Investigation of the Synergistic Toxicity of Binary Mixtures of Pesticides and Pharmaceuticals on Aliivibrio fischeri in Major River Basins in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Target Pesticides and Pharmaceuticals
2.2. Test Reagents, Chemicals and Sample Preparation
2.3. Testing Organism and Culture
2.4. Single Chemical Toxicity Test
2.5. Mixture toxicity test
2.6. Statistical Analysis of the Mixture Toxicity
3. Results and Discussion
3.1. DRCs for Single Compounds
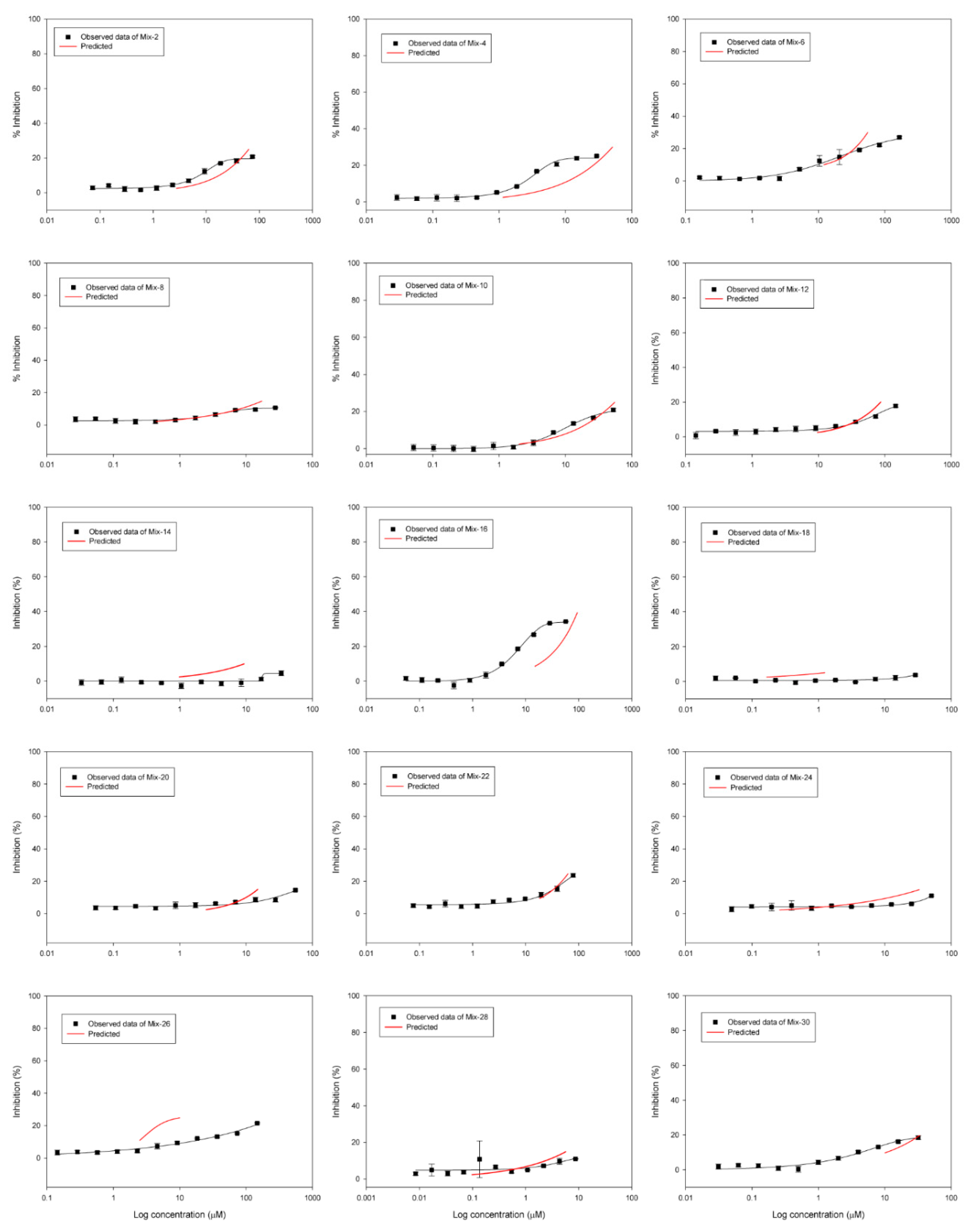
3.2. DRCs of Binary Mixtures
3.3. Statistical Analysis of Mixture Toxicity to Investigate Synergism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kortenkamp, A.; Altenburger, R. Approaches to assessing combination effects of oestrogenic environmental pollutants. Sci. Total Environ. 1999, 233, 131–140. [Google Scholar] [CrossRef]
- Junghans, M.; Backhaus, T.; Faust, M.; Scholze, M.; Grimme, L.H. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006, 76, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Plackett, R.L.; Hewlett, P.S. Quantal responses to mixtures of poisons. J. R. Stat. Soc. Ser. B (Methodol.) 1952, 14, 141–163. [Google Scholar] [CrossRef]
- German Federal Environment Agency. Ecotoxicological Combined Effects from Chemical Mixtures Part 1—Relevance and Adequate Consideration in Environmental Risk Assessment of Plant Protection Products and Biocides; Umweltbundesamt: Dessau-Roßlau, Germany, 2014. [Google Scholar]
- Kim, J.; Kim, S.; Schaumann, G.E. Reliable predictive computational toxicology methods for mixture toxicity: Toward the development of innovative integrated models for environmental risk assessment. Rev. Environ. Sci. Bio-Technol. 2013, 12, 235–256. [Google Scholar] [CrossRef]
- European Commission. State of the Art Report on Mixture Toxicity-Final Report; The School of Pharmacy, University of London: London, UK, 2009; pp. 1–31. [Google Scholar]
- US EPA. Guidelines for the Health Risk Assessment of Chemical Mixtures; US Environment Protection Agency: Washington, DC, USA, 1986; pp. 1–29.
- US EPA. Technical Support Document on Health Risk Assessment of Chemical Mixtures. Office of Research and Developmen; US Environment Protection Agency: Washington, DC, USA, 1990.
- US EPA. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. Risk Assessment Forum Technical Pannel; US Environment Protection Agency: Washington, DC, USA, 2000.
- Deneer, J.W. Toxicity of mixtures of pesticides in aquatic systems. Pest Manag. Sci. 2000, 56, 516–520. [Google Scholar] [CrossRef]
