Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Element Analysis
2.2.1. Mercury
2.2.2. Zinc, Copper, Iron and Manganese
2.3. Instrumental Analysis and Quality Control
2.4. Fatty Acids Analysis
2.5. The Lipid Quality Indexes (AI and TI)
2.6. Human Health Risk Assessment
2.6.1. Estimated Daily Intake of Mercury (EDI)
2.6.2. Target Hazard Quotient (THQ)
2.6.3. The Combined Risk of Many Heavy Metals
2.7. Statistical Analysis
3. Results and Discussion
3.1. Differences in the Content of Heavy Metals
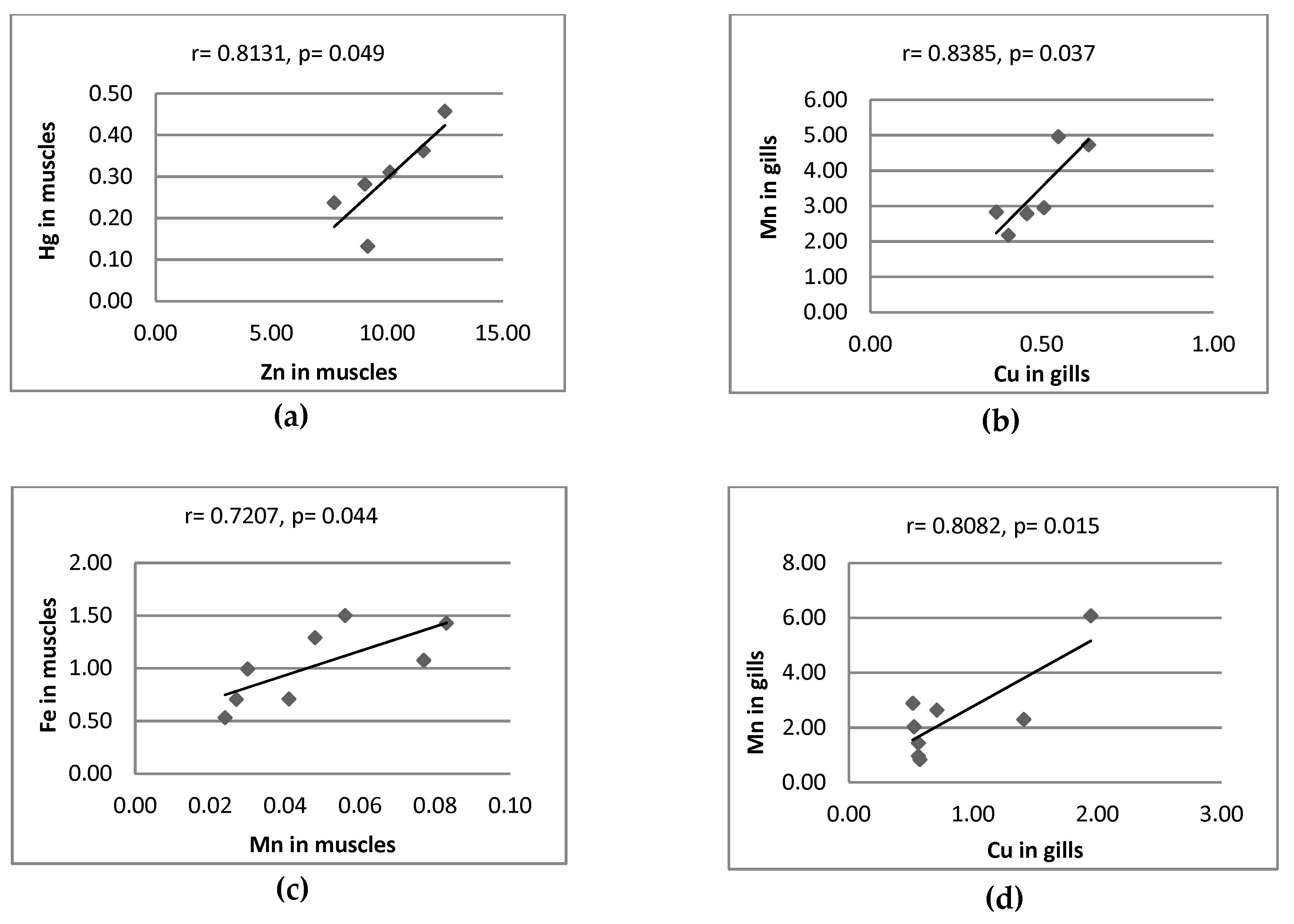
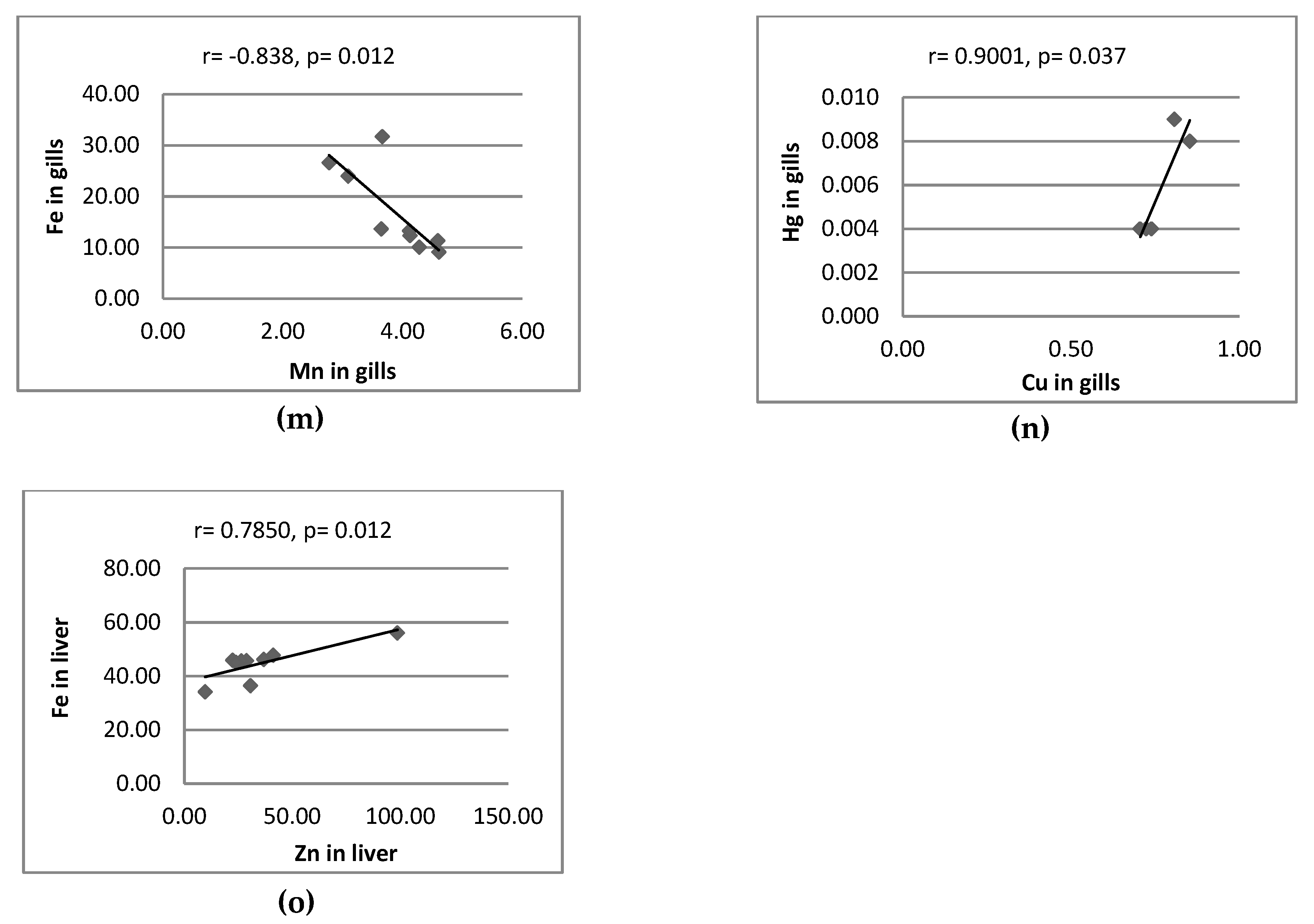
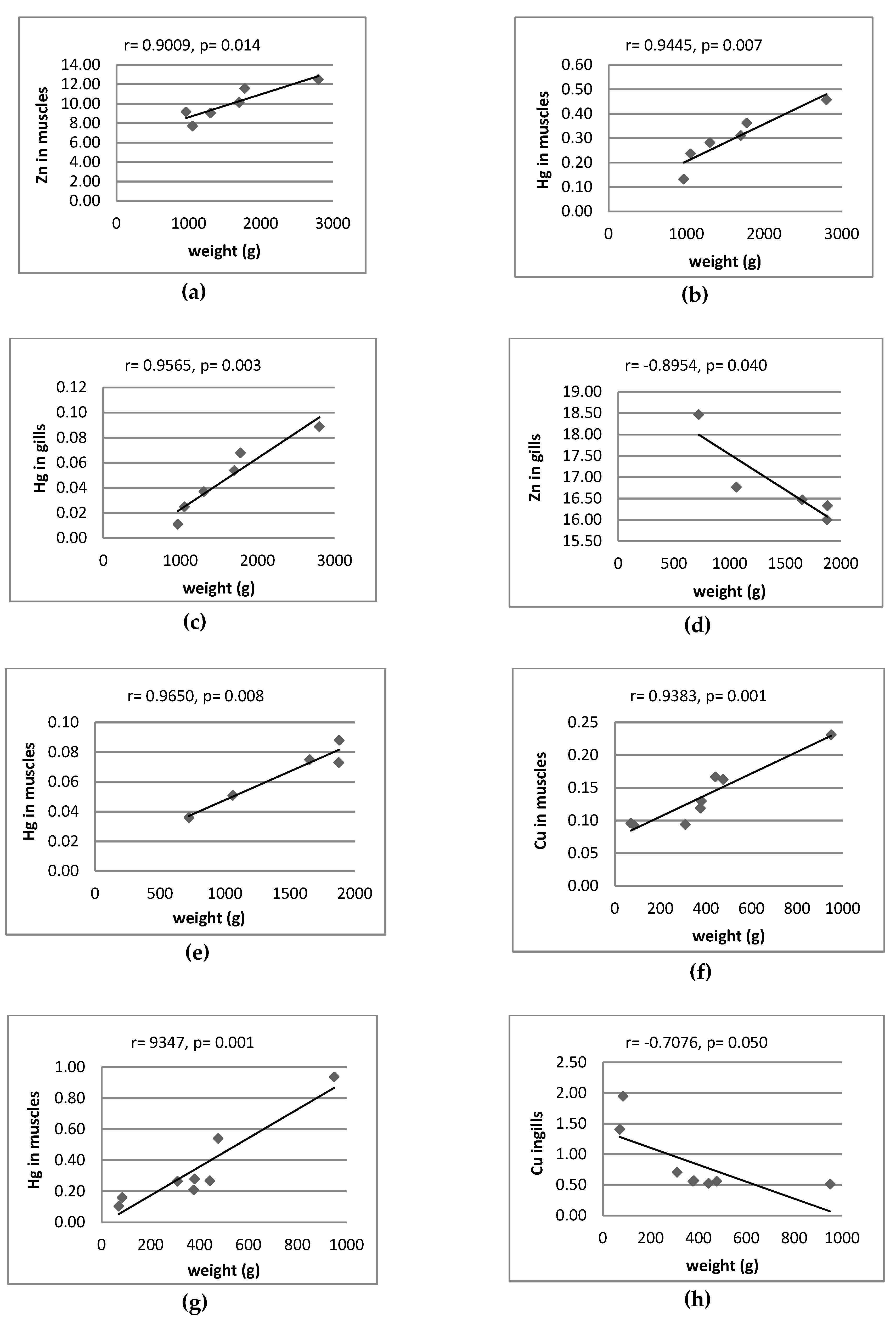
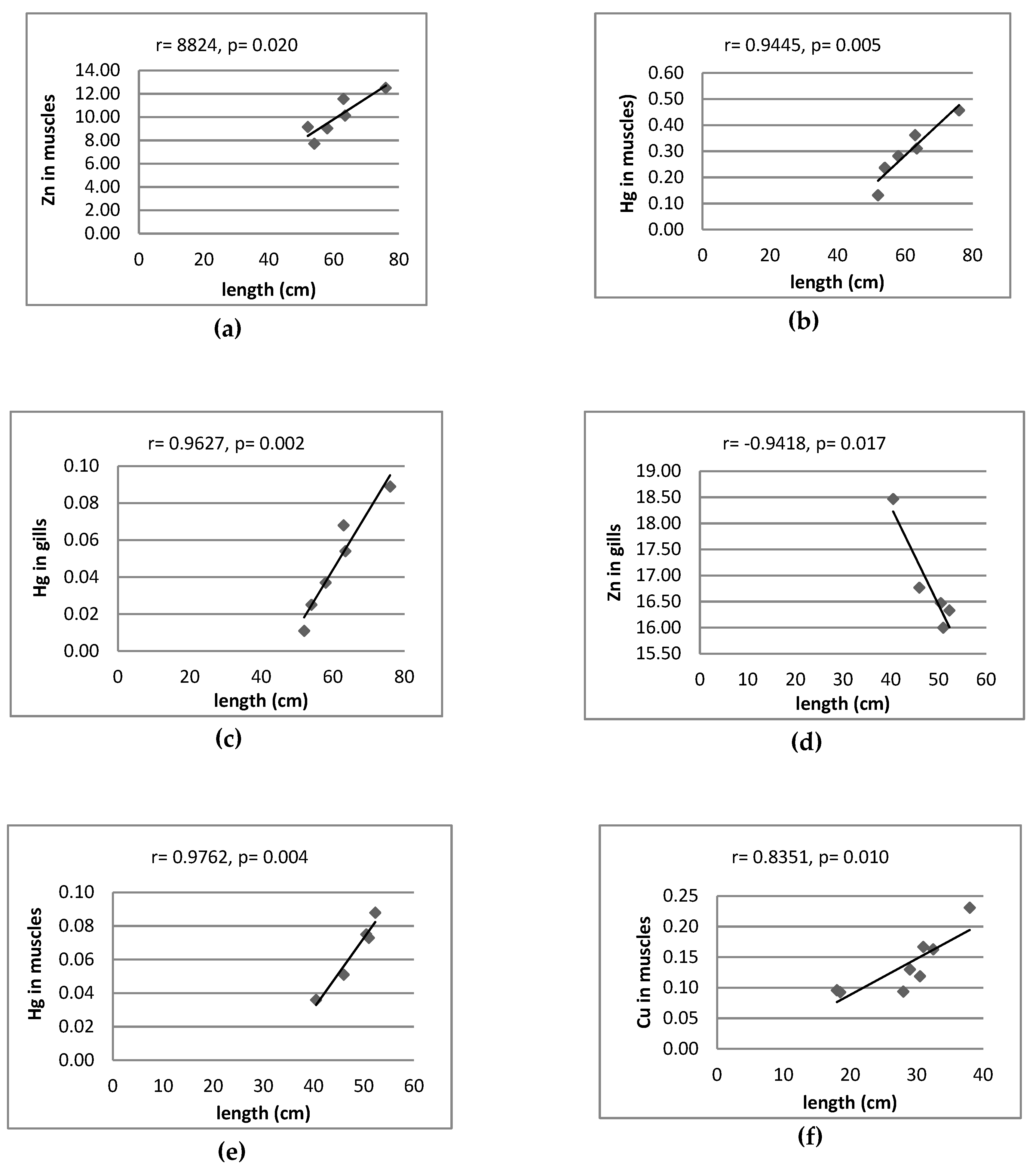
3.2. Correlation between Metal Pairs and Size
3.3. Fatty Acids and Lipid Quality Indexes
3.4. Human Health Risk
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strungaru, S.-A.; Nicoră, M.; Rău, M.A.; Plăvan, G.; Micu, D. Do you like to eat fish? An overview of the benefits of fish consumption and risk of mercury poisoning. Analele Științifice ale Universității „Alexandru Ioan Cuza” din Iași, s. Biologie animală. 2015, 61, 117–123. [Google Scholar]
- Pal, J.; Shukla, B.N.; Maurya, A.K.; Verma, H.O.; Pandey, G.; Amitha. A review on role of fish in human nutrition with special emphasis to essential fatty acid. Int. J. Fish Aqu. Stud. 2018, 6, 427–430. [Google Scholar]
- Simopoulos, A.P. Human requirement for n-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- Steffens, W. Freshwater fish—Wholesome foodstuffs. Bulg. J. Agric. Sci. 2006, 12, 320–328. [Google Scholar]
- Mohanty, B.P. Nutritional value of food fish. In Conspectus of Inland Fisheries Management; Das, A.K., Panda, D., Eds.; Barrackpore: West Bengal, India, 2015. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Candela, C.G.; López, L.M.B.; Kohen, V.L. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health. Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [CrossRef]
- Johnson, M.; Bradford, C. Omega-3, omega-6 and omega-9 fatty acids: Implications for cardiovascular and other diseases. J. Glycom. Lipidom. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Arbex, A.K.; Bizarro, V.R.; Santos, J.C.S.; Araújo, L.M.M.; de Jesus, A.L.C.; Fernandes, M.S.A.; Salles, M.M.; Rocha, D.R.T.W.; Marcadenti, A. The Impact of the Essential Fatty Acids (EFA) in Human Health. OJEMD 2015, 5, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 fatty acids and cardiovascular disease: Are there benefits. Curr. Treat. Options Cardio. Med. 2016, 18, 1–16. [Google Scholar] [CrossRef]
- Ristić-Medić, D.; Vučić, V.; Takić, M.; Karadžić, I.; Glibetić, M. Polyunsaturated fatty acids in health and disease. J. Serb. Chem. Soc. 2013, 78, 1269–1289. [Google Scholar] [CrossRef]
- FAO Food and Nutrition Paper. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation; FAO: Geneva, Switzerland, 2008. [Google Scholar]
