Evaluation of Air Contamination in Orthopaedic Operating Theatres in Hospitals in Southern Italy: The IMPACT Project
Abstract
:1. Introduction
- to determine the degree of microbial air contamination at a defined time (1-day study) in empty at rest) and working (in operation) OTs
- to assess air quality in the OTs by comparing different sampling systems
- to evaluate the association between microbiological data and particle counts with different HVAC systems.
2. Materials and Methods
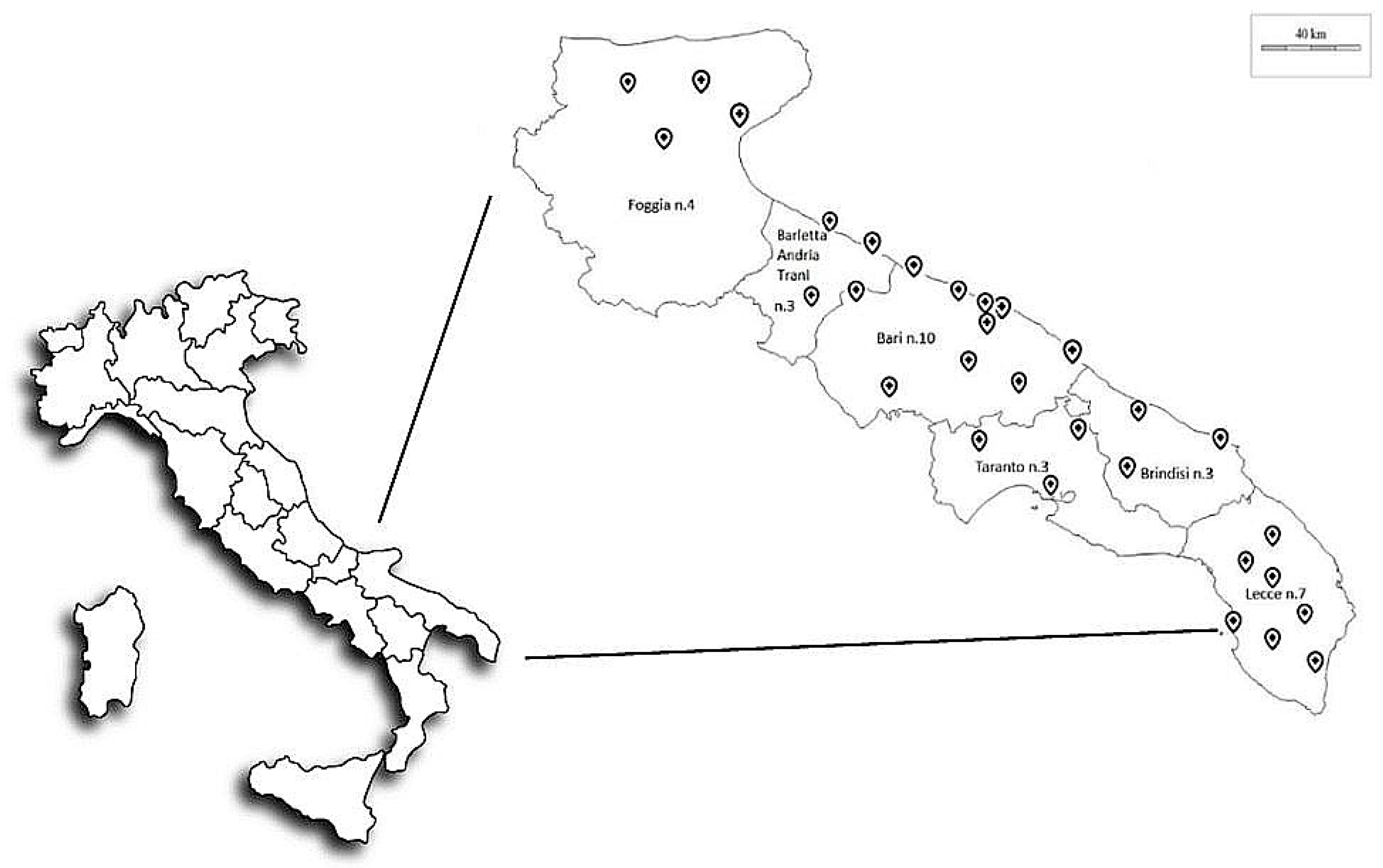
2.1. Study Design
2.2. Air Sampling
- (a)
- at rest, 1 h before the beginning of surgical activity, at the foot of the operative bed, to verify the efficiency of environmental cleaning systems and conditioner systems. All instruments started automatically 20 min after the technical team left the operating theatre.