- Belden, J.B.; Gilliom, R.J.; Lydy, M.J. How well can we predict the toxicity of pesticide mixtures to aquatic life? Integr. Environ. Assess. Manag. 2007, 3, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Cristensen, A.M.; Kamper, A.; Kudsk, P.; Mathiassen, S.K.; Streibig, J.C.; Sørensen, H. A rivew of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environ. Toxicol. Chem. 2008, 27, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk assessment of environmental mixture effects. RSC Adv. 2016, 6, 47844–47857. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Evans, R.; Faust, M.; Kalberlah, F.; Scholze, M.; Schuhmacher-Wolz, U. Investigation of the state of the science on combined actions of chemicals in food through dissimilar modes of action and proposal for science-based approach for performing related cumulative risk assessment. EFSA Support. Publ. 2012, 9. [Google Scholar] [CrossRef]
- Smith, J.W.; Seidle, L.G.; Cluff, L.E. Studies on the epideminology of adverse drug reaction (v); clinical factors influencing susceptibility. Ann. Intern. Med. 1966, 65, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Lee, Y.B. Mechanism-Based Drug Interactions; Shinilbooks: Seoul, Korea, 2005. [Google Scholar]
- Boxall, A.B.A. The environmental side effects of medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Jeong, Y.; Schaffer, A.; Smith, K. A comparison of equilibrium and kinetic passive sampling for the monitoring of aquatic organic contaminants in German rivers. Water Res. 2018, 145, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Bradley, P.M.; Iwanowicz, L.; Journey, C.A.; Walsh, H.L.; Blazer, V.S. Pharmaceuticals, hormones, pesticides, and other bioactive contaminants in water, sediment, and tissue from rocky mountain national park, 2012–2013. Sci. Total Environ. 2018, 643, 651–673. [Google Scholar] [CrossRef] [PubMed]
- ISO 11348-3. Water Qualityddetermination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria; European Committee for Standardization: Brussels, Belgium, 1998. [Google Scholar]
- ASTM D5660-96. Standard Test Method for Assessing the Microbial Detoxification of Chemically Contaminated Water and Soil Using a Toxicity Test with a Luminescent Marine Bacterium; American Society for Testing and Materials: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Scholze, M.; Boedeker, W.; Faust, M.; Backhaus, T.; Altenburger, R.; Grimme, L.H. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ. Toxicol. Chem. 2001, 20, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, J.I.; Kim, Y.H.; Choi, K.H. Prioritizing human use antibiotics for environmental health management and estimating their environmental concentrations in Korean waterway. Korean Soc. Environ. Health 2006, 10, 462–468. [Google Scholar]
- Korean National Institute of Environmental Research. Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products in Environment; Korean National Institute of Environmental Research: Seoul, Korea, 2006. [Google Scholar]
- Korea Food and Drug Administration. Human Exposure Assessment for Antibiotics in the Water Environment; Korea Food and Drug Administration: Seoul, Korea, 2007. [Google Scholar]
