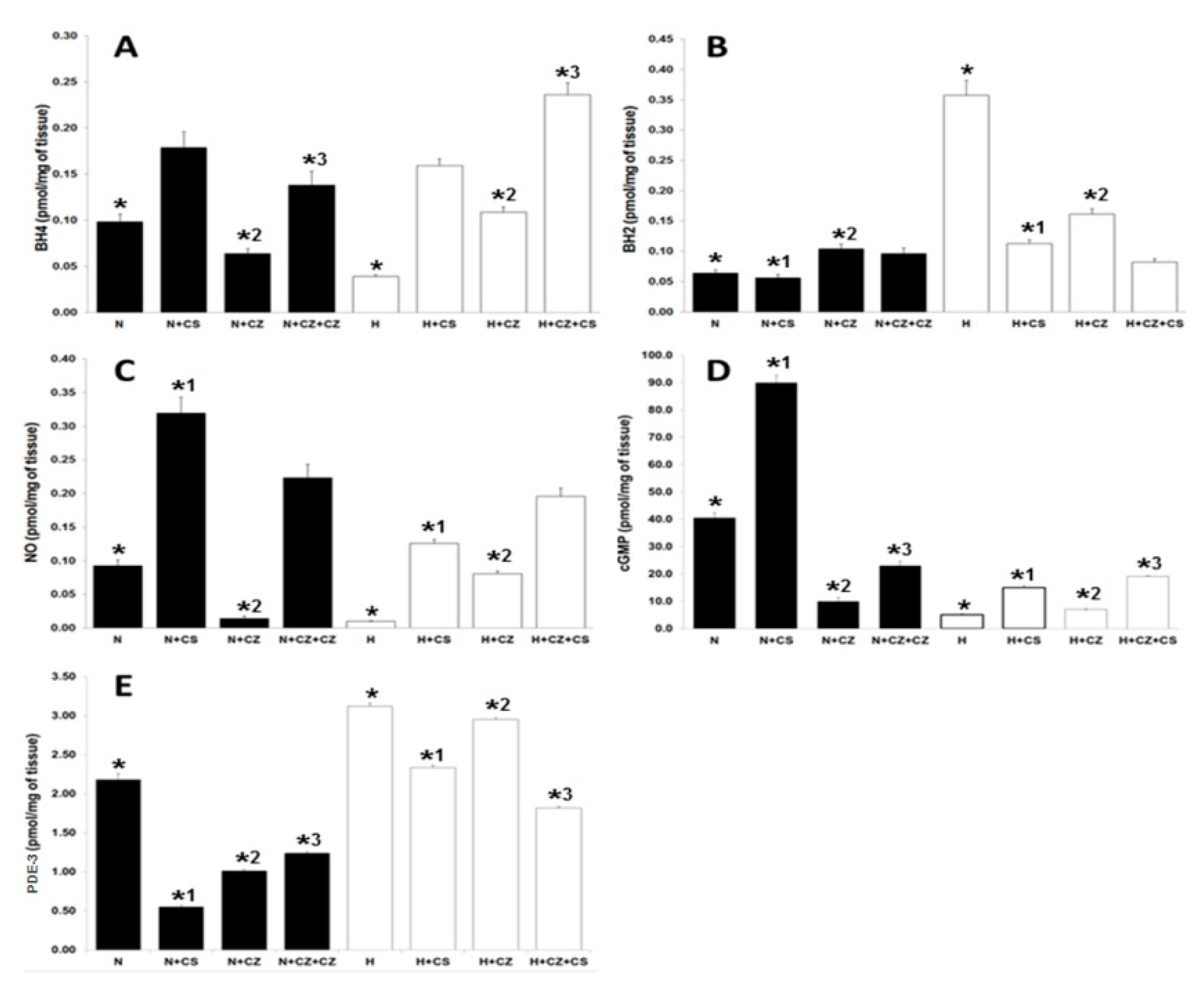
3.6. BH2 and BH4 Measured in Cardiac Tissue
3.7. NO, cGMP, PDE-3 in Ventricular Tissue
3.8. GTPCH-1 Expression
3.9. eNOS Expression
3.10. AKT Expression
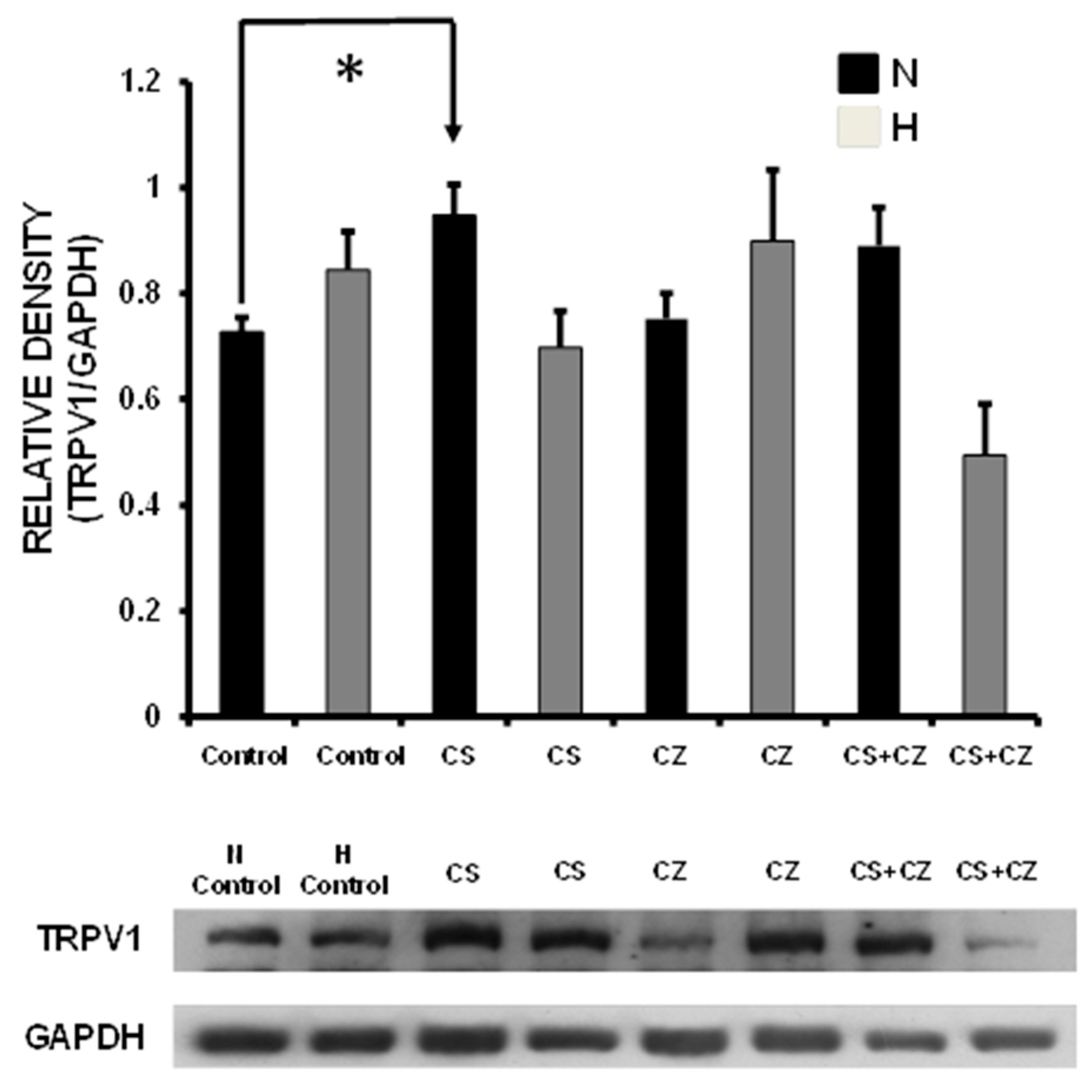
3.11. TRPV1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important patiential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Kaube, H.; Goadsby, P.J. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br. J. Pharmacol. 2003, 140, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, W.; Tsubouchi, R.; Murakami, K.; Yoshino, M. Role of vanilloid receptors in the capsaicin-mediated induction of iNOS in PC12 cells. Neurochem. Res. 2004, 29, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H. The vanilloid receptor and hypertension. Acta Pharmacol. Sinica 2005, 26, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Y.J. The vanilloid receptor TRPV1: Role in cardiovascular and gastrointestinal protection. Eur. J. Pharmcol. 2010, 627, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liang, Y.; Wang, X.; Lu, Z.; Li, L.; Zhu, S.; Liu, D.; Yan, Z.; Zhu, Z. TRPV1 activation attenuates high-salt diet-induced cardiac hypertrophy and fibrosis through PPAR-δ upregulation. PPAR Res. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Jaggi, A.S. TRPV1 channels in cardiovascular system: A double edged sword? Int. J. Cardiol. 2017, 228, 103–113. [Google Scholar] [CrossRef]
- Yao, X.; Garland, C.J. Recent developments in vascular endothelial cell transient receptor potential channels. Circ. Res. 2005, 97, 853–863. [Google Scholar] [CrossRef]
- Yoshida, T.; Inoue, R.; Mori, T.; Takahashi, N.; Yamamoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006, 2, 596–607. [Google Scholar] [CrossRef]
- Gupta, S.; Lozano-Cuenca, J.; Villalón, M.C.; Vries, R.; Garrelds, M.I.; Avezaat, J.J.C.; van Kats, J.P.; Saxena, P.R.; MaassenVanDenBrink, A. Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 29–38. [Google Scholar] [CrossRef]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef]
- Ching, L.C.; Kou, Y.R.; Shyue, S.K. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potencial vanilloid type 1. Cardiovasc. Res. 2011, 91, 492–501. [Google Scholar] [CrossRef]