- Kris-Etherton, P.M.; Yu, S. Individual fatty acid effects on plasma lipids and lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Thorgilsson, B.; Nunes, M.L.; Gunnlaungsdóttir, H. Review of Evidence for the Beneficial Effect of Fish Consumption; Skýrsla Matís 51-10; Matísohf/Matis-Food Research, Innovation & Safety: Reykjavík, Iceland, 2010. [Google Scholar]
- Jezierska, B.; Witeska, M. Metal Toxicity to Fish; Monographs No 42; Akademia Podlaska: Siedlce, Poland, 2001; 318p. [Google Scholar]
- Elbeshti, R.T.A.; Elderwish, N.M.; Abdelali, K.M.K.; Tastan, Y. Effects of Heavy Metals on Fish. MENBA J. Fish. Fac. Sayfa 2018, 4, 36–47. [Google Scholar]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 187. [Google Scholar] [CrossRef]
- Vidyavati, S.D.; Sneha, A.; Katti, S.M. Zinc: The Importance in Human Life. IJHBR 2016, 4, 18–20. [Google Scholar]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [Green Version]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855–858. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V. Essential Trace Elements in Human Health: A Physician’s View; Publishing House of Tomsk State University: Tomsk, Russia, 2018; p. 224. [Google Scholar]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Łuczyński, M.J.; Paszczyk, B.; Tońska, E. Concentration of mercury in muscles of predatory and non-predatory fish from lake Pluszne (Poland). J. Vet. Res. 2016, 60, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. The isolation of lipids from tissues. Recommended Procedures. Chloroform-methanol (2:1, v/v) extraction and “Folch” wash. In Lipid Analysis. Isolation, Separation, Identification and Structural Analysis of Lipids; Christie, W.W., Ed.; Pergamon Press Oxford: New York, NY, USA; Toronto, ON, Canada; Sydney, Austrilia; Braunschweig, Germany, 1973; pp. 39–40. [Google Scholar]
- Żegarska, Z.; Jaworski, J.; Borejszo, Z. Evaluation of the Peisker modified method for extracting methyl esters from fatty acids. Acta Acad. Agric. Technol. 1991, 24, 25–33. (In Polish) [Google Scholar]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices, of raw roe of blue fin tuna (Thunnus thunnus L.) and their salted product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Agradi, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla anguilla). Comp. Biochem. Physiol. 1992, 101B, 79–81. [Google Scholar] [CrossRef]
- Senso, L.; Suárez, M.D.; Ruiz-Cara, T.; Garcia-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- Polak-Juszczak, L.; Nermer, T. Methylmercury and total mercury in eels, Anguilla anguilla, from Lakes in Northeastern Poland: Health risk assessment. EcoHealth 2016, 13, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Baki, M.A.; Kundu, G.K.; Islam, S.; Islam, M. Human health risks from heavy metals in fish of Buriganga river, Bangladesh. SpringerPlus 2016, 5, 1697. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Regional Screening Level (RSL) Summary Table; United States Environmental Protection Agency: Washington, DC, USA, 2017. [Google Scholar]