- Korean National Institute of Environmental Research. Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products in Environment (II); Korean National Institute of Environmental Research: Seoul, Korea, 2007. [Google Scholar]
- Park, J.; Kim, M.-H.; Choi, K.; Kim, Y.; Kim, M.-Y. Environmental Risk Assessment of Pharmaceutical: Model. Application for Estimating Pharmaceutical Exposures in the Han River Basin; Korea Environment Institute: Seoul, Korea, 2007. [Google Scholar]
- Choi, K.; Kim, Y.; Jung, J.; Kim, M.H.; Kim, C.S.; Kim, N.H.; Park, J. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han river, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, C.-K.; Kim, M.-Y.; Ahn, S.-G. Contamination of verterinary antibiotics and antimirobials in Han river basin. J. Korean Soc. Environ. Anal. 2008, 11, 109–118. [Google Scholar]
- Korean National Institute of Environmental Research. Environmental Risk Assessment of Pharmaceutical Residues; Korean National Institute of Environmental Research: Seoul, Korea, 2008. [Google Scholar]
- Lee, J.-H.; Park, B.-J.; Kim, J.-K.; Kim, W.-I.; Hong, S.-M.; Im, G.-J.; Hong, M.-K. Risk assessment for aquatic organisms of pesticides detected in water phase of six major rivers in Korea. Korean J. Pestic. Sci. 2011, 15, 48–54. [Google Scholar]
- Könemann, H. Fish toxicity tests with mixtures of more than two chemicals: A proposal for a quantitative approach and experimental results. Toxicology 1981, 19, 229–238. [Google Scholar] [CrossRef]
- Altenburger, R.; Backhaus, T.; Boedeker, W.; Faust, M.; Scholze, M.; Grimme, L.H. Predictability of the toxicity of multiple chemical mixtures to vibrio fischeri: Mixtures composed of similarly acting chemicals. Environ. Toxicol. Chem. 2000, 19, 2341–2347. [Google Scholar] [CrossRef]
- Backhaus, T.; Altenburger, R.; Boedeker, W.; Faust, M.; Scholze, M.; Grimme, L.H. Predictability of the toxicity of a multiple mixture of dissimilarly acting chemicals to vibrio fischeri. Environ. Toxicol. Chem. 2000, 19, 2348–2356. [Google Scholar] [CrossRef]
- Payne, J.; Rajapakse, N.; Wilkins, M.; Kortenkamp, A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environ. Health Perspect. 2000, 108, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Huang, L.; Wang, Y.; Cai, X.; Qiao, X.; Dong, Y. Integrated fuzzy concentration addition-independent action (IFCA-IA) model outperforms two-stage prediction (TSP) for predicting mixture toxicity. Chemosphere 2009, 74, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Schaumann, G.E. Development of a partial least squares–based integrated addition model for predicting mixture toxicity. Hum. Ecol. Risk Assess. 2014, 20, 174–200. [Google Scholar] [CrossRef]
