Effects of Five Substances with Different Modes of Action on Cathepsin H, C and L Activities in Zebrafish Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Cultivation and Fish Egg Collection
2.2. Fish Embryo Toxicity Test (FET)
2.3. Danio Rerio Embryo Collection for Enzymatic Measurements during Development
2.4. Exposure Conditions to Study In Vivo Effects on Cathepsin Activities
2.5. Protein Extraction from Danio rerio Embryos
2.6. Cathepsin Assays (CatH, CatC, CatL)
2.7. Ethical Consent
3. Results
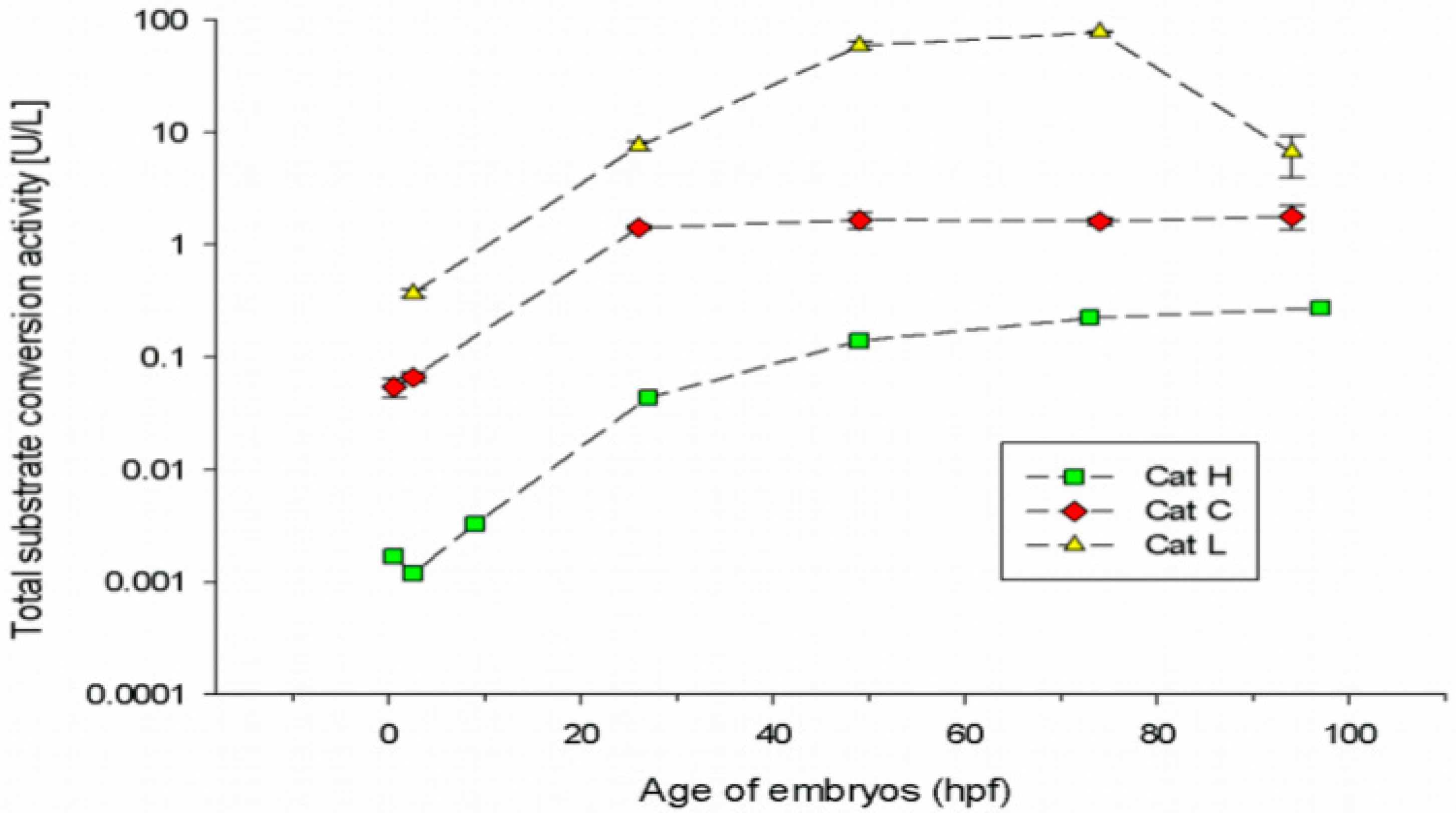
3.1. Cathepsin Activities in Embryonic Stages of the Zebrafish
3.2. Toxicant Effects on Zebrafish Embryo Mortality and on Cathepsin Activities after 48 h of Exposure
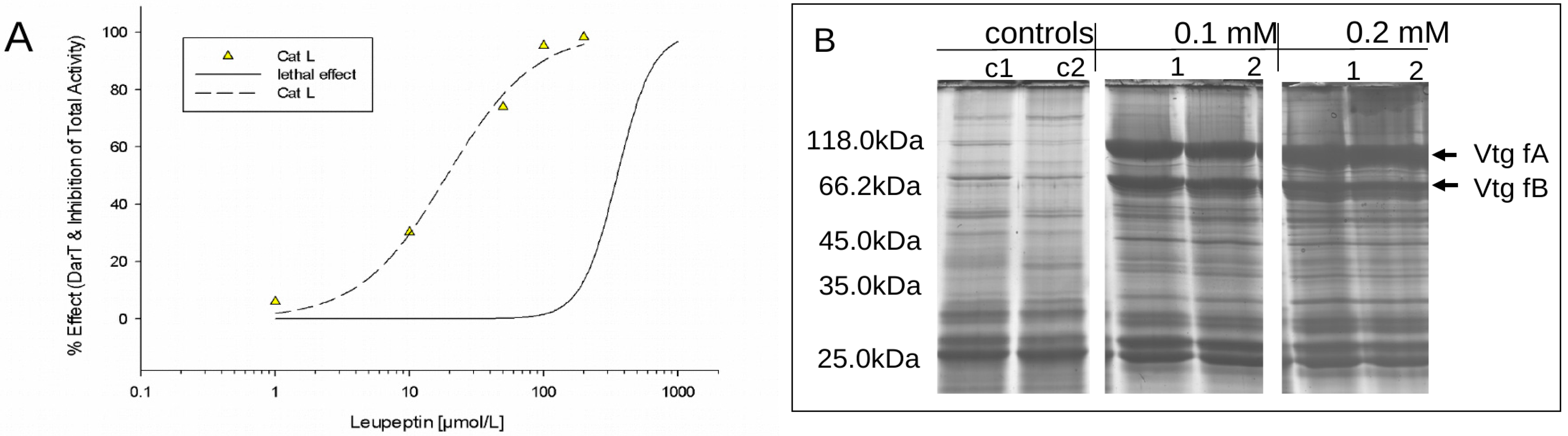
3.2.1. Leupeptin Exposure as a Positive Control for Direct in vivo Cathepsin Inhibition
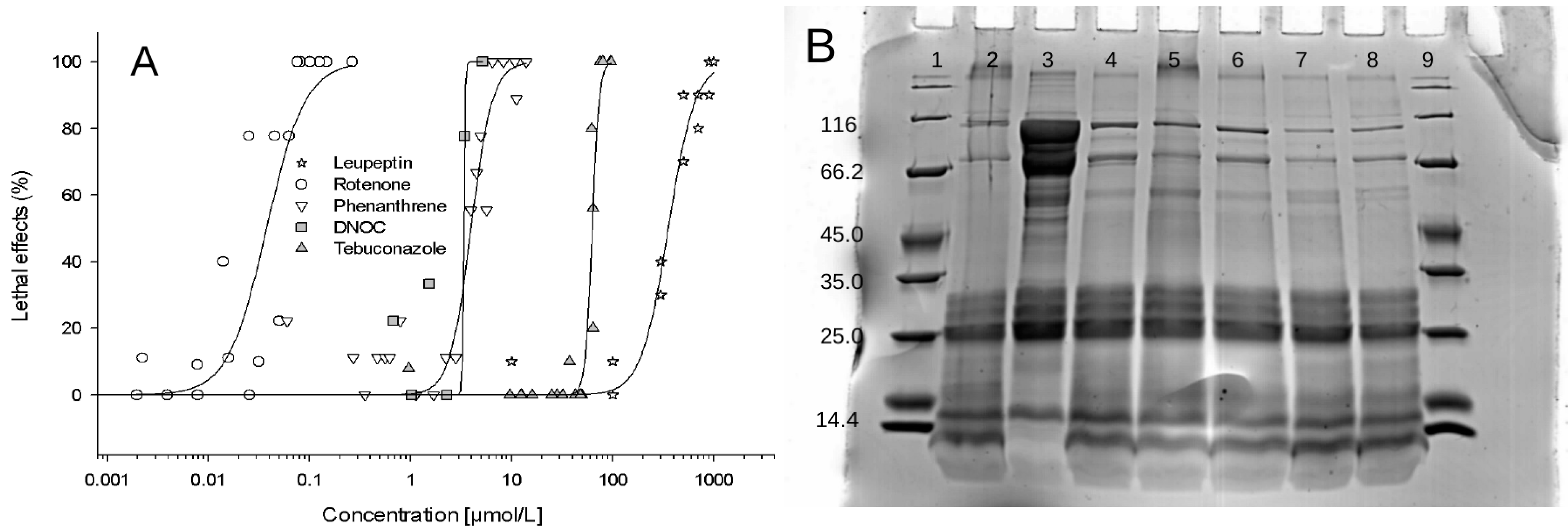
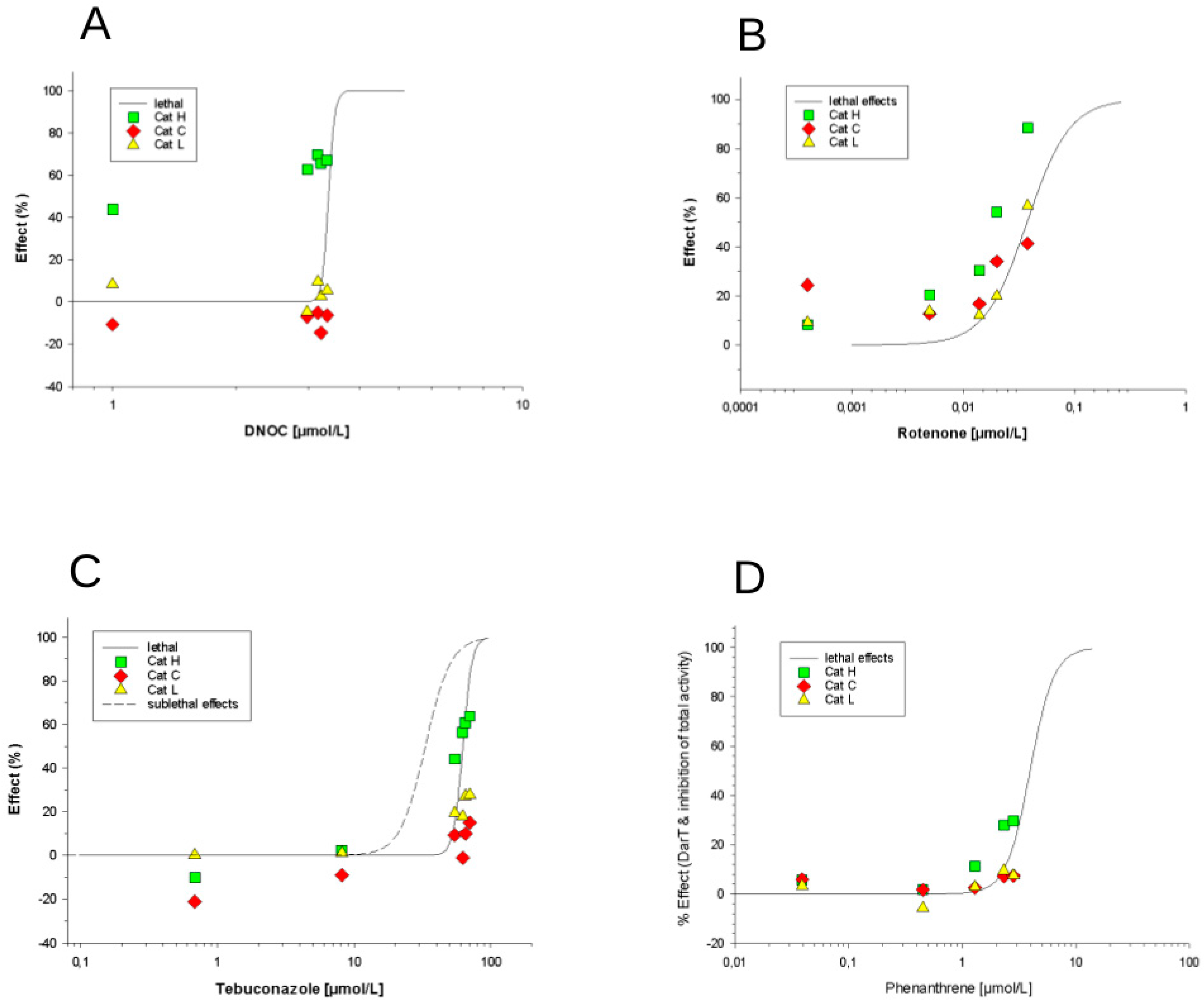
3.2.2. Effects of Phenanthrene, Rotenone, DNOC and Tebuconazole
4. Discussion
4.1. Methodological and Biological Validation of Measured Cathepsin Activities
4.2. Toxicity-Related Changes in Cathepsin Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagger, J.A.; Jones, M.B.; Leonard, D.R.P.; Owen, R.; Galloway, T.S. Biomarkers and integrated environmental risk assessment: Are there more questions than answers? Integr. Environ. Assess. Manag. 2006, 2, 312–329. [Google Scholar] [CrossRef]
- Labaer, J. So, You Want to Look for Biomarkers. J. Proteome Res. 2005, 4, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.C. Interpretation of fish biomarker data for identification, classification, risk assessment and testing of endocrine disrupting