Socio-Economic and Environmental Factors Related to Spatial Differences in Human Non-Tuberculous Mycobacterial Diseases in the Czech Republic
Abstract
:1. Introduction
2. Materials and Methods
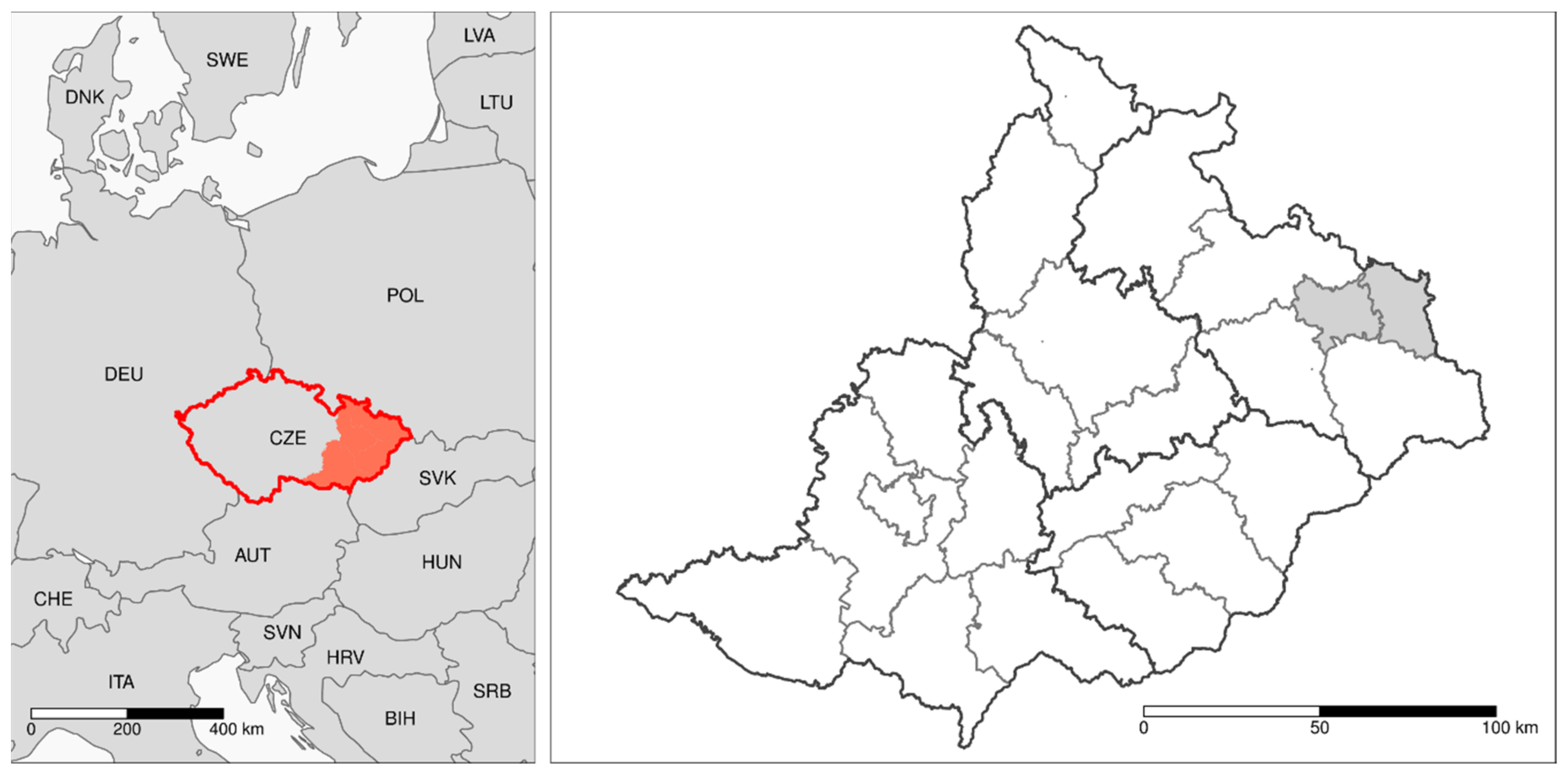
2.1. Area Description and Study Design
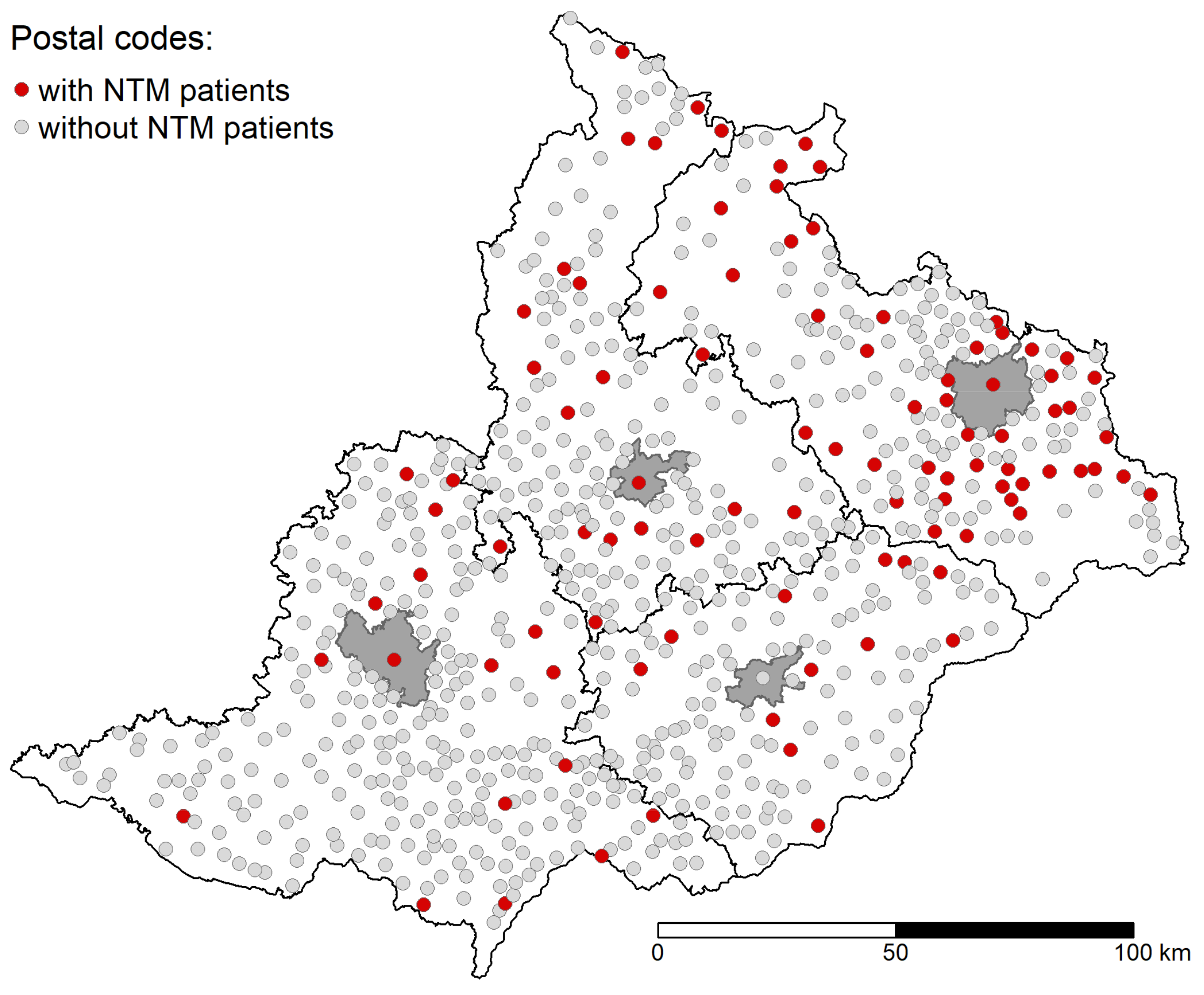
2.2. Data Source
2.3. NTM Identification
2.4. Data Analysis and Statistical Methods
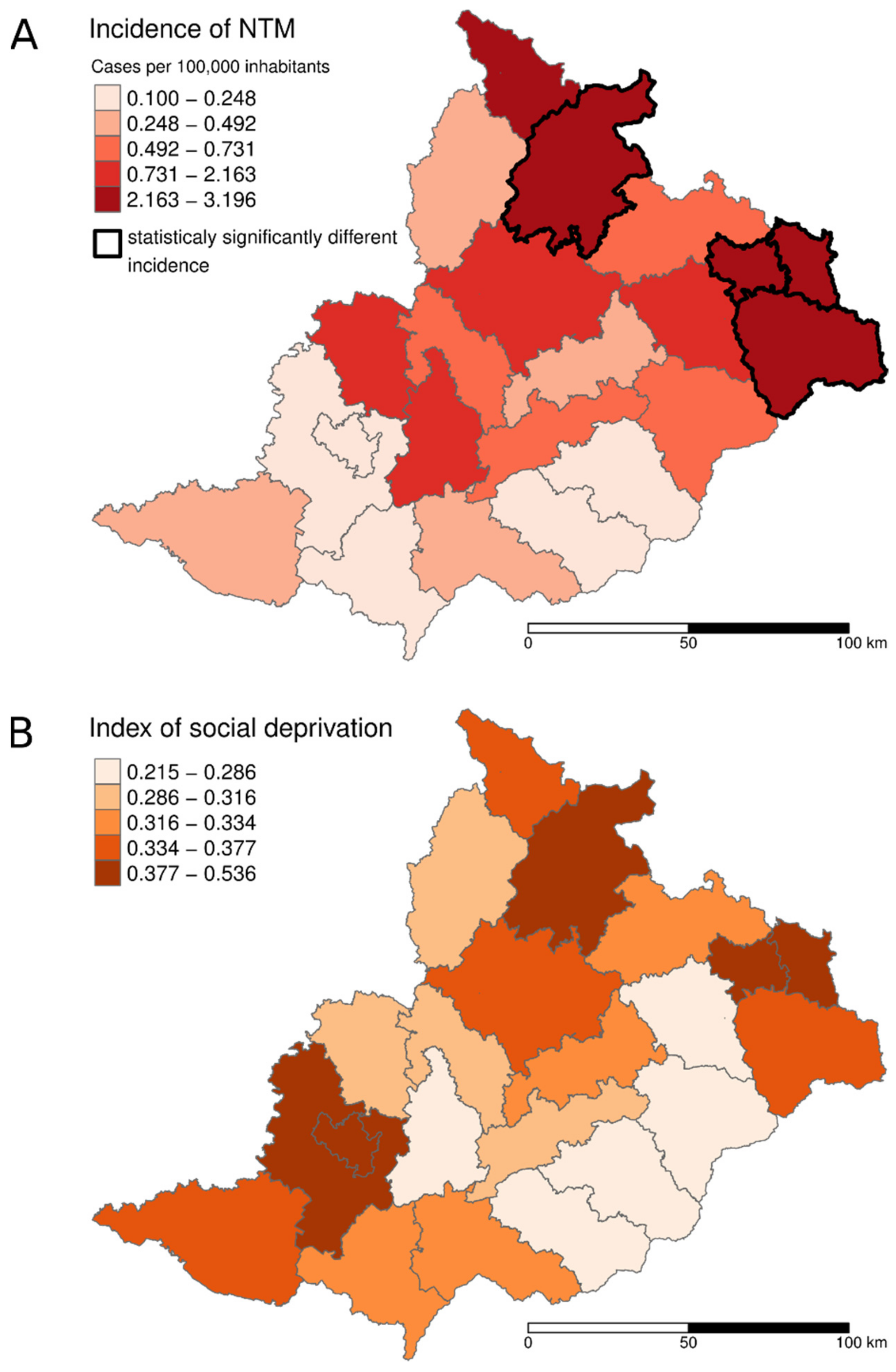
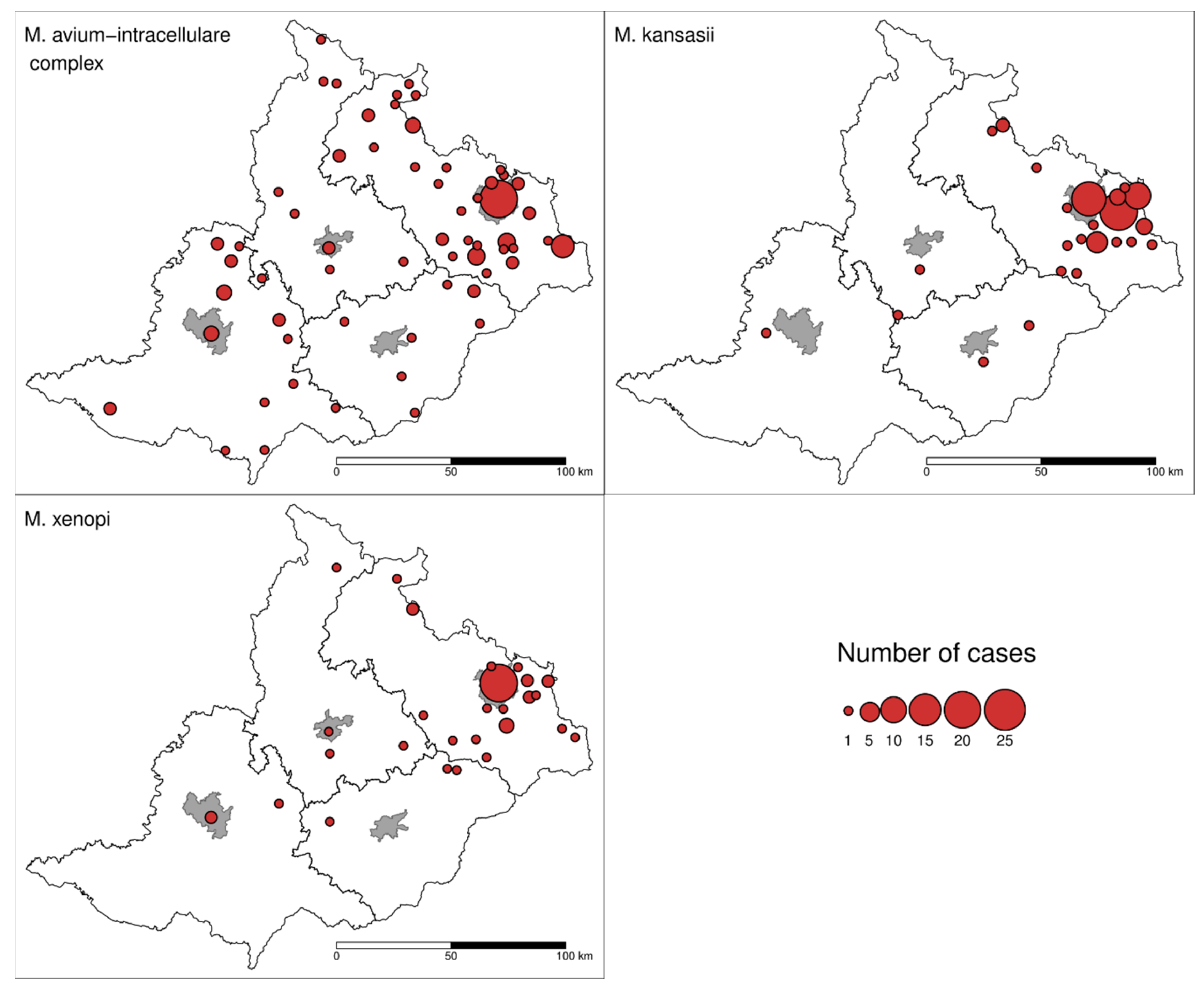
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| District | NTM | MAIC | M. kansasii | M. xenopi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Female | Male | Total | Female | Male | Total | Female | Male | Total | Female | Male | |
| Blansko | 1.058 | 0.783 | 1.341 | 1.058 | 0.783 | 1.341 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brno-city | 0.227 | 0.219 | 0.234 | 0.113 | 0.073 | 0.157 | 0 | 0 | 0 | 0.075 | 0.146 | 0 |
| Brno | 0.131 | 0.130 | 0.131 | 0 | 0 | 0 | 0.065 | 0 | 0.131 | 0 | 0 | 0 |
| Bruntál | 3.196 | 3.000 | 3.399 | 1.828 | 1.799 | 1.857 | 0.455 | 0 | 0.920 | 0.459 | 0.294 | 0.622 |
| Břeclav | 0.247 | 0.486 | 0 | 0.247 | 0.486 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frýdek-Mistek | 2.479 | 2.507 | 2.451 | 1.272 | 1.847 | 0.680 | 0.671 | 0.264 | 1.091 | 0.402 | 0.265 | 0.544 |
| Hodonín | 0.369 | 0.183 | 0.561 | 0.369 | 0.183 | 0.561 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jeseník | 2.186 | 1.445 | 2.937 | 1.091 | 1.445 | 0.733 | 0 | 0 | 0 | 0.367 | 0 | 0.739 |
| Karviná | 2.798 | 1.427 | 4.219 | 0.276 | 0.218 | 0.337 | 1.904 | 0.987 | 2.856 | 0.450 | 0.112 | 0.800 |