- Saha, N.; Zaman, M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2013, 185, 3867–3878. [Google Scholar] [CrossRef]
- Subotić, S.; Višnjić-Jeftić, Ž.; Spasić, S.; Hegediš, A.; Krpo-Ćetković, J.; Lenhardt, M. Concentrations of 18 elements in muscle, liver, gills, and gonads of Sichel (Pelecus cultratus), ruffe (Gymnocephalus cernua), and European perch (Perca fluviatilis) in the Danube River near Belgrade (Serbia). Water Air Soil Pollut. 2015, 226–287. [Google Scholar] [CrossRef]
- Al Sayegh-Petkovšek, S.; Mazej Grudnik, Z.; Pokorny, B. Heavy metals and arsenic concentrations in ten fish species from the Šalek lakes (Slovenia): Assessment of potential human health risk due to fish consumption. Environ. Monit. Assess. 2012, 184, 2647–2662. [Google Scholar] [CrossRef]
- Pintaeva, E.T.; Bazarsadueva, S.V.; Radnaeva, L.D.; Petrov, E.A.; Smirnova, O.G. Content and character of metal accumulation in fish of the Kichera River (a Tributary of Lake Baikal). Contemp. Probl. Ecol. 2011, 4, 64–68. [Google Scholar] [CrossRef]
- Ðikanović, V.; Skorić, S.; Gačić, Z. Concentrations of metals and trace elements in different tissues of nine fish species from the Meduvrsje Reservoir (West Morava River Basin, Serbia). Arch. Biol. Sci. 2016, 68, 811–819. [Google Scholar] [CrossRef]
- Rakocevic, J.; Sukovic, D.; Maric, D. Distribution and relationships of eleven trace elements in muscle of six fish species from Skadar Lake (Montenegro). Turk. J. Fish Aquat. Sci. 2018, 18, 647–657. [Google Scholar] [CrossRef]
- Maršálek, P.; Svobodová, Z.; Randák, T.; Švehla, J. Mercury and methylmercury contamination of fish from the Skalka reservoir: A case study. Acta Vet. Brno 2005, 74, 427–434. [Google Scholar] [CrossRef]
- Mazej, Z.; Al Sayegh-Petkovšek, S.; Pokorny, B. Heavy metal concentrations in food chain of Lake Velenjsko jezero, Slovenia: An artificial lake from mining. Arch. Environ. Contam. Toxicol. 2010, 58, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Alipour, H.; Pourkhabbaz, A.; Hassanpour, M. Estimation of potential health risks for some metallic elements by consumption of fish. Water Qual. Expo. Health 2015, 7, 179–185. [Google Scholar] [CrossRef]
- Stanek, M.; Janicki, B.; Kupcewicz, B. Content of selected heavy metals in the organs of fish from Żnin Duże Lake. Folia Biol. (Kraków) 2005, 53, 115–119. [Google Scholar] [CrossRef]
- Lidwin-Kaźmierkiewicz, M.; Pokorska, K.; Protasowicki, M.; Rajkowska, M.; Wechterowicz, Z. Content of selected essential and toxic metals in meat of freshwater fish from West Pomerania, Poland. Pol. J. Food Nutr. Sci. 2009, 59, 219–224. [Google Scholar]
- Łuczyńska, J.; Tońska, E. The effect of fish size on the content of zinc, iron, copper, and manganese in the muscles of perch (Perca fluviatilis L.) and pike (Esox lucius L.). Arch. Pol. Fish 2006, 14, 5–13. [Google Scholar]
- Łuczyńska, J. The influence of weight and length on the mercury content in the muscle tissue of fish from four lakes in the Olsztyn Lake District (Poland). Arch. Pol. Fish 2005, 13, 51–61. [Google Scholar]
