Effects of Phenolic Pollution on Interspecific Competition between Microcystis aeruginosa and Chlorella pyrenoidosa and their Photosynthetic Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chemical Reagents
2.2. Strains and Culture Conditions
2.3. Experimental Design
2.4. Cell Counting
2.5. Measurement of In Vivo Chlorophyll-a Fluorescence
2.6. Measurement of Dissolved Phenol Concentration
2.7. Statistical Analyses
3. Results and Discussion
3.1. Effects of Phenol on Algal Growth in Mono- and Co-Cultures
3.2. Growth Parameters and Inflection Points
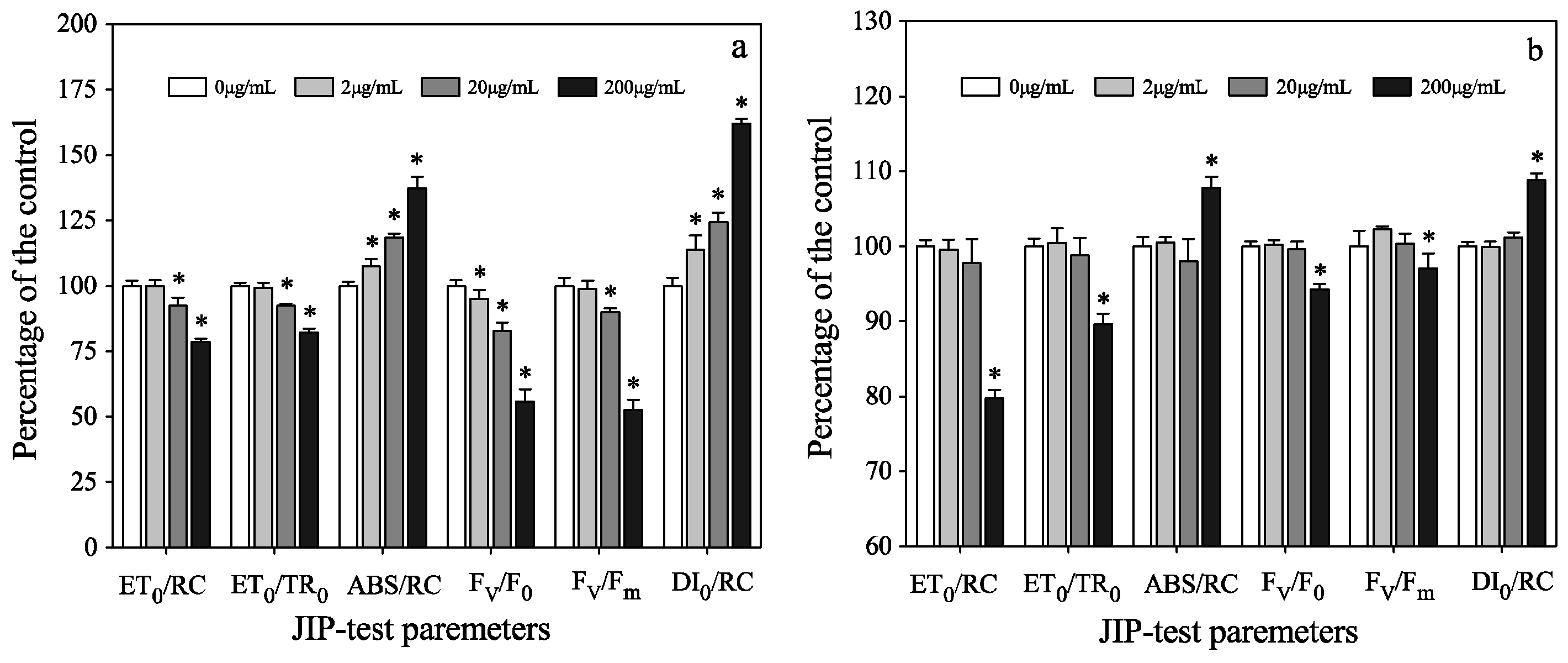
3.3. Photosynthetic Activities of Two Algae in Mono-Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Newsted, J.L. Effect of light, temperature, and pH on the accumulation of phenol by Selenastrum capricornutum, a green alga. Ecotoxicol. Environ. Saf. 2004, 59, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R.; Sethunathan, N. The impacts of environmental pollutants on microalgae and cyanobacteria. Crit. Rev. Environ. Sci. Technol. 2010, 40, 699–821. [Google Scholar] [CrossRef]
- Das, B.; Mandal, T.K.; Patra, S. A comprehensive study on Chlorella pyrenoidosa for phenol degradation and its potential applicability as biodiesel feedstock and animal feed. Appl. Biochem. Biotechnol. 2015, 176, 1382–1401. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Bhattacharya, S.; Kumar, V.; Moitra, J. Biological significance of [14C] phenol accumulation in different organs of a murrel, Channa punctatus, and the common carp, Cyprinus carpio. Biomed. Environ. Sci. 1990, 3, 337–342. [Google Scholar]
- Aleksieva, Z.; Ivanova, D.; Godjevargova, T.; Atanasov, B. Degradation of some phenol derivatives by Trichosporon cutaneum, R57. Process Biochem. 2002, 37, 1215–1219. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, L.; Yang, K.; Ge, F.; Chen, Y. Concentration and pollution characteristics of phenolic compounds in Qiantang River. China Environ. Sci. 2005, 25, 729–732. [Google Scholar]
- Song, H.; Wang, D.; Xu, X.; Wang, H.; Chen, X.; Luo, Q.; Wang, Z.; Du, Y. Occurrence of 14 phenols in 24 typical drinking water sources of China. Acta Sci. Circumstantiae 2014, 34, 355–362. [Google Scholar]
- Ying, P.J.; Tang, Y.; Hu, L.J. A survey on an accident of phenolic pollution. Mod. Prev. Med. 2006, 33, 594–595. [Google Scholar]
- Scragg, A.H. The effect of phenol on the growth of Chlorella vulgaris, and Chlorella VT-1. Enzym. Microb. Technol. 2006, 39, 796–799. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Kim, Y.J.; Choi, H.; Kim, M.S.; Park, A.; Park, J.; Shin, W.; Han, T. A rapid phenol toxicity test based on photosynthesis and movement of the freshwater flagellate, Euglena agilis Carter. Aquat. Toxicol. 2014, 155, 9–14. [Google Scholar] [CrossRef]