| Kroměříž | 0.539 | 0 | 1.103 | 0.134 | 0 | 0.274 | 0 | 0 | 0 | 0.135 | 0 | 0.276 |
| Nový Jičín | 2.072 | 2.043 | 2.102 | 1.036 | 1.115 | 0.956 | 0.282 | 0.186 | 0.382 | 0.376 | 0.370 | 0.382 |
| Olomouc | 0.733 | 0.475 | 1.005 | 0.244 | 0.357 | 0.125 | 0.062 | 0 | 0.126 | 0.123 | 0 | 0.253 |
| Opava | 0.728 | 1.111 | 0.330 | 0.567 | 0.794 | 0.330 | 0.081 | 0.158 | 0 | 0.081 | 0.158 | 0 |
| Ostrava | 2.770 | 1.877 | 3.719 | 1.012 | 1.194 | 0.818 | 0.704 | 0.341 | 1.088 | 1.011 | 0.339 | 1.722 |
| Prostějov | 0.526 | 0 | 1.078 | 0.263 | 0 | 0.539 | 0.132 | 0 | 0.270 | 0 | 0 | 0 |
| Přerov | 0.434 | 0.212 | 0.664 | 0.110 | 0 | 0.223 | 0 | 0 | 0 | 0.109 | 0 | 0.222 |
| Šumperk | 0.470 | 0.231 | 0.718 | 0.117 | 0.231 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Uh. Hradiště | 0.100 | 0 | 0.204 | 0.100 | 0 | 0.204 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vsetín | 0.695 | 0.978 | 0.403 | 0.398 | 0.783 | 0 | 0.099 | 0 | 0.202 | 0.198 | 0.194 | 0.201 |
| Vyškov | 0.945 | 1.249 | 0.633 | 0.473 | 0.936 | 0 | 0 | 0 | 0 | 0.156 | 0 | 0.316 |
| Zlín | 0.223 | 0.146 | 0.304 | 0.149 | 0 | 0.304 | 0.075 | 0.146 | 0 | 0 | 0 | 0 |
| Znojmo | 0.252 | 0.497 | 0 | 0.252 | 0.497 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Studied area overall | 1.096 | 0.869 | 1.333 | 0.467 | 0.535 | 0.396 | 0.285 | 0.163 | 0.436 | 0.218 | 0.152 | 0.331 |

References
- Falkinham, J.O., III. Surrounded by mycobacteria: Nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 2009, 107, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., III. Environmental sources of nontuberculous mycobacteria. Clin. Chest Med. 