chemicals. Environ. Int. 2016, 92–93, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.H. Integrating effects of contaminants across levels of biological organization: An overview. J. Aquat. Ecosyst. Stress Recovery 2000, 7, 113–116. [Google Scholar] [CrossRef]
- Gündel, U.; Benndorf, D.; von Bergen, M.; Altenburger, R.; Küster, E. Vitellogenin cleavage products as indicators for toxic stress in zebra fish embryos: A proteomic approach. Proteomics 2007, 7, 4541–4554. [Google Scholar] [CrossRef]
- Galloway, T.S.; Brown, R.J.; Browne, M.A.; Dissanayake, A.; Lowe, D.; Jones, M.B.; Depledge, M.H. A multibiomarker approach to environmental assessment. Environ. Sci. Technol. 2004, 38, 1723–1731. [Google Scholar] [CrossRef]
- Muncke, J.; Junghans, M.; Eggen, R.I.L. Testing Estrogenicity of Known and Novel (Xeno-)Estrogens in the MolDarT Using Developing Zebrafish (Danio rerio). Environ. Toxicol. 2007, 22, 185–193. [Google Scholar] [CrossRef]
- Moore, M.N.; Depledge, M.H.; Readman, J.W.; Paul Leonard, D.R. An integrated biomarker-based strategy for ecotoxicological evaluation of risk in environmental management. Mutat. Res. 2004, 552, 247–268. [Google Scholar] [CrossRef]
- Van Der Oost, R.; Beyer, J.; Vermeulen, N.P.E.; Vanderoost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Stegeman, J.J.; Brouwer, M.; Richard, T.D.G.; Förlin, L.; Fowler, B.A.; Sanders, B.M.; VanVeld, P.A. Molecular responses to environmental contamination: Enzyme and protein systems as indicators of chemical exposure and effect. In Biomarkers-Biochemical, Physiological and Histological Markers of Anthropogenic Stress; Hugget, R.J., Kimley, R.A., Mehrle, P.M., Bergmann, J., Bergmann, H.L., Eds.; Lewis Publishers: Chelsea, MA, USA, 1992; pp. 235–335. [Google Scholar]