- Matorin, D.N.; Plekhanov, S.E.; Bratkovskaia, L.B.; Yakovleva, O.V.; Alekseev, A.A. The effect of phenols on the parameters of chlorophyll fluorescence and reaction of P700 in the green algae Scenedesmus quadricauda. Biophysics 2014, 59, 374–379. [Google Scholar] [CrossRef]
- Chen, C.P.; Zhang, T.T.; He, M.; Wu, A.P.; Nie, L.W. Effects of aniline and phenol on freshwater algae growth. Chin. J. Appl. Ecol. 2007, 18, 219–223. [Google Scholar]
- Dziga, D.; Suda, M.; Bialczyk, J.; Czaj-Prokop, U.; Lechowski, Z. The alteration of Microcystis aeruginosa biomass and dissolved microcystin-LR concentration following exposure to plant-producing phenols. Environ. Toxicol. 2007, 22, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, J.; Sheng, L.; Jin, M. Interspecies competition between Microcystis aeruginosa and Scenedesmus obliquus under phenanthrene stress. Pol. J. Environ. Stud. 2014, 23, 1609–1616. [Google Scholar]
- Zhu, W.; Chen, H.M.; Guo, L.L.; Li, M. Effects of linear alkylbenzene sulfonate (LAS) on the interspecific competition between Microcystis and Scenedesmus. Environ. Sci. Pollut. Res. 2016, 23, 16194–16200. [Google Scholar] [CrossRef]
- De Morais, P.; Stoichev, T.; Basto, M.C.P.; Ramos, V.; Vasconcelos, V.M.; Vasconcelos, M.T.S.D. Pentachlorophenol toxicity to a mixture of Microcystis aeruginosa and Chlorella vulgaris cultures. Aquat. Toxicol. 2014, 150, 159–164. [Google Scholar] [CrossRef]
- Bi, R.; Wang, Y.; Wang, R.; Li, W.; Tang, X. Effect of anthracene on the interaction between Platymonas helgolandica var. tsingtaoensis and Heterosigma akashiwo in laboratory cultures. J. Ocean Univ. China 2015, 14, 105–113. [Google Scholar] [CrossRef]
- Yang, J.; Tang, H.; Zhang, X.; Zhu, X.; Huang, Y.; Yang, Z. High temperature and pH favor Microcystis aeruginosa to outcompete Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2018, 25, 4794–4802. [Google Scholar] [CrossRef]
- De Morais, P.; Stoichev, T.; Basto, M.C.P.; Ramos, V.; Vasconcelos, V.M.; Vasconcelos, M.T.S.D. Cyanobacterium Microcystis aeruginosa response to pentachlorophenol and comparison with that of the microalga Chlorella vulgaris. Water Res. 2014, 52, 63–72. [Google Scholar] [CrossRef]
- Kong, Q.X.; Zhu, L.Z.; Shen, X.Y. The toxicity of naphthalene to marine Chlorella vulgaris under different nutrient conditions. J. Hazard. Mater. 2010, 178, 282–286. [Google Scholar] [CrossRef]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; pp. 1–37. [Google Scholar]