- Łuczyńska, J.; Paszczyk, B.; Łuczyński, M.J. Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotox. Environ. Safe 2018, 153, 60–67. [Google Scholar] [CrossRef]
- Nozari, M.; Esmaili-Sari, A.; Riyahi-Bakhtiyari, A.; Aazami, J. Mercury Concentration in Muscle and Liver of Pike (Esoxlucius) Collected from Anzali International Wetland, Iran. Iran. J. Toxicol. 2011, 5, 516–520. [Google Scholar]
- Miller, A.; Bignert, A.; Porvari, P.; Danielsson, S.; Verta, M. Mercury in Perch (Perca fluviatilis) from Swedenand Finland. Water Air Soil Pollut. 2013, 224, 1–12. [Google Scholar] [CrossRef]
- Polak-Juszczak, L.; Komar-Szymczak, K. Fatty acid profiles and fat contents of commercially important fish from Vistula Lagoon. Pol. J. Food Nutr. Sci. 2009, 59, 225–229. [Google Scholar]
- Łuczyńska, J.; Borejszo, Z.; Łuczyński, M.J. The composition of fatty acids in muscles of six freshwater fish species from the Mazurian Great Lakes (northeastern Poland). Arch. Pol. Fish 2008, 16, 167–178. [Google Scholar] [CrossRef]
- Cengiz, E.I.; Ünlü, E.; Baṣhan, M. Fatty acid composition of total lipids in muscle tissues of nine freshwater fish from the River Tigris (Turkey). Turk. J. Biol. 2010, 34, 433–438. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Borejszo, Z.; Tarkowski, Ł. Fatty acid profile of muscles of freshwater fish from Olsztyn markets. Pol. J. Food Nutr. Sci. 2012, 62, 51–55. [Google Scholar] [CrossRef]
- Ljubojevic, D.; Trbovic, D.; Lujic, J.; Bjelic-Cabrilo, O.; Kostic, D.; Novakov, N.; Cirkovic, M. Fatty acid composition of fishes from Inland waters. Bulg. J. Agric. Sci. 2013, 19, 62–71. [Google Scholar]
- Łuczyńska, J.; Paszczyk, B.; Łuczyński, M.J. Fatty acid profiles in marine and freshwater fish from fish markets in northeastern Poland. Arch. Pol. Fish 2014, 22, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Kainz, M.J.; Hager, H.H.; Rasconi, S.; Kahilainen, K.K.; Amundsen, P.-A.; Hayden, B. Polyunsaturated fatty acids in fishes increase with total lipids irrespective of feeding sources and trophic position. Ecosphere 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M.J. Mercury, fatty acids content and lipid quality indexes in muscles of freshwater and marine fish on the Polish market, risk assessment of fish consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef]
- Linhartová, Z.; Krejsa, J.; Zajíc, T.; Másílko, J.; Sampels, S.; Mráz, J. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquacult. Int. 2018, 26, 695–711. [Google Scholar] [CrossRef]
- Tilami, S.K.; Sampels, S.; Zajic, T.; Krejsa, J.; Másílko, J.; Mráz, J. Nutritional value of several commercially important river fish species from the Czech Republic. PEER J. 2018, 6, e5729. [Google Scholar] [CrossRef] [PubMed]
- Statistical Yearbook of Agriculture. 2016; p. 460. Available online: https://stat.gov.pl/files/gfx/portalinformacyjny/en/defaultaktualnosci/3328/6/11/1/statistical_yearbook_of_agriculture_2016.pdf (accessed on 15 December 2016). (In Polish)