2015, 36, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Revetta, R.P.; Gomez-Alvarez, V.; Gerke, T.L.; Santo Domingo, J.W.; Ashbolt, N.J. Changes in bacterial composition of biofilm in a metropolitan drinking water distribution system. J. Appl. Microbiol. 2016, 121, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roguet, A.; Therial, C.; Saad, M.; Boudahmane, L.; Moulin, L.; Lucas, F.S. High mycobacterial diversity in recreational lakes. Antonie Van Leeuwenhoek 2016, 109, 619–631. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.J. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect. Dis. 2018, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Henkle, E.; Hedberg, K.; Schafer, S.; Novosad, S.; Winthrop, L. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann. Am. Thorac. Soc. 2015, 12, 642–647. [Google Scholar] [CrossRef]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of pulmonary nontuberculous mycobacterial disease in Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef]
- Shah, N.M.; Davidson, J.A.; Anderson, L.F.; Lator, M.K.; Kim, J.; Thomas, H.L.; Lipman, M.; Abubakar, I. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect. Dis. 2016, 16, 195. [Google Scholar] [CrossRef]
- Diel, R.; Jacob, J.; Lampenius, N.; Loebinger, M.; Nienhaus, A.; Rabe, K.F.; Ringshausen, F.C. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur. Respir. J. 2017, 49, 1602109. [Google Scholar] [CrossRef] [Green Version]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Ängeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, M.; Samarzija, M.; Sabol, I.; Jakopovic, M.; Katalinic