- Su, K.H.; Lee, K.I.; Shyue, S.K.; Chen, H.Y.; Wei, J.W.; Lee, T.S. Implication of transient receptor potential vanilloid type I in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. Int. J. Biol. Sci. 2014, 10, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Jensen, L.J.; Shi, J.; Morita, H.; Nishida, M.; Honda, A.; Ito, Y. Transient receptor potential channels in cardiovascular function and disease. Cir. Res. 2006, 99, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kaminski, E.N.; Wang, D.H. Endocannabinoid regulates blood pressure via activation of the transient receptor potential vanilloid type 1 in Wistar rats fed a high-salt diet. J. Pharmacol. Exp. Ther. 2007, 321, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Chapnick, B.M.; Howlett, A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am. J. Physiol. Heart. Circ. Physiol. 2002, 82, H2046–H2054. [Google Scholar] [CrossRef]
- Morales-Lázaro, S.L.; Simon, S.A.; Rosenbaum, T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J. Physiol. 2013, 591, 3109–3121. [Google Scholar] [CrossRef]
- Del Valle-Mondragón, L.; Tenorio-López, F.A.; Zarco-Olvera, G.; Pastelín-Hernández, G. Vulgarenol, a sesquiterpene isolated from Magnolia grandiflora, induces nitric oxide synthases II and III overexpression in guinea pig hearts. Z. Naturforsch. C 2007, 62, 725–730. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Alp, J.N. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin. Sci. 2007, 113, 47–63. [Google Scholar] [CrossRef]
- Craige, S.M.; Kant, S.; Keaney, J.F. Reactive oxygen species in endothelial function. Circ. J. 2015, 79, 1145–1155. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Yamashiro, S.; Noguchi, K.; Matsuzaki, T.; Miyagi, K.; Nakasone, J.; Sakanashi, M.; Koja, K.; Sakanashi, M. Benefical effect of tetrahydrobiopterin on ischemia-reperfusion injury in isolated perfused rat hearts. J. Thoracic. Cardiovasc. Surgery 2002, 124, 775–784. [Google Scholar] [CrossRef][Green Version]
- Alp, J.N.; Channon, K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 413–420. [Google Scholar] [CrossRef]
- Claeson, K.; Aberg, F.; Karlberg, B. Free malondialdehyde determination in rat brain tissue by capillary zone electrophoresis: Evaluation of two protein removal procedures. J. Chromatogr. B 2000, 740, 87–92. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Yan, C.; Miller, C.L.; Abe, J.I. Regulation of phosphodiesterase 3 and inducible cAMP early represor in the heart. Cir. Res. 2007, 100, 489–501. [Google Scholar] [CrossRef]
- de Aluja, A.S. Animales de laboratorio y la Norma Oficial Mexicana (NOM-062-ZOO-1999). Gac. Méd. Méx. 2002, 138, 295–298. [Google Scholar]