- Nagel, R.; Dar, T. The embryo test with the zebrafish Danio rerio—A general model in ecotoxicology and toxicology. Altex Altern. Tierexp. 2002, 19, 38–48. [Google Scholar]
- Schulte, C.; Nagel, R. Testing acute toxicity in the embryo of zebrafish, Brachydanio rerio, as an alternative to the acute fish test: Preliminary results. Atla 1994, 22, 12–19. [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). OECD Guidelines for the Testing of Chemicals-Fish Embryo Acute Toxicity (FET) Test (TG 236); OECD: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Kamler, E. Resource allocation in yolk-feeding fish. Rev. Fish Biol. Fish. 2008, 18, 143–200. [Google Scholar] [CrossRef]
- Wallace, R.A.; Jared, D.W. Studies on Amphibian Yolk VIII. The Estrogen-induced Synthesis of a Serum Lipophosphoprotein and Its Selective Uptake by the Ovary and Transformation Hepatic into Yolk Platelet Proteins in Xenopus laevis. Dev. Biol. 1969, 19, 498–526. [Google Scholar] [CrossRef]
- Tyler, C.R.; Sumpter, J.P. Oocyte growth and development in teleosts. Rev. Fish Biol. Fish. 1996, 6, 287–318. [Google Scholar] [CrossRef]
- Tay, T.L.; Lin, Q.; Seow, T.K.; Tan, K.H.; Hew, C.L.; Gong, Z. Proteomic analysis of protein profiles during early development of the zebrafish, Danio rerio. Proteomics 2006, 6, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Carletta, R.; Cambi, A.; Vita, A.; Bromage, N. Yolk formation and degradation during oocyte maturation in seabream Sparus aurata: Involvement of two lysosomal proteinases. Biol. Reprod. 1999, 60, 140–146. [Google Scholar] [CrossRef]