Jankovic, V.; Zmak, L.; Ticac, B.; Marusic, A.; Obrovac, M.; van Ingen, J. Geographical distribution and clinical relevance of non-tuberculous mycobacteria in Croatia. Int. J. Tuberc. Lung Dis. 2013, 17, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaulding, A.B.; Lai, Y.L.; Zelazny, A.M.; Olivier, K.N.; Kadri, S.S.; Prevots, D.R.; Adjemian, J. Geographic distribution of nontuberculous mycobacterial species identified among clinical isolates in the United States, 2009–2013. Ann. Am. Thorac. Soc. 2017, 14, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Frankland, T.B.; Daida, Y.G.; Honda, J.R.; Olivier, K.N.; Zelazny, A.; Honda, S.; Prevots, D.R. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerg. Infect. Dis. 2017, 23, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, P.; Murray, J.; Glynn, J.R.; Thomas, R.G.; Godfrey-Faussett, P.; Shearer, S. Risk factors for pulmonary disease due to culture-positive M. tuberculosis or nontuberculous mycobacteria in South African gold miners. Eur. Respir. J. 2000, 15, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Koh, W.-J.; Yew, W.W. Update on pulmonary disease due to non-tuberculous mycobacteria. Int. J. Infect. Dis. 2016, 45, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria infections in immunosuppressed host. Clin. Chest Med. 2015, 36, 91–99. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.-C.; Lung, S.-C.C.; Sun, Z.; Chen, C.; Chen, J.-K.; Zou, Q.; Lin, Y.-H.; Lin, C.-H. Polycyclic aromatic hydrocarbons are associated with increased risk of chronic obstructive pulmonary disease during haze events in China. Sci. Total Environ. 2017, 574, 1649–1658. [Google Scholar] [CrossRef]