- Henrion, D.; Dowell, F.J.; Levy, B.I.; Michel, J.B. In vitro alteration of aortic vascular reactivity in hypertension induced by chronic NG-nitro-L-arginine methyl ester. Hypertension 1996, 28, 361–366. [Google Scholar] [CrossRef]
- Baños, G.; Carvajal, K.; Cardoso, G.; Zamora, J.; Franco, M. Vascular reactivity and effect of serum in a rat model of hypertriglyceridemia and hypertension. Am. J. Hypertens. 1997, 10(Pt. 1), 379–388. [Google Scholar] [CrossRef]
- Zhou, F.W.; Li, Y.J.; Lu, R.; Deng, H.W. Protection of calcitonin gene-related peptide-mediated preconditioning against coronary endothelial dysfunction induced by reperfusion in the isolated rat heart. Life Sci. 1999, 64, 1091–1097. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, L.E.; Ladas, T.P.; Chiang, C.C.; Duran, D.M. TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 2013, 250, 321–332. [Google Scholar] [CrossRef]
- Shah, V.; Haddad, F.G.; Garcia-Cadena, G.; Frangos, J.A.; Mennone, A.; Groszmann, R.J.; Sessa, W.C. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Investig. 1997, 100, 2923–2930. [Google Scholar] [CrossRef]
- Lugnier, C.; Muller, B.; Bec, A.L.; Beaudry, C.; Rousseau, E. Characterization of indolidan and rolipram sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J. Pharm. Exp. Ther. 1993, 265, 1142–1151. [Google Scholar]
- Ohba, Y.L.; Soda, K.; Zaitsu, K. A sensitive assay of human blood platelet cyclic nucleotide phosphodiesterase activity by HPLC using fluorescence derivatization and its application to assessment of cyclic nucleotide phosphodiesterase inhibitors. Biol. Pharm. Bull. 2001, 24, 567–569. [Google Scholar] [CrossRef][Green Version]
- González, G.; Alvarado-Vasquez, N.; Fernández-G, J.M.; Cruz-Robles, D.; Del Valle, L.; Pinzón, E.; Torres, I.; Rodriguez, E.; Zapata, E.; Gómez-Vidales, V.; et al. The antithrombotic effect of the aminoestrogen prolame (n-(3-hydroxy-1,3,5(10)-estratrien-17b-yl)-3-hydroxypropylamine) is linked to an increase in nitric oxide production by platelets and endothelial cells. Atherosclerosis 2010, 208, 62–68. [Google Scholar]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Han, F.; Huynh, B.H.; Shi, H.; Lin, B.; Ma, Y. Pteridine analysis in urine by capillary electrophoresis using laser-induced fluorescence detection. Anal. Chem. 1999, 71, 1265–1269. [Google Scholar] [CrossRef]