- Addo-Bediako, A.; Marr, S.M.; Jooste, A.; Luus-Powell, W.J. Human health risk assessment for silver catfish Schilbe intermedius Rϋppell, 1832, from two impoundments in the Olifants River, Limpopo, South Africa. Water SA 2014, 40, 607–614. [Google Scholar] [CrossRef]
- Küpeli, T.; Altundađ, H.; Imamođlu, M. Assessment of trace element levels in muscle tissues of fish species collected from a River, Stream, Lake, and Sea in Sakarya, Turkey. Hindawi 2014, 2014, 496107. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, K.; Lenhardt, M.; Višnjić-Jeftić, Ž.; Ɖikanović, V.; Skorić, S.; Smederevac-Lalić, M.; Jaćimović, M.; Gačić, Z.; Jarić, I.; Hegediš, A. Assessment of fish stocks and elemental pollution in the Danube, Sava and Kolubara rivers on the territory of the city of Belgrade, Serbia. Acta Zool. Bulg. 2014, 7, 179–184. [Google Scholar]






| Bream Abramis brama L. (n = 5) | Roach Rutilus rutilus L. (n = 9) | Pike Esox lucius L. (n = 6) | Perch Perca fluviatilis L. (n = 8) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Mg/kg Wet Weight | ||||||||
| Length (cm) | 48.1 | 4.8 | 30.8 | 7.2 | 61.1 | 8.7 | 28.2 | 6.8 |
| Weight (g) | 1438.0 | 521.7 | 426.2 | 272.9 | 1601.7 | 675.3 | 386.0 | 274.2 |
| Muscles Cu | 0.210 c | 0.053 | 0.208 c | 0.127 | 0.155 c | 0.029 | 0.137 c | 0.048 |
| Fills Cu | 0.766 b | 0.062 | 1.366 b | 1.181 | 0.486 b | 0.099 | 0.849 b | 0.535 |
| Liver Cu | 11.92 a | 9.313 | 5.749 a | 4.718 | 3.475 a | 1.710 | 2.085 a | 1.211 |
| Muscles Zn | 4.183 c | 0.915 | 4.522 c | 1.035 | 10.020 c | 1.763 | 4.352 b | 0.520 |
| Gills Zn | 16.81 b | 0.968 | 78.33 a | 31.67 | 115.1 a | 22.95 | 22.21 a | 6.316 |
| Liver Zn | 34.46 a | 10.08 | 35.38 b | 25.38 | 35.41 b | 8.095 | 23.72 a | 3.331 |
| Muscles Mn | 0.122 c | 0.032 | 0.106 c | 0.054 | 0.090 c | 0.039 | 0.048 c | 0.022 |
| Gills Mn | 5.148 a | 1.751 | 3.874 a | 0.638 | 3.402 a | 1.150 | 2.394 a | 1.666 |
| Liver Mn | 1.230 b | 0.481 | 0.843 b | 0.413 | 1.095 b | 0.092 | 1.149 b | 0.355 |
| Muscles Fe | 1.309 b | 0.286 | 1.005 c | 0.435 | 0.833 b | 0.170 | 1.030 c | 0.360 |
| Gills Fe | 20.91 a | 2.571 | 16.89 b | 8.253 | 23.38 a | 12.01 | 17.59 b | 4.211 |
| Liver Fe | 30.65 a | 18.56 | 44.74 a | 6.364 | 21.16 a | 6.163 | 26.40 a | 7.352 |
| Muscles Hg | 0.065 a | 0.021 | 0.140 a | 0.033 | 0.297 a | 0.111 | 0.346 a | 0.271 |
| Gills Hg | 0.006 b | 0.002 | 0.025 c | 0.008 | 0.047 c | 0.029 | 0.050 c | 0.028 |
| Liver Hg | 0.050 a | 0.045 | 0.059 b | 0.030 | 0.121 b | 0.015 | 0.147 b | 0.055 |
| Species | Hg | Zn | Cu | Fe | Mn | References | |
|---|---|---|---|---|---|---|---|
| Muscles | |||||||
| Roach, Rutilus rutilus (L.) | Skalka Reservoir | 0.81 | - | - | - | - | [40] |
| Bream, Abramis brama (L.) | West Morava River Basin | 1.22 | 27.30 | 1.45 | 21.13 | 0.82 | [38] |
| Roach, Rutilus rutilus (L.) | West Morava River Basin | 0.95 | 47.77 | 1.47 | 25.51 | 1.02 | [38] |