- Fang, C.J.; Duan, C.Y.; He, C.; Han, G.; Meng, Q.J. A supramolecular analog of cyclohexane sustained by aromatic c-h center dot center dot center dot pi interactions between ferrocene moieties: Molecular packing of ferrocene-containing thiosemicarbazato metal complexes. New J. Chem. 2000, 24, 697–701. [Google Scholar]
- Liang, Y.Z.; Fang, K.T.; Xu, Q.S. Uniform design and its applications in chemistry and chemical engineering. Chemom. Intell. Lab. Syst. 2001, 58, 43–57. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, S.S.; Liu, H.L.; Liu, Z.Z. Evaluation of the combined toxicity of 15 pesticides by uniform design. Pest Manag. Sci. 2010, 66, 879–887. [Google Scholar] [CrossRef]
- Loewe, S.; Muischnek, H. Über Kombinationswirkungen i. Mitteilung: Hilfsmittel der Fragestellung. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Wieczerzak, M.; Kudłak, B.; Yotova, G.; Nedyalkova, M.; Tsakovski, S.; Simeonov, V.; Namieśnik, J. Modeling of pharmaceuticals mixtures toxicity with deviation ratio and best-fit functions models. Sci. Total Environ. 2016, 571, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Warne, M.S.J.; Hawker, D.W. The number of components in a mixture determines whether synergistic and antagonistic or additive toxicity predominate: The funnel hypothesis. Ecotoxicol. Environ. Saf. 1995, 31, 23–28. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Opinion on Toxicity and Assessment of Chemical Mixtures; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Wang, Z.; Chen, Q.; Hu, L.; Wang, M. Combined effects of binary antibiotic mixture on growth, microcystin production, and extracellular release of microcystis aeruginosa: Application of response surface methodology. Environ. Sci. Pollut. Res. Int. 2018, 25, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Di Nica, V.; Villa, S.; Finizio, A. Toxicity of individual pharmaceuticals and their mixtures to aliivibrio fischeri: Evidence of toxicological interactions in binary combinations. Environ. Toxicol Chem. 2017, 36, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Rodea-Palomares, I.; Petre, A.L.; Boltes, K.; Leganes, F.; Perdigon-Melon, J.A.; Rosal, R.; Fernandez-Pinas, F. Application of the combination index (ci)-isobologram equation to study the toxicological interactions of lipid regulators in two aquatic bioluminescent organisms. Water Res. 2010, 44, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganes, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernandez-Pinas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Wang, D.; Lin, Z.; An, Q.; Yin, C.; Liu, Y. The joint effects of sulfonamides and quorum sensing inhibitors on vibrio fischeri: Differences between the acute and chronic mixed toxicity mechanisms. J. Hazard. Mater. 2016, 310, 56–67. [Google Scholar] [CrossRef]



| Mixture No. | Substance A | Substance B | Mixture Design |
|---|---|---|---|
| 1 | Tetracycline | Sulfamethoxazole | EC50 + EC50 |