- Carnevali, O.; Cionna, C.; Tosti, L.; Lubzens, E.; Maradonna, F. Role of cathepsins in ovarian follicle growth and maturation. Gen. Comp. Endocrinol. 2006, 146, 195–203. [Google Scholar] [CrossRef]
- Qiu, G.F.; Yamano, K.; Unuma, T. Cathepsin C transcripts are differentially expressed in the final stages of oocyte maturation in kuruma prawn Marsupenaeus japonicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 171–181. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Prat, F.; Randall, C.; Tyler, C.R. Molecular characterization of putative yolk processing enzymes and their expression during oogenesis and embryogenesis in rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 2001, 65, 1701–1709. [Google Scholar] [CrossRef]
- Fagotto, F. COMMENTARY Regulation of yolk degradation, or how to make sleepy lysosomes. J. Cell Sci. 1995, 108, 3645–3647. [Google Scholar]
- Sire, M.F.; Babin, P.J.; Vernier, J.M. Involvement of the lysosomal system in yolk protein deposit and degradation during vitellogenesis and embryonic development in trout. J. Exp. Zool. 1994, 269, 69–83. [Google Scholar] [CrossRef]
- Yoshizaki, N.; Yonezawa, S.; Education, G.; Prefectural, A. Cathepsin D Activity in the Vitellogenesis of Xenopus laevis. Dev. Growth Differ. 1994, 36, 299–306. [Google Scholar] [CrossRef]
- Fabra, M.; Cerdà, J. Ovarian cysteine proteinases in the teleost Fundulus heteroclitus: Molecular cloning and gene expression during vitellogenesis and oocyte maturation. Mol. Reprod. Dev. 2004, 67, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Mosconi, G.; Cambi, A.; Ridolfi, S.; Zanuy, S.; Polzonetti-magni, A.M. Changes of lysosomal enzyme activities in sea bass (Dicentrarchus labrax) eggs and developing embryos. Aquaculture 2001, 249–256. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Ichikawa, N.; Fukada, H.; Fujita, T.; Sullivan, C.V.; Hara, A. Identification and Characterization of Proteases Involved in Specific Proteolysis of Vitellogenin and Yolk Proteins in Salmonids. J. Exp. Zool. 2002, 292, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Tingaud-Sequeira, A.; Cerdà, J. Phylogenetic relationships and gene expression pattern of three different cathepsin L (Ctsl) isoforms in zebrafish: Ctsla is the putative yolk processing enzyme. Gene 2007, 386, 98–106. [Google Scholar] [CrossRef]
- Olin, T.; von der Decken, A. Yolk proteins in salmon (Salmo salar) oocytes, eyed eggs, and alevins differing in viability. Can. J. Zool. 1990, 68, 895–900. [Google Scholar] [CrossRef]
- Hartling, R.C.; Kunkel, J.G. Developmental fate of the yolk protein lipovitellin in embryos and larvae of winter flounder, Pleuronectes americanus. J. Exp. Zool. Part A 1999, 284, 686–695. [Google Scholar] [CrossRef]
- Matsubara, T.; Ohkubo, N.; Andoh, T.; Sullivan, C.V. Two Forms of Vitellogenin, Yielding Two Distinct Lipovitellins, Play Different Roles during Oocyte Maturation and Early Development of Barfin Flounder, Verasper moseri, a Marine Teleost that Spawns Pelagic Eggs. Dev. Biol. 1999, 213, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.X.; Xu, Y.; Hui, Y. Reproductive effects of prenatal exposure to nonylphenol on zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 77–84. [Google Scholar] [CrossRef]