- Duan, Z.P.; Tan, X.; Li, N.G. Ultrasonic selectivity on depressing photosynthesis of cyanobacteria and green algae probed by chlorophyll-a fluorescence transient. Water Sci. Technol. 2017, 76, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.S.; Jajoo, A. A Quick investigation of the detrimental effects of environmental pollutant polycyclic aromatic hydrocarbon fluoranthene on the photosynthetic efficiency of wheat (Triticum aestivum). Ecotoxicology 2013, 22, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; He, N.; Chen, Q.; Duan, S. Effects of lanthanum on the photosystem II energy fluxes and antioxidant system of Chlorella Vulgaris and Phaeodactylum Tricornutum. Int. J. Environ. Res. Public Health 2019, 16, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.M.; Dixon, J.B. Preparation and application of organo-minerals as sorbents of phenol, benzene and toluene. Appl. Clay Sci. 2001, 18, 111–122. [Google Scholar] [CrossRef]
- Othman, H.; Leboulanger, C.; Le Floc’h, E.; Mabrouk, H.H.; Hlaili, A.S. Toxicity of benz (a) anthracene and fluoranthene to marine phytoplankton in culture: Does cell size really matter? J. Hazard. Mater. 2012, 243, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Perron, M.C.; Qiu, B.; Boucher, N.; Bellemare, F.; Juneau, P. Use of chlorophyll a fluorescence to detect the effect of microcystins on photosynthesis and photosystem II energy fluxes of green algae. Toxicon 2012, 59, 567–577. [Google Scholar] [CrossRef]
- Foit, K.; Kaske, O.; Liess, M. Competition increases toxicant sensitivity and delays the recovery of two interacting populations. Aquat. Toxicol. 2012, 106–107, 25–31. [Google Scholar] [CrossRef]
- Knillmann, S.; Stampfli, N.C.; Beketov, M.A.; Liess, M. Intraspecific competition increases toxicant effects in outdoor pond microcosms. Ecotoxicology 2012, 21, 1857–1866. [Google Scholar] [CrossRef]
- Madhavi, D.R.; Umamaheswari, A.; Venkateswarlu, K. Effective concentrations of nitrophenolics toward growth yield of selected microalgae and cyanobacteria isolated from soil. Ecotoxicol. Environ. Saf. 1995, 32, 205–208. [Google Scholar] [CrossRef]
- Stebbing, A.R.D. Hormesis—The stimulation of growth by low levels of inhibitors. Sci. Total Environ. 1982, 22, 213–234. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols-sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 445–483. [Google Scholar]
- Gao, Q.T.; Tam, N.F.Y. Growth, photosynthesis and antioxidant responses of two microalgal species, Chlorella vulgaris and Selenastrum capricornutum, to nonylphenol stress. Chemosphere 2011, 82, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions: A review. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Kummerová, M.; Vanova, L. Chlorophyll fluorescence as an indicator of fluoranthene phototoxicity. Plant Soil Environ. 2007, 53, 430–436. [Google Scholar] [CrossRef]