| 2 | Tetracycline | Sulfamethoxazole | EC10 + EC10 |
| 3 | Tetracycline | Hexaconazole | EC50 + EC50 |
| 4 | Tetracycline | Hexaconazole | EC10 + EC10 |
| 5 | Tetracycline | Chlortetracycline | EC50 + EC50 |
| 6 | Tetracycline | Chlortetracycline | EC10 + EC10 |
| 7 | Tetracycline | Isoprothiolane | EC50 + EC50 |
| 8 | Tetracycline | Isoprothiolane | EC10 + EC10 |
| 9 | Tetracycline | Trimethoprim | EC50 + EC50 |
| 10 | Tetracycline | Trimethoprim | EC10 + EC10 |
| 11 | Trimethoprim | Sulfamethoxazole | EC50 + EC50 |
| 12 | Trimethoprim | Sulfamethoxazole | EC10 + EC10 |
| 13 | Trimethoprim | Hexaconazole | EC50 + EC50 |
| 14 | Trimethoprim | Hexaconazole | EC10 + EC10 |
| 15 | Trimethoprim | Chlortetracycline | EC50 + EC50 |
| 16 | Trimethoprim | Chlortetracycline | EC10 + EC10 |
| 17 | Trimethoprim | Isoprothiolane | EC50 + EC50 |
| 18 | Trimethoprim | Isoprothiolane | EC10 + EC10 |
| 19 | Sulfamethoxazole | Hexaconazole | EC50 + EC50 |
| 20 | Sulfamethoxazole | Hexaconazole | EC10 + EC10 |
| 21 | Sulfamethoxazole | Chlortetracycline | EC50 + EC50 |
| 22 | Sulfamethoxazole | Chlortetracycline | EC10 + EC10 |
| 23 | Sulfamethoxazole | Isoprothiolane | EC50 + EC50 |
| 24 | Sulfamethoxazole | Isoprothiolane | EC10 + EC10 |
| 25 | Hexaconazole | Chlortetracycline | EC50 + EC50 |
| 26 | Hexaconazole | Chlortetracycline | EC10 + EC10 |
| 27 | Hexaconazole | Isoprothiolane | EC50 + EC50 |
| 28 | Hexaconazole | Isoprothiolane | EC10 + EC10 |
| 29 | Chlortetracycline | Isoprothiolane | EC50 + EC50 |
| 30 | Chlortetracycline | Isoprothiolane | EC10 + EC10 |
| Substance | EC50 (μM) | EC10 (μM) | RM 1 | r2 | Model Parameter | ||
|---|---|---|---|---|---|---|---|
| A 2 | Β 3 | Γ 4 | |||||
| Hexaconazole | 51.65 (50.97–52.33) | 3.06 (2.38–3.74) | C | 0.995 | 1.4335 | 0.0035 | 0.5869 |
| Isoprothiolane | 137.07 (136.14–138.0) | 1.05 (0.12–1.85) | H | 0.971 | 97.3866 | 0.3312 | 1.10 × 109 |
| Tetracycline | 150.08 (148.85–151.30) | 10.60 (9.38–11.83) | L | 0.981 | 1.4806 | –0.7364 | 374.5933 |
| Trimethoprim | 338.81 (338.05–339.56) | 26.20 (25.45–26.95) | L | 0.990 | 1.2286 | –0.7997 | 542.4834 |
| Sulfamethoxazole | 254.20 (253.25–255.15) | 47.79 (46.83–48.74) | C | 0.994 | 0.9561 | 0.0034 | 1.1932 |
| Chlortetracycline | 91.32 (90.52–92.12) | 12.32 (11.52–13.12) | G | 0.993 | 0.9902 | 65.2541 | 66.4673 |
| Mixture No. | EC50 (μM) | EC10 (μM) | RM 1 | r2 | Model Parameter | ||
|---|---|---|---|---|---|---|---|
| A 2 | Β 3 | Γ 4 | |||||
| 1 | 313.95 (313.07–314.82) | 16.62 (15.74–17.50) | C | 0.987 | 2528.4458 | 2.47 × 10−6 | 0.5477 |
| 2 | n.a.5 | 7.43 (6.99–7.88) | G | 0.968 | 19.5735 | 6.4890 | 4.8497 |
| 3 | 133.58 (132.69–134.46) | 2.89 (2.01–3.78) | C | 0.989 | 915.2173 | 7.38 × 10−6 | 0.4200 |
| 4 | n.a. | 2.17 (1.75–2.59) | G | 0.983 | 23.9224 | 2.0367 | 1.8951 |