- Raudoniute, J.; Stasiulaitiene, I.; Kulvinskiene, I.; Bagdonas, E.; Garbaras, A.; Krugly, E.; Martuzevicius, D.; Bironaite, D.; Aldonyte, R. Pro-inflammatory effects of extracted urban fine particulate matter on human bronchial epithelial cells BEAS-2B. Environ. Sci. Pollut. Res. 2018, 25, 32277–32291. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Ranjit, S.; Midde, N.M.; Sinha, N.; Patters, B.J.; Rahman, M.A.; Cory, T.J.; Rao, P.S.S.; Kumar, S. Effect of polyaryl hydrocarbons on cytotoxicity in monocytic cells: Potential role of cytochromes P450 and oxidative stress pathways. PLoS ONE 2016, 11, e0163827. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., III. Mycobacterial aerosols and respiratory disease. Emerg. Infect. Dis. 2003, 9, 763–767. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.E.; Bardsley, P. An association between Mycobacterium malmoense and coal workers´ pneumoconiosis. Lung 2009, 187, 51–54. [Google Scholar] [CrossRef]
- Jiřík, V.; Machaczka, O.; Miturová, H.; Tomášek, I.; Šlachtová, H.; Janoutová, J.; Velická, H.; Janout, V. Air pollution and potential health risk in Ostrava Region—A review. Cent. Eur. J. Public Health 2016, 24, S4–S17. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, P.; Hovorka, J.; Klán, M.; Hopke, P.K. Source apportionment of size resolved particulate matter at a European air pollution hot spot. Sci. Total Environ. 2015, 502, 172–183. [Google Scholar] [CrossRef]
- OKD Ltd. Annual Reports 2005–2015. Available online: https://www.okd.cz/en/about-us/annual-reports (accessed on 10 September 2018).