- Volterra, V. Fluctuation in the abundance of a species considered mathmatically. Nature 1926, 118, 558–560. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Stefanov, M.A.; Dobrikova, A.G.; Apostolova, E.L. Different sensitivities of photosystem II in green algae and cyanobacteria to phenylurea and phenol-type herbicides: Effect on electron donor side. Z. Nat. C 2017, 72, 315–324. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Stamatakis, K.; Papageorgiou, G.C. Dark-to-light transition in Synechococcus sp. PCC 7942 cells studied by fluorescence kinetics assesses plastoquinone redox poise in the dark and photosystem II fluorescence component and dynamics during state 2 to state 1 transition. Photosynth. Res. 2009, 99, 243–255. [Google Scholar] [CrossRef]
- Ni, L.; Acharya, K.; Hao, X.; Li, S.; Li, Y.; Li, Y. Effects of artemisinin on photosystem II performance of Microcystis aeruginosa by in vivo chlorophyll fluorescence. Bull. Environ. Contam. Toxicol. 2012, 89, 1165–1169. [Google Scholar] [CrossRef]
- Barber, J. Crystal structure of the oxygen-evolving complex of photosystem II. Inorg. Chem. 2008, 47, 1700–1710. [Google Scholar] [CrossRef]


| Formulae or terms | Description |
|---|---|
| F0 | Minimum fluorescence |
| Fm | Maximum fluorescence |
| VJ = (F2ms − F0)/(Fm − F0) | Relative variable fluorescence at 2 ms |
| M0 = 4 (F300μs − F0)/(Fm − F0) | Approximated initial slope (in ms −1) of the fluorescence transient V = f (t); It is a measure of the rate of the primary photochemistry |
| VK = (F300μs − F0)/(Fm − F0) | Relative variable fluorescence at 300 μs |
| Fv/Fm | The maximum quantum yield of primary photochemistry at t = 0 |
| ET0/TR0 = 1 − VJ | Probability (at time 0) that a trapped exciton moves an electron into the electron transport chain beyond QA− |
| ET0/RC = (M0/VJ) × (1 − VJ) | Electron transport from QA to QB flux per RC (reaction center) |
| ABS/RC = [M0 (1/VJ)/(Fv/Fm)] | Absorption flux per RC |
| Fv/F0 | An indicator of the efficiency in the primary photochemical reaction |
| Fraction of OEC = [1 − (VK/VJ)]treated/[1 − (VK/VJ)]control | The fraction of OEC (oxygen-evolving complex) in comparison with the control |
| DI0/RC = ABS/RC − TR0/RC | Dissipated energy flux per RC at t = 0 |
| Species | Culture Type | EC50 (μg mL−1) | Probit Regression Equation i | ||
|---|---|---|---|---|---|
| a | b | r2 | |||
| M. aeruginosa | mono-culture | 80.8 ± 0.16 | 1.16 | 2.2 | 0.979 |
| co-culture | 54.7 ± 0.25 | 1.03 | 1.58 | 0.996 | |