| 5 | 129.70 (128.75–130.66) | 5.98 (5.02–6.93) | C | 0.989 | 102.0025 | 0.0025 | 0.5494 |
| 6 | n.a. | 9.54 (8.83–10.25) | L | 0.954 | 30.2768 | −0.8861 | 21.1859 |
| 7 | 203.46 (202.88–204.04) | 36.83 (36.25–37.41) | G | 0.996 | 84.6072 | 118.9205 | 127.0557 |
| 8 | n.a. | 11.66 (11.25–12.06) | G | 0.874 | 10.4177 | 3.3048 | 1.0944 |
| 9 | 265.16 (264.62–265.69) | 15.55 (15.01–16.09) | C | 0.996 | 99.5885 | 0.0015 | 0.6049 |
| 10 | n.a. | 8.64 (8.18–9.10) | L | 0.970 | 22.0641 | −1.4308 | 9.8530 |
| 11 | 779.94 (779.31–780.56) | 62.99 (62.36–63.61) | C | 0.989 | 83.7765 | 0.0009 | 0.7231 |
| 12 | n.a. | 50.31 (49.80–50.81) | G | 0.914 | 19.2041 | 49.3054 | 29.2598 |
| 13 | 488.97 (488.44–489.49) | 59.282 (58.76–59.81) | H | 0.991 | 131.1544 | 0.9527 | 812.9558 |
| 14 | n.a. | n.a. | G | 0.455 | 4.5523 | 0.5916 | 17.0836 |
| 15 | 222.30 (220.95–223.65) | 4.56 (3.22–5.91) | H | 0.964 | 63.8649 | 0.7633 | 41.4132 |
| 16 | n.a. | 3.91 (3.38–4.44) | C | 0.988 | 33.8541 | 0.1489 | 1.4924 |
| 17 | 637.32 (637.01–637.64) | 154.31 (153.99–154.63) | G | 0.997 | 60.7316 | 216.8352 | 282.2291 |
| 18 | n.a. | n.a. | G | 0.429 | 8.9924 | 25.6941 | 25.7707 |
| 19 | 209.14 (208.45–209.83) | 34.75 (34.06–35.44) | G | 0.992 | 78.4915 | 114.7884 | 117.7289 |
| 20 | n.a. | 26.94 (26.43–27.44) | G | 0.817 | 17.7472 | 30.9625 | 9.7285 |
| 21 | 206.10 (205.57–206.64) | 37.41 (36.87–37.95) | G | 0.997 | 96.2390 | 135.9703 | 148.5324 |
| 22 | n.a. | 15.85 (15.31–16.38) | G | 0.9331 | 27.4285 | 34.3506 | 16.1526 |
| 23 | 339.80 (339.23–340.37) | 106.78 (106.22–107.36) | G | 0.9951 | 83.3055 | 163.6608 | 229.7587 |
| 24 | n.a. | 45.77 (45.31–46.24) | S | 0.6981 | 1804.3798 | 51.2073 | 311.5280 |
| 25 | 119.13 (118.31–119.96) | 8.40 (7.57–9.23) | H | 0.9884 | 1.37 × 105 | 0.6069 | 5.52 × 107 |
| 26 | n.a. | 13.89 (13.46–14.33) | C | 0.9560 | 158.6840 | 9.12 × 10-6 | 0.3080 |
| 27 | 204.18 (203.53–204.83) | 45.75 (45.10–46.40) | G | 0.9901 | 107.4683 | 139.9025 | 166.7380 |
| 28 | n.a. | 5.82 (4.61–7.04) | G | 0.2571 | 12.1655 | 3.7398 | –0.2706 |
| 29 | 247.79 (246.98–248.59) | 28.44 (27.64–29.24) | H | 0.9870 | 4.73 × 105 | 0.7435 | 5.52 × 107 |
| 30 | n.a. | 4.38 (3.84–4.91) | C | 0.9435 | 18.6241 | 0.1128 | 0.6594 |
| Mixture No. | EC50mix 1 | EC10mix | ||||||
|---|---|---|---|---|---|---|---|---|
| Observed (EC50, μM) | Predicted 2 | MDR 3 | Type 4 | Observed (EC10, μM) | Predicted | MDR | Type | |
| EC50 ratio mixtures 5 | ||||||||
| 1 | 313.95 (313.07–314.82) | 169.63 | 0.54 | Add.6 | 16.62 (15.74–17.50) | 13.55 | 0.82 | Add. |
| 3 | 133.58 (132.69–134.46) | 128.47 | 0.96 | Add. | 2.89 (2.01–3.78) | 8.71 | 3.01 | Syn. |
| 5 | 129.70 (128.75–130.66) | 132.37 | 1.02 | Add. | 5.98 (5.02–6.93) | 10.92 | 1.83 | Add. |
| 7 | 203.46 (202.88–204.04) | 147.27 | 0.72 | Add. | 36.83 (36.25–37.41) | 3.84 | 0.10 | Anta. 7 |