| Perch, Perca fluviatilis (L.) | West Morava River Basin | 2.06 | 22.69 | 0.02 | 9.236 | 0.45 | [38] |
| Pike, Esox lucius (L.) | West Morava River Basin | 1.04 | 48.57 | 0.84 | 18.304 | 1.249 | [38] |
| Bream, Abramis brama (L.) | Skalka Reservoir | 0.96 | - | - | - | - | [40] |
| Perch, Perca fluviatilis (L.) | Velenjsko Lake | 0.12 | 12.5 | - | - | - | [41] |
| Perch, Perca fluviatilis (L.) | Šalek lakes | 0.12 | 12.5 | - | - | - | [36] |
| Roach, Rutilus rutilus (L.) | Šalek lakes | 0.08 | 13.4 | - | - | - | [36] |
| Bream, Abramis brama danubii | Šalek lakes | 0.16 | 7.79 | - | - | - | [36] |
| Roach, Rutilus rutilus (L.) | Miankaleh wetland | - | 7.2 | 1.6 | 28 | - | [42] |
| Perch, Perca fluviatilis (L.) | Danube River near Belgrade | 2.72 | 18.89 | 0.45 | 11.85 | 0.69 | [35] |
| Roach, Rutilus rutilus (L.) | Kirchera River | 0.01 | 4.81 | - | - | 0.22 | [37] |
| Perch, Perca fluviatilis (L.) | Kirchera River | 0.04 | 4.33 | - | - | 0.18 | [37] |
| Pike, Esox lucius (L.) | Kirchera River | 0.07 | 2.88 | - | - | 0.11 | [37] |
| Bream, Abramis brama (L.) | Żnin Duże Lake | - | 1.516 | 0.889 | 1.704 | 0.807 | [43] |
| Roach, Rutilus rutilus (L.) | Żnin Duże Lake | - | 1.761 | 0.659 | 1.519 | 0.996 | [43] |
| Perch, Perca fluviatilis (L.) | Żnin Duże Lake | - | 1.546 | 0.980 | 1.572 | 0.768 | [43] |
| Perch, Perca fluviatilis (L.) | Skadar Lake | 0.121 | 5.83 | 0.81 | 4.77 | 0.22 | [39] |
| Pike, Esox lucius (L.) | Ińsko Lake | 0.01 | 9.4 | 0.14 | 1.4 | 0.24 | [44] |
| Bream, Abramis brama (L.) | Miedwie | - | 2.3 | 0.14 | 1.3 | 0.11 | [44] |
| Perch, Perca fluviatilis (L.) | Miedwie | 0.01 | 4.6 | 0.14 | 1.2 | 0.18 | [44] |
| Liver | |||||||
| Bream, Abramis brama (L.) | Skalka Reservoir | 1.50 | - | - | - | - | [40] |
| Roach, Rutilus rutilus (L.) | Skalka Reservoir | 0.88 | - | - | - | - | [40] |
| Bream, Abramis brama (L.) | West Morava River Basin | 1.05 | 91.81 | 44.75 | 428.11 | 5.82 | [38] |
| Roach, Rutilus rutilus (L.) | West Morava River Basin | 0.95 | 80.51 | 30.43 | 177.23 | 5.05 | [38] |
| Perch, Perca fluviatilis (L.) | West Morava River Basin | 1.85 | 71.80 | 17.41 | 355.07 | 2.1 | [38] |
| Pike, Esox lucius (L.) | West Morava River Basin | 0.81 | 90.72 | 13.88 | 261.38 | 1.85 | [38] |
| Bream, Abramis brama (L.) | Żnin Duże Lake | - | 2.098 | 1.755 | 2.436 | 1.143 | [43] |
| Roach, Rutilus rutilus (L.) | Żnin Duże Lake | - | 2.382 | 2.169 | 2.631 | 1.405 | [43] |
| Perch, Perca fluviatilis (L.) | Żnin Duże Lake | - | 2.012 | 1.395 | 2.358 | 1.143 | [43] |
| Perch, Perca fluviatilis (L.) | Danube River near Belgrade | 2.52 | 77.66 | 18.20 | 225.0 | 4.24 | [35] |
| Perch, Perca fluviatilis (L.) | Velenjsko Lake | 0.22 | 29.3 | - | - | - | [41] |
| Perch, Perca fluviatilis (L.) | Šalek lakes | 0.22 | 29.3 | - | - | - | [36] |
| Roach, Rutilus rutilus (L.) | Šalek lakes | 0.09 | 29.2 | - | - | - | [36] |
| Bream, Abramis brama danubii | Šalek lakes | 0.31 | 28.0 | - | - | - | [36] |
| Roach, Rutilus rutilus (L.) | Kirchera River | 0.02 | 18.26 | - | - | 4.09 | [37] |