| C. pyrenoidosa | mono-culture | 565.9 ± 0.41 | 0.49 | 1.36 | 0.980 |
| co-culture | NC | NC | NC | NC | |
| Species | Culture Type | Phenol Treatment (μg mL−1) | K (* 105 cells mL−1) | a | r | R2 | Tp (day) |
|---|---|---|---|---|---|---|---|
| M. aeruginosa | mono-culture | 0 | 408.1 | 5.73 | 0.41 | 0.96 | 13.9 |
| 2 | 433.6 | 5.71 | 0.41 | 0.95 | 13.9 | ||
| 20 | 401.2 | 5.71 | 0.43 | 0.98 | 13.3 | ||
| 200 | 128.6 | 5.78 | 0.40 | 0.98 | 14.5 | ||
| co-culture | 0 | 348.8 | 5.55 | 0.37 | 0.98 | 15.0 | |
| 2 | 268.8 | 5.17 | 0.37 | 0.96 | 14.0 | ||
| 20 | 283.6 | 5.52 | 0.38 | 0.98 | 14.5 | ||
| 200 | 41.4 | 4.00 | 0.22 | 0.95 | 18.2 | ||
| C. pyrenoidosa | mono-culture | 0 | 281.8 | 4.25 | 0.43 | 0.96 | 9.8 |
| 2 | 301.6 | 4.44 | 0.45 | 0.98 | 9.9 | ||
| 20 | 450.6 | 4.98 | 0.49 | 0.98 | 10.2 | ||
| 200 | 433.6 | 5.36 | 0.48 | 0.97 | 11.2 | ||
| co-culture | 0 | 133.4 | 3.22 | 0.41 | 0.95 | 7.9 | |
| 2 | 139.6 | 3.25 | 0.36 | 0.98 | 9.0 | ||
| 20 | 153.2 | 3.61 | 0.37 | 0.97 | 9.8 | ||
| 200 | 279.1 | 4.28 | 0.39 | 0.92 | 11.0 |
| Phenol Treatment (μg mL−1) | α | β |
|---|---|---|
| 0 | −1.26 ± 0.12 | 1.75 ± 0.07 |
| 2 | −0.84 ± 0.08 n | 1.58 ± 0.22 |
| 20 | −0.94 ± 0.18 n | −0.60 ± 0.05 |
| 200 | −1.12 ± 0.26 | −2.19 ± 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Dai, K.; Parajuli, K.; Hang, X.; Duan, Z.; Hu, Y. Effects of Phenolic Pollution on Interspecific Competition between Microcystis aeruginosa and Chlorella pyrenoidosa and their Photosynthetic Responses. Int. J. Environ. Res. Public Health 2019, 16, 3947. https://doi.org/10.3390/ijerph16203947
Tan X, Dai K, Parajuli K, Hang X, Duan Z, Hu Y. Effects of Phenolic Pollution on Interspecific Competition between Microcystis aeruginosa and Chlorella pyrenoidosa and their Photosynthetic Responses. International Journal of Environmental Research and Public Health. 2019; 16(20):3947. https://doi.org/10.3390/ijerph16203947
Chicago/Turabian StyleTan, Xiao, Kaiwen Dai, Keshab Parajuli, Xiaoshuai Hang, Zhipeng Duan, and Yue Hu. 2019. "Effects of Phenolic Pollution on Interspecific Competition between Microcystis aeruginosa and Chlorella pyrenoidosa and their Photosynthetic Responses" International Journal of Environmental Research and Public Health 16, no. 20: 3947. https://doi.org/10.3390/ijerph16203947
APA StyleTan, X., Dai, K., Parajuli, K., Hang, X., Duan, Z., & Hu, Y. (2019). Effects of Phenolic Pollution on Interspecific Competition between Microcystis aeruginosa and Chlorella pyrenoidosa and their Photosynthetic Responses. International Journal of Environmental Research and Public Health, 16(20), 3947. https://doi.org/10.3390/ijerph16203947