| 9 | 265.16 (264.62–265.69) | 189.17 | 0.71 | Add. | 15.55 (15.01–16.09) | 13.61 | 0.88 | Add. |
| 11 | 779.94 (779.31–780.56) | 281.92 | 0.36 | Anta. | 62.99 (62.36–63.61) | 36.51 | 0.58 | Add. |
| 13 | 488.97 (488.44–489.49) | 129.65 | 0.27 | Anta. | 59.282 (58.76–59.81) | 8.20 | 0.14 | Anta. |
| 15 | 222.30 (220.95–223.65) | 139.68 | 0.63 | Add. | 4.56 (3.22–5.91) | 16.45 | 3.61 | Syn. 8 |
| 17 | 637.32 (637.01–637.64) | 194.16 | 0.30 | Anta. | 154.31 (153.99–154.63) | 2.00 | 0.01 | Anta. |
| 19 | 209.14 (208.45–209.83) | 101.47 | 0.49 | Anta. | 34.75 (34.06–35.44) | 7.21 | 0.21 | Anta. |
| 21 | 206.10 (205.57–206.64) | 119.87 | 0.58 | Add. | 37.41 (36.87–37.95) | 17.01 | 0.45 | Anta. |
| 23 | 339.80 (339.23–340.37) | 167.07 | 0.49 | Anta. | 106.78 (106.22–107.36) | 1.70 | 0.02 | Anta. |
| 25 | 119.13 (118.31–119.96) | 66.93 | 0.56 | Add. | 8.40 (7.57–9.23) | 5.04 | 0.60 | Add. |
| 27 | 204.18 (203.53–204.83) | 74.93 | 0.37 | Anta. | 45.75 (45.10–46.40) | 1.57 | 0.03 | Anta. |
| 29 | 247.79 (246.98–248.59) | 100.21 | 0.40 | Anta. | 28.44 (27.64–29.24) | 3.18 | 0.11 | Anta. |
| EC10 ratio mixtures | ||||||||
| 2 | n.a. 9 | n.a. | - | - | 7.43 (6.99–7.88) | 17.53 | 2.36 | Syn |
| 4 | n.a. | n.a. | - | - | 2.17 (1.75–2.59) | 8.96 | 4.13 | Syn. |
| 6 | n.a. | n.a. | - | - | 9.54 (8.83–10.25) | 12.03 | 1.26 | Add. |
| 8 | n.a. | n.a. | - | - | 11.66 (11.25–12.06) | 8.57 | 0.73 | Add. |
| 10 | n.a. | n.a. | - | - | 8.64 (8.18–9.10) | 13.84 | 1.60 | Add. |
| 12 | n.a. | n.a. | - | - | 50.31 (49.80–50.81) | 41.16 | 0.82 | Add. |
| 14 | n.a. | n.a. | - | - | n.a. | n.a. | - | - |
| 16 | n.a. | n.a. | - | - | 3.91 (3.38–4.44) | 15.03 | 3.84 | Syn. |
| 18 | n.a. | n.a. | - | - | n.a. | n.a. | - | - |
| 20 | n.a. | n.a. | - | - | 26.94 (26.43–27.44) | 14.00 | 0.52 | Add. |
| 22 | n.a. | n.a. | - | - | 15.85 (15.31–16.38) | 18.52 | 1.17 | Add. |
| 24 | n.a. | n.a. | - | - | 45.77 (45.31–46.24) | 12.98 | 0.28 | Anta. |
| 26 | n.a. | n.a. | - | - | 13.89 (13.46–14.33) | 11.18 | 0.80 | Add. |
| 28 | n.a. | n.a. | - | - | 5.82 (4.61–7.04) | 2.52 | 0.43 | Anta. |
| 30 | n.a. | n.a. | - | - | 4.38 (3.84–4.91) | 10.07 | 2.30 | Syn. |
| Mixture | Experimental Design | Species | Endpoint | Convind Effect | Quantification Methods | Ref. | |
|---|---|---|---|---|---|---|---|
| High Level | Low Level | ||||||
| Two antibiotics | Binary equitoxic mixture ratio (5:1, 1:1, 1:5) | Bacteria Microcystis aeruginosa (MA) | EC50 and EC5 for MA cell from equitoxic ratio SP/Amp (5:1, 1:5, 1:1) | Antagonism 1:1 ratio >0.7 ug/L | Synergism 1:1 ratio <0.5 ug/L | Departure from additivity model (CA, IA) | [47] |
| Five veterinary pharmaceuticals | Binary and multicomponent mixture | Bacteria A. fischeri | Applying the combination index method from active pharmaceutical compound interactions for bacteria | Antagonism | Synergism | Departure from combination index (CA, IA) | [48] |