- (b)
- in operation, 15 min after the surgical incision, at the foot of the operative bed, to verify the human impact on environmental pollution. During each operation, we collected detailed information concerning the number of staff in the OTs and the number of door openings.
2.3. Particle Counts
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, B.M.; Solheim, N. Occlusive scrub suits in operating theaters during cataract surgery: Effect on airborne contamination. Infect. Control Hosp. Epidemiol. 2002, 23, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, S.; Cencetti, S.; Rovesti, S.; Marchesi, I.; Bargellini, A.; Borella, P. Risk factors for particulate and microbial contamination of air in operating theatres. J. Hosp. Infect. 2007, 66, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.H.; Chung, F.F.; Tang, C.S. Long-term surveillance of air quality in medical center operating rooms. Am. J. Infect. Control 2011, 39, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, A.M.; Ottria, G.; Amicizia, D.; Perdelli, F.; Cristina, M.L. Operating theatre quality and prevention of surgical site infections. J. Prev. Med. Hyg. 2013, 54, 131–137. [Google Scholar] [PubMed]
- Cristina, M.L.; Sartini, M.; Schinca, E.; Ottria, G.; Spagnolo, A.M. Operating room environment and surgical site infections in arthroplasty procedures. J. Prev. Med. Hyg. 2016, 57, 142–148. [Google Scholar]
- Gelaw, K.A.; Aweke, A.M.; Astawesegn, F.H.; Demissie, B.W.; Zeleke, L.B. Surgical site infection and its associated factors following cesarean section: A cross sectional study from a public hospital in Ethiopia. Patient Saf. Surg. 2017, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection; WHO: Geneva, Switzerland, 2016; p. 158. [Google Scholar]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplasty 2012, 27, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Ong, K.L.; Schmier, J.; Mowat, F.; Saleh, K.; Dybvik, E.; Kärrholm, J.; Garellick, G.; Havelin, L.I.; Furnes, O. Future clinical and economic impact of revision total hip and knee arthroplasty. J. Bone Joint Surg. Am. 2007, 89, 144–151. [Google Scholar] [PubMed]
- Torre, M.; Carrani, E.; Luzi, I.; Ceccarelli, S.; Laricchiuta, P. Registro Italiano ArtroProtesi. Report Annuale 2018, 1st ed.; Il Pensiero Scientifico Editore: Roma, Italy, 2018. [Google Scholar]
- Friberg, B.; Friberg, S.; Burman, L.G. Inconsistent correlation between aerobic bacterial surface and air counts in operating rooms with ultraclean laminar air flows: Proposal of a new bacteriological standard for surface contamination. J. Hosp. Infect. 1999, 42, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, P.; Kubilay, N.Z.; Allegranzi, B.; Egger, M.; Gastmeier, P. Effect of laminar airflow ventilation on surgical site infections: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 553–561. [Google Scholar] [CrossRef]
- Pasquarella, C.; Barchitta, M.; D’Alessandro, D.; Cristina, M.L.; Mura, I.; Nobile, M.; Auxilia, F.; Agodi, A.; Avondo, S.; Basile, G.; et al. Heating, ventilation and air conditioning (HVAC) system, microbial air contamination and surgical site infection in hip and knee arthroplasties: The GISIO-SItI Ischia study. Ann. Ig. 2018, 30, 22–35. [Google Scholar] [PubMed]
- Health Technical Memorandum 03-01. Specialised Ventilation for Healthcare Premises—Part A: Design and Validation; TSO: Edinburgh, UK, 2007. [Google Scholar]
- ISPESL (Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro). Linee Guida Sugli Standard di Sicurezza e di Igiene Del Lavoro Nel Reparto Operatorio; ISPESL: Rome, Italy, 2009. [Google Scholar]
- Die Spitäler der Schweiz. Anhang 4 zur KlatAS. Beschreibung der IMA-Methode.Stand. 2007. Available online: http://www.hplus.ch/fileadmin/user_upload/Betriebswirtschaft/Spitalinfrastruktur/deutsch/Anhang%204%20Standard%20IMA.pdf (accessed on 29 June 2019).