- Zhang, T.; Rawson, D.M.; Tosti, L.; Carnevali, O. Cathepsin activities and membrane integrity of zebrafish (Danio rerio) oocytes after freezing to -196 degrees C using controlled slow cooling. Cryobiology 2008, 56, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Raldua, D.; Andre, M.; Babin, P.J. Clofibrate and gemfibrozil induce an embryonic mal. Toxicol. Appl. Pharmacol. 2008, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, K.; Küster, E.; Altenburger, R.; Gündel, U. Proteomic Signatures of the Zebrafish (Danio rerio) Embryo: Sensitivity and Specificity in Toxicity Assessment of Chemicals. Int. J. Proteom. 2010, 2010, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, F.; Casida, J.E. Functional coupling of PSST and ND1 subunits in NADH:ubiquinone oxidoreductase established by photoaffinity labeling. Biochim. Et Biophys. Acta 2001, 1506, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Pelfrene, A.F. DINITRO- ortho -CRESOL. Environ. Health Criteria 2000, 220, 1–105. [Google Scholar]
- Han, Q.M.; Kang, Z.S.; Buchenauer, H.; Huang, L.L.; Zhao, J. Cytological and immunocytochemical studies on the effects of the fungicide tebuconazole on the interaction of wheat with stripe rust. J. Plant Pathol. 2006, 88, 263–271. [Google Scholar] [CrossRef]
- Rauch, G.J.; Granato, M.; Haffter, P. A polymorphic zebrafish line for genetic mapping using SSLPs on high-percentage agarose gels. Tech. Tips Online 1997, 2, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Küster, E. Cholin- and carboxylesterase activities in developing zebrafish embryos (Danio rerio) and their potential use for insecticide hazard assessment. Aquat. Toxicol. 2005, 75, 76–85. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book, A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000; Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 15 May 2018).