| Diclofenac : Sulfamethizole | EC50 1.13 | EC10 0.61 | |||||
| Acetylsalycilic acid : Sulfamethizole | EC50 2.58 | EC10 0.85 | |||||
| Chlortetracycline : Amoxicillin | EC50 2.16 | EC10 0.08 | |||||
| Acetylsalycilic acid : Diclofenac | EC50 1.13 | EC10 0.73 | |||||
| Sulfamethizole : Amoxicillin | EC50 1.57 | EC10 0.41 | |||||
| Acetylsalycilic acid : Amoxicillin | EC50 2.17 | EC10 0.72 | |||||
| Predicted no-effect concentration (five pharmaceutical compound mixture) | EC50 1.36 | EC10 0.61 | |||||
| Three pharmaceuticals | Binary and ternary combinations | Bacteria A. fischeriCyanobacteria Anabaena CPB4337 | Applying the combination index with isobologram equation methods from pharmaceutical compounds for in vitro and in vivo bioassay | Antagonism | Synergism | Departure from combination index (CA and IA) with isobologram equation | [49] |
| Fenofibrate : Bezafibrate | EC90 2.59 EC50 1.19 | EC10 0.55 | |||||
| Fenofibrate : Gemfibrozil | EC90 12.9 EC50 1.29 | EC10 0.13 | |||||
| Fenofibrate : Gemfibrozil : Bezafibrate | EC90 3.92 | EC50 0.57 EC10 0.09 | |||||
| Five antibiotics | Binary and multicomponent mixture | Cyanobacteria Anabaena CPB4337 Microalgae Raphidocelis subcapitata | Applying combination index with isobologram equation methods from pharmaceutical compound for in vitro and in vivo bioassay | Antagonism | Synergism | Departure from combination index (CA and IA) with isobologram equation | [50] |
| Levofloxacin : Tetracycline | EC50 1.6 | EC10 0.37 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, I.-H.; Kim, Y.; Baik, S.; Kim, J. Investigation of the Synergistic Toxicity of Binary Mixtures of Pesticides and Pharmaceuticals on Aliivibrio fischeri in Major River Basins in South Korea. Int. J. Environ. Res. Public Health 2019, 16, 208. https://doi.org/10.3390/ijerph16020208
Baek I-H, Kim Y, Baik S, Kim J. Investigation of the Synergistic Toxicity of Binary Mixtures of Pesticides and Pharmaceuticals on Aliivibrio fischeri in Major River Basins in South Korea. International Journal of Environmental Research and Public Health. 2019; 16(2):208. https://doi.org/10.3390/ijerph16020208
Chicago/Turabian StyleBaek, In-Hyuk, Youngjun Kim, Seungyun Baik, and Jongwoon Kim. 2019. "Investigation of the Synergistic Toxicity of Binary Mixtures of Pesticides and Pharmaceuticals on Aliivibrio fischeri in Major River Basins in South Korea" International Journal of Environmental Research and Public Health 16, no. 2: 208. https://doi.org/10.3390/ijerph16020208
APA StyleBaek, I.-H., Kim, Y., Baik, S., & Kim, J. (2019). Investigation of the Synergistic Toxicity of Binary Mixtures of Pesticides and Pharmaceuticals on Aliivibrio fischeri in Major River Basins in South Korea. International Journal of Environmental Research and Public Health, 16(2), 208. https://doi.org/10.3390/ijerph16020208