- Pittet, D.; Ducel, G. Infectious risk factors related to operating rooms. Infect. Control Hosp. Epidemiol. 1994, 15, 456–462. [Google Scholar] [CrossRef]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Panatto, D.; Gasparini, R.; Orlando, P.; Ottria, G.; Perdelli, F. Can Particulate Air Sampling Predict Microbial Load in Operating Theatres Arthroplasty? PLoS ONE 2012, 7, e52809. [Google Scholar] [CrossRef] [PubMed]
- Birgand, G.; Toupet, G.; Rukly, S.; Antoniotti, G.; Dechamps, M.N.; Lepelletier, D.; Pornet, C.; Stern, J.B.; Vandamme, Y.M.; Van der Mee-Marquet, N. Air contamination for predicting wound contamination in clean surgery: A large multicenter study. Am. J. Infect. Control 2015, 43, 516–521. [Google Scholar] [CrossRef]
- D’Amico, A.; Montagna, M.T.; Caggiano, G.; De Giglio, O.; Rutigliano, S.; Lopuzzo, M.; Mascipinto, S.; Napoli, C.; Currà, E.; D’Alessandro, D. Observational study on hospital building heritage and microbiological air quality in the orthopedic operating theater: The IM.PA.C.T. Project. Ann. Ig. 2019, 31, 482–495. [Google Scholar]
- Standardization IOS. Cleanrooms and Associated Controlled Environments: Biocontamination Control, Part 1: General Principles and Methods, ISO 14698-1:2003; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Carvalho, E.; Sindt, C.; Verdier, A.; Galan, C.; O’Donoghue, L.; Parks, S.; Thibaudon, M. Performance of the Coriolis air sampler, a high-volume aerosol-collection system for quantification of airborne spores and pollen grains. Aerobiologia 2008, 24, 191–201. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Schulz, J.; Hartung, J. Air samplings in a Campylobacter jejuni positive laying hen flock. Ann. Agric. Environ. Med. 2013, 20, 16–20. [Google Scholar]
- Pasquarella, C.; Pitzurra, O.; Savino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef] [Green Version]
- IOS. Determination of Particle Size Distribution-Single Particle Light Interaction Methods-Part 4: Light Scattering Airborne Particle Counter for Clean Spaces. ISO 21501-1:2007 (E); ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Montagna, M.T.; De Giglio, O.; Cristina, M.L.; Napoli, C.; Pacifico, C.; Agodi, A.; Baldovin, T.; Casini, B.; Coniglio, M.A.; D’Errico, M.M.; et al. Evaluation of Legionella air contamination in healthcare facilities by different sampling methods: An Italian multicenter study. Int. J. Environ. Res. Public Health. 