| Perch, Perca fluviatilis (L.) | Kirchera River | 0.02 | 53.56 | - | - | 2.24 | [37] |
| Pike, Esox lucius (L.) | Kirchera River | 0.02 | 36.18 | - | - | 0.59 | [37] |
| Gills | |||||||
| Bream, Abramis brama (L.) | West Morava River Basin | 1.31 | 68.46 | 2.36 | 389.61 | 21.45 | [38] |
| Roach, Rutilus rutilus (L.) | West Morava River Basin | 1.27 | 196.69 | 3.1 | 180.98 | 20.57 | [38] |
| Perch, Perca fluviatilis (L.) | West Morava River Basin | 1.8 | 71.2 | 0.99 | 138.52 | 12.29 | [38] |
| Pike, Esox lucius (L.) | West Morava River Basin | 1.27 | 558.11 | 1.56 | 96.69 | 26.83 | [38] |
| Perch, Perca fluviatilis (L.) | Šalek lakes | 0.06 | 24.9 | - | - | - | [36] |
| Roach, Rutilus rutilus (L.) | Šalek lakes | 0.06 | 78.7 | - | - | - | [36] |
| Bream, Abramis brama danubii | Šalek lakes | 0.03 | 16.5 | - | - | - | [36] |
| Perch, Perca fluviatilis (L.) | Danube River near Belgrade | 1.84 | 64.82 | 0.66 | 189.39 | 10.57 | [35] |
| Bream, Abramis brama (L.) | Żnin Duże Lake | - | 1.937 | 0.650 | 2.436 | 1.745 | [43] |
| Roach, Rutilus rutilus (L.) | Żnin Duże Lake | - | 3.030 | 0.956 | 2.364 | 1.632 | [43] |
| Perch, Perca fluviatilis (L.) | Żnin Duże Lake | - | 1.945 | 0.663 | 2.212 | 1.680 | [43] |
| Perch, Perca fluviatilis (L.) | Velenjsko Lake | 0.06 | 24.9 | - | - | - | [41] |
| Fatty Acids | Bream Abramis brama L. | Roach Rutilus rutilus L. | Pike Esox lucius L. | Perch Perca fluviatilis L. | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| n | 5 | 9 | 6 | 8 | ||||
| C12:0 | 0.43 a | 0.72 | 0.10 a | 0.02 | 0.13 a | 0.01 | 0.11 a | 0.04 |
| C14:0 | 1.32 a | 0.46 | 1.19 a | 0.22 | 1.30 a | 0.37 | 1.57 a | 0.27 |
| C15:0 | 0.73 a | 0.33 | 0.45 b | 0.07 | 0.43 b | 0.04 | 0.42 b | 0.08 |
| C16:0 | 22.31 a | 1.43 | 22.74 a | 1.53 | 23.36 a | 1.98 | 22.55 a | 4.83 |
| C17:0 | 1.03 a | 0.68 | 0.59 b | 0.13 | 0.48 b | 0.06 | 0.62 b | 0.06 |
| C18:0 | 6.94 a | 1.26 | 6.55 a | 0.78 | 6.57 a | 0.38 | 6.81 a | 1.51 |
| C20:0 | 0.18 a | 0.03 | 0.11 b | 0.03 | 0.08 b | 0.02 | 0.16 a | 0.05 |
| C14:1 | 0.05 a | 0.05 | 0.04 ab | 0.03 | 0.00 b | 0.00 | 0.03 ab | 0.04 |
| C16:1 | 6.03 a | 3.43 | 4.81 ab | 1.73 | 1.94 c | 0.36 | 3.52 bc | 0.90 |
| C17:1 | 0.75 a | 0.20 | 0.46 b | 0.19 | 0.37 b | 0.09 | 0.49 b | 0.06 |
| C18:1 | 16.32 a | 6.68 | 12.93 ab | 3.47 | 9.85 b | 1.40 | 12.11 ab | 2.78 |
| C20:1 (n-7) | 0.20 a | 0.05 | 0.22 a | 0.05 | 0.12 b | 0.01 | 0.11 b | 0.03 |
| C20:1 (n-9) | 0.46 a | 0.24 | 0.52 a | 0.18 | 0.39 a | 0.19 | 0.32 a | 0.12 |
| C20:1 (n-11) | 0.31 a | 0.10 | 0.43 a | 0.19 | 0.05 b | 0.08 | 0.00 b | 0.00 |
| C18:2(n-6) | 3.36 a | 0.54 | 2.28 a | 0.76 | 2.95 a | 0.24 | 3.13 a | 1.83 |
| C18:3γ-lin (n-6) | 0.32 a | 0.08 | 0.20 b | 0.06 | 0.21 b | 0.02 | 0.30 a | 0.06 |
| C20:2(n-6) | 0.70 a | 0.19 | 0.67 a | 0.22 | 0.56 a | 0.09 | 0.24 b | 0.10 |
| C20:3(n-6) | 0.45 a | 0.08 | 0.38 a | 0.10 | 0.14 b | 0.06 | 0.21 b | 0.12 |
| C20:4(n-6) | 7.63 ab | 2.03 | 8.51 a | 1.93 | 6.44 b | 1.85 | 8.71 a | 0.80 |