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 7346-3:1996: Water Quality—Determination of the Acute Lethal Toxicity of Substances to a Freshwater Fish [Brachydanio Rerio Hamilton-Buchanan (Teleostei, Cyprinidae)] —Part 3: Flow-Through Method; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). OECD Guideline for the Testing of Chemicals-Draft Proposal for a New Guideline_Fish Embryo Toxicity (FET) Test; OECD: Paris, France, 2006; pp. 1–11. Available online: https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-2-effects-on-biotic-systems_20745761?page=2 (accessed on 15 October 2019).
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Riedl, J.; Altenburger, R. Physicochemical substance properties as indicators for unreliable exposure in microplate-based bioassays. Chemosphere 2007, 67, 2210–2220. [Google Scholar] [CrossRef]
- Schreiber, R.R.R.; Altenburger, R.; Paschke, A.; Kuester, E.; Küster, E. How to deal with lipophilic and volatile organic substances in microtiter plate assays. Environ. Toxicol. Chem. 2008, 27, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin Phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Laemmli, U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hachicho, N.; Reithel, S.; Miltner, A.; Heipieper, H.J.; Küster, E.; Luckenbach, T. Body Mass Parameters, Lipid Profiles and Protein Contents of Zebrafish Embryos and Effects of 2,4-Dinitrophenol Exposure. PLoS ONE 2015, 10, e0134755. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Toursakissian, K.; Sweeney, J.; Jones, T.H.D. Dipeptidyl-Aminopeptidases and Aminopeptidases in Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 1985, 127, 962–968. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; 1620p, ISBN 9780367384173. [Google Scholar]
- Carnevali, O.; Maradonna, F. Exposure to xenobiotic compounds: Looking for new biomarkers. Gen. Comp. Endocrinol. 2003, 131, 203–209. [Google Scholar] [CrossRef]
- McGrath, M.E. The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 181–204. [Google Scholar] [CrossRef]
- Kaivarainen, E.; Nemova, N.; Krupnova, M.; Bondareva, L. The effect of toxic factors on intracellular proteinase activity in freshwater fish. Acta Vet. BRNO 1998, 67, 309–316. [Google Scholar] [CrossRef]
- Link, V.; Shevchenko, A.; Heisenberg, C. BMC Developmental Biology. Cell 2006, 9, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Cal, Y.; Xu, Y. Oxidative Damage in Unfertilized Eggs of Chinese Rare Minnow (Gobiocypris Rarus) Exposed to Nonylphenol. Environ. Toxicol. Chem. 2008, 27, 213–219. [Google Scholar] [CrossRef]
- Raldúa, D.; Fabra, M.; Bozzo, M.G.; Weber, E.; Cerdà, J.; Raldua, D.; Raldúa, D.; Fabra, M.; Bozzo, M.G.; Weber, E.; et al. Cathepsin B-mediated yolk protein degradation during killifish oocyte maturation is blocked by an H+-ATPase inhibitor: Effects on the hydration mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R456–R466. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.W.A.; Matsumoto, J.; Van Der Kraak, G. Thyroglobulin type-1 domain protease inhibitors exhibit specific expression in the cortical ooplasm of vitellogenic rainbow trout oocytes. Mol. Reprod. Dev. 2004, 69, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, A.J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem. J. 1980, 187, 909–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyagi, T.; Miyata, S.; Nanbo, M.; Kojima, F.; Matsuzaki, M. Biological activities of leupeptins. J. Antibiot. 1969, 22, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Ambroso, J.L.; Harris, C. In Vitro Embryotoxicity of the Cysteine Proteinase Inhibitors Benzyloxycarbonyl-Phenylalanine- Alanine-Diazomethane (Z-Phe-Ala-CHN,) and Benzyloxycarbonyl-Phenylalanine-Phenylalanine- Diazomethane (Z-Phe-Phe-CHN). Teratology 1994, 50, 214–228. [Google Scholar] [CrossRef]