2017, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Bartlett, K.H.; Brauer, M.; Stephens, G.M.; Black, W.A.; Teschke, K. A field comparison of four samplers for enumerating fungal aerosols I. Sampling characteristics. Indoor Air. 2004, 14, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Pasquarella, C.; Albertini, R.; Dall’aglio, P.; Saccani, E.; Sansebastiano, G.E.; Signorelli, C. Air microbial sampling: The state of the art. Ig. Sanita Pubbl. 2008, 64, 79–120. [Google Scholar] [PubMed]
- Landrin, A.; Bissery, A.; Kac, G. Monitoring air sampling in operating theatres: Can particle counting replace microbiological sampling? J. Hosp. Infect. 2005, 61, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, L.J.; Taylor, J.; Cloutman-Green, E.A.; Hartley, J.C.; Lai, K.M. Can clean-room particle counters be used as an infection control tool in hospital operating theatres? Indoor Built Environ. 2012, 21, 381–391. [Google Scholar] [CrossRef]
- Napoli, C.; Marcotrigiano, V.; Montagna, M.T. Air sampling procedures to evaluate microbial contamination: A comparison between active and passive methods in operating theatres. BMC Public Health. 2012, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Tafuri, S.; Montenegro, L.; Cassano, M.; Notarnicola, A.; Lattarulo, S.; Montagna, M.T.; Moretti, B. Air sampling methods to evaluate microbial contamination in operating theatres: Results of a comparative study in an orthopaedics department. J. Hosp. Infect. 2012, 80, 128–132. [Google Scholar] [CrossRef]
- Pasquarella, C.; Veronesi, L.; Napoli, C.; Castiglia, P.; Liguori, G.; Rizzetto, R.; Torre, I.; Righi, E.; Farruggia, P.; Tesauro, M.V.; et al. Microbial environmental contamination in Italian dental clinics: A multicenter study yielding recommendations for standardized sampling methods and threshold values. Sci. Total Environ. 2012, 420, 289–299. [Google Scholar] [CrossRef]
- Caggiano, G.; Napoli, C.; Coretti, C.; Lovero, G.; Scarafile, G.; De Giglio, O.; Montagna, M.T. Mold contamination in a controlled hospital environment: A 3-year surveillance in southern Italy. BMC Infect. Dis. 2014, 14, 595. [Google Scholar] [CrossRef]
- Shaw, B.H.; Whyte, W. Air movement through doorways: The influence of temperature and its control by forced airflow. Build Serv. Eng. 1974, 42, 210–218. [Google Scholar]