- Friedecky, D.; Tomková, J.; Maier, V.Z.; Janostáková, A.; Procházka, M.; Adam, T. Capillary electrophoretic method for nucleotide analysis in cells: Application on inherited metabolic disorders. Electrophoresis 2007, 28, 373–380. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Döring, H.J.; Dehvert, H. The isolated perfused warm-blooded heart according to Langendorff. In Biological Measurement Techniques, 1st ed.; Dehnert, H., Ed.; Biomes Technik-Verlag: Baden-Wurtemberg, Germany, 1988; pp. 1–131. [Google Scholar]
- Pfeiffer, S.; Leopold, E.; Schmidt, K.; Brunner, F.; Mayer, B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): Requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br. J. Pharmacol. 1996, 118, 1433–1440. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Jin, J.S.; Cho, Y.A.; Lee, J.H.; Park, S.; Jeong, S.W.; Kim, Y.H.; Lim, C.S.; Abd El-Aty, A.M.; Kim, G.S.; et al. Determination of polyphenols in three Capsicum annuum L. (bell pepper) varieties using high-performance liquid chromatographytandem mass spectrometry: Their contribution to overall antioxidant and anticancer activity. J. Sep. Sci. 2011, 34, 2967–2974. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar]
- Alyane, M.; Kebsa, M.B.W.; Boussenane, H.N.; Rouibah, H.; Lahouel, M. Cardioprotective effects and mechanism of action of polyphenol extracted from propolis against doxorubin toxicity. Pak. J. Pharm. Sci. 2008, 21, 201–209. [Google Scholar]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Zenebe, W.; Pechanova, O. Effects of red wine polyphenolic compounds on the cardiovascular system. Bratisl. Lek. Listy. 2002, 103, 159–165. [Google Scholar]
- Zenebe, W.; Pechánova, O.; Andriantsitohaina, R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol. Res. 2003, 52, 425–432. [Google Scholar]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Bernátova, I.; Pechánova, O.; Babál, P.; Kysela, S.; Stvrtina, S.; Andriantsitohaina, R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H942–H948. [Google Scholar] [CrossRef]
- Heiss, E.H.; Dirsh, V.M. Regulation of eNOS enzyme activity by posttranslational modification. Curr. Pharm. Des. 2014, 20, 3503–3513. [Google Scholar] [CrossRef]
- Wang, Y.; Szaba, T.; Welter, J.D.; Toth, A.; Tran, R.; Lee, J.; Kang, S.U.; Suh, Y.G.; Blumbergand, P.M.; Lee, J. High Affinity Antagonists of the Vanilloid Receptor. Mol. Pharmacol. 2002, 62, 947–956. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Pleiotropic pharmacological actions of capsazepine, a synthetic analogue of capsaicin, against various cancers and inflammatory diseases. Molecules 2019, 24, 995. [Google Scholar] [CrossRef]