- Chobot, S.; Mališ, J.; Šebáková, H.; Pelikán, M.; Zatloukal, O.; Palička, P.; Kocurová, D. Endemic incidence of infections caused by Mycobacterium kansasii in the Karviná District in 1968–1995. Cent. Eur. J. Public Health 1997, 5, 164–173. [Google Scholar]
- Sosnovcová, T. The Impacts of Coal Mining in the Ostrava Region. Bachelor’s Thesis, Faculty of Science, Masaryk University in Brno, Brno, Czech Republic, 2016. (In Czech). [Google Scholar]
- Czech Statistical Office. 2019. Available online: https://www.czso.cz/csu/czso/home (accessed on 12 May 2019).
- Czech Hydrometeorological Institute. 2019. Available online: http://portal.chmi.cz/?l=en (accessed on 18 May 2019).
- OECD. Handbook on Constructing Composite Indicators; Methodology and User Guide; OECD Publications: Paris, France, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. Available online: https://www.R-project.org/ (accessed on 24 April 2019).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Package version 0.7.7. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 24 April 2019).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Tennekes, M. Tmap: Thematic Maps in R. J. Stat. Softw. 2018, 84, 1–39. [Google Scholar] [CrossRef]
- Andréjak, C.; Thomsen, V.Ø.; Johansen, I.S.; Riis, A.; Benfield, T.L.; Duhaut, P.; Sørensen, H.T.; Lescure, F.-X.; Thomsen, R.W. Nontuberculous pulmonary mycobacteriosis in Denmark: Incidence and prognostic factor. Am. J. Respir. Crit. Care Med. 2010, 181, 514–521. [Google Scholar] [CrossRef]
- Gerogianni, I.; Papala, M.; Kostikas, K.; Petinaki, E.; Gourgoulianis, K.I. Epidemiology and clinical significance of mycobacterial respiratory infections in Central Greece. Int. J. Tuberc. Lung Dis. 2008, 12, 807–812. [Google Scholar]
- Jankovic, M.; Sabol, I.; Zmak, L.; Katalinic Jankovic, V.; Jakopovic, M.; Obrovac, M.; Ticac, B.; Kardum Bulat, L.; Grle, S.P.; Marekovic, I.; et al. Microbiological criteria in non-tuberculous mycobacteria pulmonary disease: A tool for diagnosis and epidemiology. Int. J. Tuberc. Lung Dis. 2016, 20, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.P.; O’Connor, T.M.; Ryan, C.; Sheehan, S.; Cryan, B.; Bredin, C. Nontuberculous mycobacteria: Incidence in Southwest Ireland from 1987 to 2000. Respir. Med. 2003, 97, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with non-tuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Bendien, S.A.; de Lange, W.C.M.; Hoefsloot, W.; Dekhuijzen, P.N.R.; Boeree, M.J.; van Soolingen, D. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax 2009, 64, 502–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlik, I.; Gersl, M.; Bartos, M.; Ulmann, V.; Kaucka, P.; Caha, J.; Unc, A.; Hubelova, D.; Konecny, O.; Modra, H. Nontuberculous mycobacteria in the environment of Hranice Abyss, the world’s deepest flooded cave (Hranice karst, Czech Republic). Environ. Sci. Pollut. Res. 2018, 25, 23712–23724. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Falkinham, J.O., III; Holland, S.M.; Prevots, D.R. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am. J. Respir. Crit. Care Med. 2012, 186, 553–558. [Google Scholar] [CrossRef]
- Iivanainen, E.K.; Martikainen, P.J.; Räisänen, M.L.; Katila, M.-L. Mycobacteria in boreal coniferous forest soils. FEMS Microbiol. Ecol. 1997, 23, 325–332. [Google Scholar] [CrossRef]
- Kirschner, R.A.; Parker, B.C.; Falkinham, J.O., III. Epidemiology of infection by nontuberculous mycobacteria. Am. Rev. Respir. Dis. 1992, 145, 271–275. [Google Scholar] [CrossRef]
- Dirac, M.A.; Horan, K.L.; Doody, D.R.; Meschke, J.S.; Park, D.R.; Jackson, L.A.; Weiss, N.S.; Winthrop, K.L.; Cangelosi, G.A. Environment or host? A case-control study of risk factors for Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2012, 186, 684–691. [Google Scholar] [CrossRef]
- Glassroth, J. Pulmonary disease due to nontuberculous mycobacteria. Chest 2008, 133, 243–251. [Google Scholar] [CrossRef]
- Lake, M.A.; Ambrose, L.R.; Lipman, M.C.I.; Lowe, D.M. “Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Claeys, T.A.; Robinson, R.T. The many lives of nontuberculous mycobacteria. J. Bacteriol. 2018, 200, e00739-e17. [Google Scholar] [CrossRef] [PubMed]