- Wood, A.W.; Van der Kraak, G. Yolk proteolysis in rainbow trout oocytes after serum-free culture: Evidence for a novel biochemical mechanism of atresia in oviparous vertebrates. Mol. Reprod. Dev. 2003, 65, 219–227. [Google Scholar] [CrossRef]
- Maradonna, F.; Carnevali, O. Vitellogenin, zona radiata protein, cathepsin D and heat shock protein 70 as biomarkers of exposure to xenobiotics. Biomarkers 2007, 12, 240–255. [Google Scholar] [CrossRef]
| Leupeptin | Rotenone | DNOC | Phenanthrene | Tebuconazole | |
|---|---|---|---|---|---|
| CAS RN | 24365-47-7 | 83-79-4 | 534-52-1 | 85-01-8 | 107534-96-3 |
| MW (1) (g/mol) | 426.55 | 394.42 | 198.13 | 178.23 | 307.82 |
| LogKOW (2) | −0.23 | 4.1 | 2.13 | 4.46 | 3.70 (exp.) |
| Wsol (3) (mg/L) | 161.7 @ 25 °C | 0.2 @ 20 °C, 0.17 @ 25 °C (4) | 198/678.4 @ 25 °C | 0.677/1.15 @ 25 °C | 36 @ 20 °C |
| Usage (1) | Protease inhibitor | Insecticide (acaricide) | Insecticide (fungicide, herbicide, and antibacterial drug) | Environmental pollutant | Triazole fungicide (and antibacterial drug) |
| Mode of action | Inhibitor of cathepsin L | Inhibition of Complex I of the electron transport chain | Decoupler | Narcotic toxicity | Inhibition of sterol biosynthesis |
| Parameters (lethal effects) | a = 100 b = 3.2691 c = x50 = 353.76 R2 = 0.9691 | a = 100 b = 2.2794 c = x50= 0.0376 R2 = 0.8049 | a = 100 b = 56.5262 c = x50 = 3.3646 R2 = 0.8374 | a = 100 b = 4.1481 c = x50 = 3.9560 R2 = 0.9146 | a = 100 b = 12.9412 c = x50 = 62.4523 R2 = 0.9275 |
| Sublethal effects | Not significantly different from lethal effect “curve” | Not significantly different from lethal effect “curve” | Not significantly different from lethal effect “curve” | Not significantly different from lethal effect “curve” | a = 100 b = 1.34 c = x50 = EC50 = 19.09 R² = 0.989 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küster, E.; Kalkhof, S.; Aulhorn, S.; von Bergen, M.; Gündel, U. Effects of Five Substances with Different Modes of Action on Cathepsin H, C and L Activities in Zebrafish Embryos. Int. J. Environ. Res. Public Health 2019, 16, 3956. https://doi.org/10.3390/ijerph16203956
Küster E, Kalkhof S, Aulhorn S, von Bergen M, Gündel U. Effects of Five Substances with Different Modes of Action on Cathepsin H, C and L Activities in Zebrafish Embryos. International Journal of Environmental Research and Public Health. 2019; 16(20):3956. https://doi.org/10.3390/ijerph16203956
Chicago/Turabian StyleKüster, Eberhard, Stefan Kalkhof, Silke Aulhorn, Martin von Bergen, and Ulrike Gündel. 2019. "Effects of Five Substances with Different Modes of Action on Cathepsin H, C and L Activities in Zebrafish Embryos" International Journal of Environmental Research and Public Health 16, no. 20: 3956. https://doi.org/10.3390/ijerph16203956
APA StyleKüster, E., Kalkhof, S., Aulhorn, S., von Bergen, M., & Gündel, U. (2019). Effects of Five Substances with Different Modes of Action on Cathepsin H, C and L Activities in Zebrafish Embryos. International Journal of Environmental Research and Public Health, 16(20), 3956. https://doi.org/10.3390/ijerph16203956