- Wilson, D.J.; Kiel, D.E. Gravity-driven counterflow through an open door in a sealed room. Build Environ. 1990, 25, 379–388. [Google Scholar] [CrossRef]
- Ritter, M.A.; Eitzen, H.; French, M.L.; Hart, J.B. The operating room environment as affected by people and the surgical face mask. Clin. Orthop. Relat. Res. 1975, 111, 147–150. [Google Scholar] [CrossRef] [PubMed]

| Variables | M-OTs (N = 17) | T-OTs (N = 18) | p-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| DOORS | 2 | 1–2 | 2 | 1–3 | 0.9721 |
| Sliding doors | 1 | 1–1 | 2 | 1–2 | 0.1697 |
| Swinging doors | 0 | 0–1 | 0 | 0–0 | 0.0899 |
| Volume of OTs | 114 | 105–123 | 140 | 114–170 | 0.0729 |
| Surface of OTs | 38 | 34–42 | 42 | 39–47.5 | 0.1922 |
| Number of air changes/h | 15 | 15–26 | 18.29 | 15–18.5 | 0.8162 |
| Method | At Rest | In Operation | ||||||
|---|---|---|---|---|---|---|---|---|
| Mixed | Turbulent | Mixed | Turbulent | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| SAS (cfu/m3) | 0 $ | 0–2 | 0.5 # | 0–3 | 15 $ | 7.5–60 | 23.5 # | 17–58 |
| Coriolis®μ (cfu/m3) | 0 ^ | 0–0 | 0 | 0–0 | 48 ^ | 24–67.75 | 10.5 | 0–52 |
| Settling plates (IMA) | 0 * | 0–1 | 0 § | 0–2 | 4 * | 2.75–6 | 4.5 § | 4–8 |
| Variables | Mixed Airflow | |||||
| SAS | Coriolis®μ | Settling Plates | ||||
| Spearman Coeff. | p-Value | Spearman Coeff. | p-Value | Spearman Coeff. | p-Value | |
| Particles ≥ 0.5 µm | −0.071 | 0.818 | 0.045 | 0.8839 | −0.125 | 0.6848 |
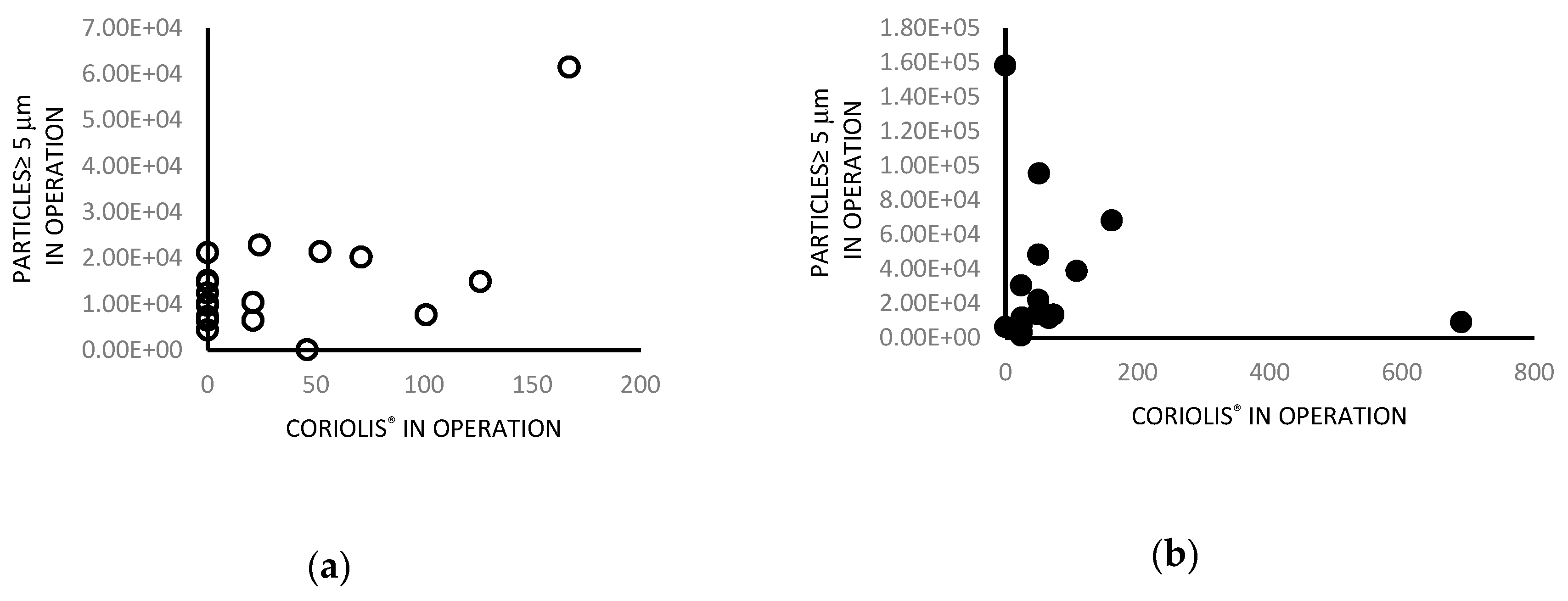
| Particles ≥ 5 µm | 0.015 | 0.9631 | 0.68 | 0.0158 | 0.006 | 0.9854 |
| Number of doors | 0.048 | 0.8822 | 0.32 | 0.3147 | −0.063 | 0.8452 |
| Number of people | 0.44 | 0.1496 | −0.15 | 0.6489 | 0.26 | 0.4228 |
| Variables | Turbulent Airflow | |||||
| SAS | Coriolis®μ | Settling plates | ||||
| Spearman Coeff. | p-Value | Spearman Coeff. | p-Value | Spearman Coeff. | p-Value | |
| Particles ≥ 0.5 µm | 0.24 | 0.4542 | 0.62 | 0.0316 | 0.23 | 0.4786 |
| ≥ 5 µm | 0.47 | 0.1015 | 0.47 | 0.1041 | 0.16 | 0.5988 |
| Number of doors | −0.12 | 0.6972 | −0.27 | 0.3666 | 0.31 | 0.3002 |
| Number of people | 0.17 | 0.5775 | 0.34 | 0.2542 | 0.34 | 0.4315 |
| Mixed Airflow | |||||
| Door Openings (OTs = 10) | Doors Kept Open (OTs = 7) | p-Value | |||
| No. | % | No. | % | ||
| SETTLING PLATES (IMA/plate) | |||||
| 0 | rs | rs | 0 | 0.0 | 1 |
| >0 | 10 | 100.0 | 7 | 100.0 | |