- Hashish, E.; Merwad, A.; Elgaml, S.; Amer, A.; Kamal, H.; Elsadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management: A review. Vet. Q. 2018, 38, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Prevots, D.R. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010–2014. Ann. Am. Thorac. Soc. 2018, 15, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: Implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 2007, 37, 748–755. [Google Scholar] [CrossRef]
- Tan, Y.; Su, B.; Shu, W.; Cai, X.; Kuang, S.; Kuang, H.; Liu, J.; Pang, Y. Epidemiology of pulmonary disease due to nontuberculous mycobacteria in Southern China, 2013–2016. BMC Pulm. Med. 2018, 18, 168. [Google Scholar] [CrossRef]
- Kaustová, J.; Málková, V. Results of ten-year follow-up of patients with repeated isolation of M. gordonae in the Ostrava District. Stud. Pneumol. Phtiseol. Cech. 1982, 42, 443–447. [Google Scholar]
- Bakuła, Z.; Kościuch, J.; Safianowska, A.; Proboszcz, M.; Bielecki, J.; van Ingen, J.; Krenke, R.; Jagielski, T. Clinical, radiological and molecular features of Mycobacterium kansasii pulmonary disease. Respir. Med. 2018, 139, 91–100. [Google Scholar] [CrossRef]
- Hoefsloot, W.; Boeree, M.J.; van Ingen, J.; Bendien, S.; Magis, C.; de Lange, W.; Dekhuijzen, P.N.R.; van Soolingen, D. The rising incidence and clinical relevance of Mycobacterium malmoense: A review of the literature. Int. J. Tuberc. Lung Dis. 2008, 12, 987–993. [Google Scholar]
- Haverkamp, M.H.; Arend, S.M.; Lindeboom, J.A.; Hartwig, N.G.; van Dissel, J.T. Nontuberculous mycobacterial infection in children: A 2-year prospective surveillance study in the Netherlands. Clin. Infect. Dis. 2004, 39, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Reuss, M.A.; Wiese-Posselt, M.; Weißmann, N.; Siedler, A.; Zuschneid, I.; An der Heiden, M.; Claus, H.; von Kries, R.; Haas, W.H. Incidence rate of nontuberculous mycobacterial disease in immunocompetent children: A prospective nationwide surveillance study in Germany. Pediatr. Infect. Dis. J. 2009, 28, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, E. Mycobacterial lymphadenitis in children: A prospective study of 105 nontuberculous cases with long-term follow-up. Clin. Infect. Dis. 1995, 20, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Piau, C.; Lanotte, P.; Carricajo, A.; Guillouzouic, A.; Peuchant, O.; Cady, A.; Dupin, C.; Fangous, M.-S.; Martin, C.; et al. Emergence of nontuberculous mycobacterial lymphadenitis in children after the discontinuation of mandatory Bacillus Calmette and Guérin immunization in France. Pediatr. Infect. Dis. J. 2018, 37, e257–e260. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, T.S.; Ravn, P.; Svensson, E.; Lillebaek, T. Nontuberculous mycobacteria in Denmark, incidence and clinical importance during the last quarter-century. Sci. Rep. 2017, 7, 6696. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Kruijshaar, M.E.; Ormerod, L.P.; Drobniewski, F.; Abubakar, I. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC Public Health 2010, 10, 612. [Google Scholar] [CrossRef]
- Dailloux, M.; Abalain, M.L.; Laurain, C.; Lebrun, L.; Loos-Ayav, C.; Lozniewski, A.; Maugein, J. Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. Eur. Resp. J. 2006, 28, 1211–1215. [Google Scholar] [CrossRef]
- Marras, T.K.; Daley, C.L. Epidemiology of human pulmonary infections with nontuberculous mycobacteria. Clin. Chest Med. 2002, 23, 553–568. [Google Scholar] [CrossRef]
- Chan, E.D.; Iseman, M.D. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend. Med. 2010, 7, 5–18. [Google Scholar] [CrossRef]
- Halstrom, S.; Price, P.; Thomson, R. Review: Environmental mycobacteria as a cause of human infection. Int. J. Mycobacteriol. 2015, 4, 81–91. [Google Scholar] [CrossRef]
- Thomson, R.; Tolson, C.; Huygens, F.; Hargreaves, M. Strain variation amongst clinical and potable water isolates of M. kansasii using automated repetitive unit PCR. Int. J. Med. Microbiol. 2014, 304, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Whitaker, R.; Lechevallier, M.W.; Wen-Tso Liu, W.-L. Drinking water microbiome assembly induced by water stagnation. ISME J. 2018, 12, 1520–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bédard, E.; Laferrière, C.; Déziel, E.; Prévost, M.; Li, M. Impact of stagnation and sampling volume on water microbial quality monitoring in large buildings. PLoS ONE 2018, 13, e0199429. [Google Scholar] [CrossRef] [PubMed]