| Experimental Groups | Mean Arterial Pressure (mmHg) | |
|---|---|---|
| Treatment | Normotensive | Hypertensive |
| Control | 118 ± 3 | 165 ± 4 * |
| Capsaicin | 121 ± 2 | 147 ± 2 ** |
| Capsazepine | 130 ± 5 | 172 ± 3 |
| Capsazepine + Capsaicin | 125 ± 8 | 154 ± 9 *** |
| Experimental Groups | Coronary Vascular Resistance (mmHg/mL/min) | |
|---|---|---|
| Treatment | Normotensive | Hypertensive |
| Control | 4 ± 0.3 | 11 ± 0.9 * |
| Capsaicin | 5 ± 0.4 | 7 ± 0.4 ** |
| Capsazepine | 6 ± 0.4 | 9 ± 0.4 |
| Capsazepine + Capsaicin | 6 ± 0.5 | 6 ± 0.5 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Narváez, J.C.; Pérez-Torres, I.; Castrejón-Téllez, V.; Varela-López, E.; Oidor-Chan, V.H.; Guarner-Lans, V.; Vargas-González, Á.; Martínez-Memije, R.; Flores-Chávez, P.; Cervantes-Yañez, E.Z.; et al. The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats. Int. J. Environ. Res. Public Health 2019, 16, 3576. https://doi.org/10.3390/ijerph16193576
Torres-Narváez JC, Pérez-Torres I, Castrejón-Téllez V, Varela-López E, Oidor-Chan VH, Guarner-Lans V, Vargas-González Á, Martínez-Memije R, Flores-Chávez P, Cervantes-Yañez EZ, et al. The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats. International Journal of Environmental Research and Public Health. 2019; 16(19):3576. https://doi.org/10.3390/ijerph16193576
Chicago/Turabian StyleTorres-Narváez, Juan Carlos, Israel Pérez-Torres, Vicente Castrejón-Téllez, Elvira Varela-López, Víctor Hugo Oidor-Chan, Verónica Guarner-Lans, Álvaro Vargas-González, Raúl Martínez-Memije, Pedro Flores-Chávez, Etzna Zizith Cervantes-Yañez, and et al. 2019. "The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats" International Journal of Environmental Research and Public Health 16, no. 19: 3576. https://doi.org/10.3390/ijerph16193576
APA StyleTorres-Narváez, J. C., Pérez-Torres, I., Castrejón-Téllez, V., Varela-López, E., Oidor-Chan, V. H., Guarner-Lans, V., Vargas-González, Á., Martínez-Memije, R., Flores-Chávez, P., Cervantes-Yañez, E. Z., Soto-Peredo, C. A., Pastelín-Hernández, G., & del Valle-Mondragón, L. (2019). The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats. International Journal of Environmental Research and Public Health, 16(19), 3576. https://doi.org/10.3390/ijerph16193576