| C22:5(n-6) | 1.10 b | 0.50 | 2.94 a | 1.43 | 2.03 ab | 0.49 | 1.57 b | 0.47 |
| C18:3(n-3) | 1.40 a | 0.49 | 1.24 a | 0.67 | 1.68 a | 0.29 | 2.03 a | 1.76 |
| C18:4 (n-3) | 0.19 b | 0.12 | 0.31 b | 0.21 | 0.75 a | 0.11 | 0.68 a | 0.25 |
| C20:3(n-3) | 0.48 a | 0.29 | 0.42 a | 0.16 | 0.22 b | 0.06 | 0.23 b | 0.09 |
| C20:4(n-3) | 0.54 a | 0.10 | 0.81 a | 0.44 | 0.59 a | 0.07 | 0.74 a | 0.29 |
| C20:5(n-3) EPA | 8.43 a | 0.49 | 7.12 a | 2.30 | 7.36 a | 1.25 | 9.26 a | 4.55 |
| C22:5(n-3) | 2.32 b | 0.47 | 2.72 ab | 0.29 | 3.00 ab | 0.32 | 3.20 a | 1.07 |
| C22:6(n-3) DHA | 16.03 b | 4.63 | 21.30 b | 3.41 | 29.02 a | 3.61 | 20.88 b | 5.70 |
| Σ SFA | 32.94 a | 3.28 | 31.73 a | 2.07 | 32.34 a | 2.47 | 32.24 a | 6.33 |
| Σ MUFA | 24.12 a | 10.30 | 19.40 ab | 5.45 | 12.73 b | 1.76 | 16.58 b | 3.60 |
| Σ n-6 PUFA | 13.55 a | 2.34 | 14.98 a | 2.63 | 12.32 a | 2.32 | 14.15 a | 2.16 |
| Σ n-3 PUFA | 29.39 c | 5.90 | 33.91 b | 3.25 | 42.61 a | 3.92 | 37.02 b | 1.82 |
| Σ PUFA | 42.94 c | 8.19 | 48.89 b | 4.24 | 54.93 a | 3.69 | 51.17 ab | 3.22 |
| n-3/n-6 | 2.16 b | 0.12 | 2.32 b | 0.42 | 3.56 a | 0.67 | 2.66 b | 0.35 |
| Cu | Zn | Mn | Fe | Hg | |
|---|---|---|---|---|---|
| Abramis brama L. (n = 5) | 0.117 | 2.330 | 0.068 | 0.729 | 0.036 |
| Rutilus rutilus L. (n = 9) | 0.116 | 2.519 | 0.059 | 0.560 | 0.078 |
| Esox lucius L. (n = 6) | 0.086 | 5.582 | 0.050 | 0.489 | 0.165 |
| Perca fluviatilis L. (n = 8) | 0.076 | 2.424 | 0.027 | 0.574 | 0.193 |
| Cu | Zn | Mn | Fe | Hg | |||
|---|---|---|---|---|---|---|---|
| RfD (mg/kg/day) | 4.00 × 10−2 | 3.00 × 10−1 | 1.4 × 10−1 | 7.00 × 10−1 | 3.00 × 10−4 | ||
| THQ | TTHQ | HI | |||||
| Abramis brama L. (n = 5) | 0.0029 | 0.0078 | 0.0005 | 0.0010 | 0.1200 | 0.132 | 1.630 |
| Rutilus rutilus L. (n = 9) | 0.0029 | 0.0084 | 0.0004 | 0.0008 | 0.2596 | 0.272 | |
| Esox lucius L. (n = 6) | 0.0022 | 0.0186 | 0.0004 | 0.0007 | 0.5512 | 0.573 | |
| Perca fluviatilis L. (n = 8) | 0.0019 | 0.0081 | 0.0002 | 0.0008 | 0.6418 | 0.653 | |
| TDHQ | 0.010 | 0.043 | 0.0015 | 0.003 | 1.573 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczyńska, J.; Paszczyk, B. Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland. Int. J. Environ. Res. Public Health 2019, 16, 3780. https://doi.org/10.3390/ijerph16193780
Łuczyńska J, Paszczyk B. Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland. International Journal of Environmental Research and Public Health. 2019; 16(19):3780. https://doi.org/10.3390/ijerph16193780
Chicago/Turabian StyleŁuczyńska, Joanna, and Beata Paszczyk. 2019. "Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland" International Journal of Environmental Research and Public Health 16, no. 19: 3780. https://doi.org/10.3390/ijerph16193780
APA StyleŁuczyńska, J., & Paszczyk, B. (2019). Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland. International Journal of Environmental Research and Public Health, 16(19), 3780. https://doi.org/10.3390/ijerph16193780