| SAS (cfu/m3) | |||||
| 0 | 0 | 0.0 | 0 | 0.0 | 0.134769 |
| >0 | 10 | 100.0 | 7 | 100.0 | |
| Coriolis®μ (cfu/m3) | |||||
| 0 | 0 | 0.0 | 2 | 28.6 | 0.1544 |
| >0 | 10 | 100.0 | 5 | 71.4 | |
| Turbulent Airflow | |||||
| Door Openings (OTs = 10) | Doors Kept Open (OTs = 7) | p-Value | |||
| No. | % | No. | % | ||
| SETTLING PLATES (IMA/plate) | |||||
| 0 | 0 | 0.0 | 1 | 12.5 | 0.44444 |
| >0 | 10 | 100.0 | 7 | 87.5 | |
| SAS (cfu/m3) | |||||
| 0 | 0 | 0.0 | 0 | 0.0 | 1 |
| >0 | 10 | 100.0 | 8 | 100.0 | |
| Coriolis®μ (cfu/m3) | |||||
| 0 | 6 | 60.0 | 3 | 37.5 | 0.6372 |
| >0 | 4 | 40.0 | 5 | 62.5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagna, M.T.; Rutigliano, S.; Trerotoli, P.; Napoli, C.; Apollonio, F.; D’Amico, A.; De Giglio, O.; Diella, G.; Lopuzzo, M.; Marzella, A.; et al. Evaluation of Air Contamination in Orthopaedic Operating Theatres in Hospitals in Southern Italy: The IMPACT Project. Int. J. Environ. Res. Public Health 2019, 16, 3581. https://doi.org/10.3390/ijerph16193581
Montagna MT, Rutigliano S, Trerotoli P, Napoli C, Apollonio F, D’Amico A, De Giglio O, Diella G, Lopuzzo M, Marzella A, et al. Evaluation of Air Contamination in Orthopaedic Operating Theatres in Hospitals in Southern Italy: The IMPACT Project. International Journal of Environmental Research and Public Health. 2019; 16(19):3581. https://doi.org/10.3390/ijerph16193581
Chicago/Turabian StyleMontagna, Maria Teresa, Serafina Rutigliano, Paolo Trerotoli, Christian Napoli, Francesca Apollonio, Alessandro D’Amico, Osvalda De Giglio, Giusy Diella, Marco Lopuzzo, Angelo Marzella, and et al. 2019. "Evaluation of Air Contamination in Orthopaedic Operating Theatres in Hospitals in Southern Italy: The IMPACT Project" International Journal of Environmental Research and Public Health 16, no. 19: 3581. https://doi.org/10.3390/ijerph16193581
APA StyleMontagna, M. T., Rutigliano, S., Trerotoli, P., Napoli, C., Apollonio, F., D’Amico, A., De Giglio, O., Diella, G., Lopuzzo, M., Marzella, A., Mascipinto, S., Pousis, C., Albertini, R., Pasquarella, C., D’Alessandro, D., Serio, G., & Caggiano, G. (2019). Evaluation of Air Contamination in Orthopaedic Operating Theatres in Hospitals in Southern Italy: The IMPACT Project. International Journal of Environmental Research and Public Health, 16(19), 3581. https://doi.org/10.3390/ijerph16193581