- Mirsaedi, M.; Farshidpour, M.; Ebrahimi, G.; Aliberti, S.; Falkinham, J.O., III. Management of nontuberculous mycobacterial infection in the elderly. Eur. J. Intern. Med. 2014, 25, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loret, J.F.; Dumoutier, N. Non-tuberculous mycobacteria in drinking water systems: A review of prevalence data and control means. Int. J. Hyg. Environ. Health 2019, 222, 628–634. [Google Scholar] [CrossRef]
- Gebert, M.J.; Delgado-Baquerizo, M.; Oliverio, A.M.; Webster, T.M.; Nichols, L.M.; Honda, J.R.; Chan, E.D.; Adjemian, J.; Dunn, R.R.; Fierer, N. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. MBio 2018, 9, e01614–e01618. [Google Scholar] [CrossRef]
- Kaustová, J.; Chmelík, M.; Ettlová, D.; Hudec, V.; Lazarevá, H.; Richtrová, S. Disease due to Mycobacterium kansasii in the Czech Republic: 1984–89. Tuber. Lung Dis. 1995, 76, 205–209. [Google Scholar] [CrossRef]
- Corbett, E.L.; Hay, M.; Churchyard, G.J.; Herselman, P.; Clayton, T.; Williams, B.G.; Hayes, R.; Mulder, D.; De Cock, K.M. Mycobacterium kansasii and M. scrofulaceum isolates from HIV-negative South African gold miners: Incidence, clinical significance and radiology. Int. J. Tuberc. Lung Dis. 1999, 3, 501–507. [Google Scholar]
- Ulmann, V.; Kracalikova, A.; Dziedzinska, R. Mycobacteria in water used for personal hygiene in heavy industry and collieries: A potential risk for employees. Int. J. Environ. Public Health 2015, 12, 2870–2877. [Google Scholar] [CrossRef]
- Vaerewijck, M.J.M.; Huys, G.; Palomino, J.C.; Swings, J.; Portaels, F. Mycobacteria in drinking water distribution systems: Ecology and significance for human health. FEMS Microbiol. Rev. 2005, 29, 911–934. [Google Scholar] [CrossRef]
- Bentham, R.; Whiley, H. Quantitative microbial risk assessment and opportunist waterborne infections—Are there too many gaps to fill? Int. J. Environ. Public Health 2018, 15, 1150. [Google Scholar] [CrossRef] [PubMed]
- Danelishvili, L.; Stang, B.; Bermudez, L.E. Identification of Mycobacterium avium genes expressed during in vivo infection and the role of the Oligopeptide transporter OppA in virulence. Microb. Pathog. 2014, 76, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dvorska, L.; Bartos, M.; Ostadal, O.; Kaustova, J.; Matlova, J.; Pavlik, I. IS1311 and IS1245 restriction fragment length polymorphism analyses, serotypes, and drug susceptibilities of Mycobacterium avium complex isolates obtained from a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 2002, 40, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chatterji, D. Stress responses in mycobacteria. Life 2005, 57, 149–159. [Google Scholar] [CrossRef]
- Yano, H.; Iwamoto, T.; Nishiuchi, Y.; Nakajima, C.; Starkova, D.A.; Mokrousov, I.; Narvskaya, O.; Yoshida, S.; Arikawa, K.; Nakanishi, N.; et al. Population structure and local adaptation of MAC lung disease agent Mycobacterium avium subsp. hominissuis. Genome Biol. Evol. 2017, 9, 2403–2417. [Google Scholar] [CrossRef]
- Kaustová, J.; Charvát, B.; Mudra, R.; Holendová, E. Ostrava—A new endemic focus of Mycobacteria xenopi in the Czech Republic. Cent. Eur. J. Public Health 1993, 1, 35–37. [Google Scholar]
- Marmot, M. Social justice, epidemiology and health inequalities. Eur. J. Epidemiol. 2017, 32, 537–546. [Google Scholar] [CrossRef]
- Marmot, M.; Bell, R. Fair society, healthy lives. Public Health 2012, 126, S4–S10. [Google Scholar] [CrossRef]
- Ouředníček, M.; Temelová, J.; Pospíšilová, L. (Eds.) Atlas of Socio-Spatial Differentiation of the Czech Republic, 1st ed.; Carolinum: Prague, Czech Republic, 2011; p. 137, (In Czech). ISBN 978-8-02-461889-0. [Google Scholar]
- Chou, M.P.; Clements, A.C.A.; Thomson, R. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect. Dis. 2014, 14, 279. [Google Scholar] [CrossRef]
- Bloch, K.C.; Zwerling, L.; Pletcher, M.J.; Hahn, J.A.; Gerberding, J.L.; Osroff, S.M.; Vugia, D.J.; Reingold, A.L. Incidence and clinical implication of isolation of Mycobacterium kansasii: Results of a 5-year, population-based study. Ann. Intern. Med. 1998, 129, 698–704. [Google Scholar] [CrossRef]
- Alene, K.A.; Viney, K.; McBryde, E.S.; Clements, A.C.A. Spatial patterns of multidrug resistant tuberculosis and relationships to socioeconomic, demographic and household factors in northwest Ethiopia. PLoS ONE 2017, 12, e0171800. [Google Scholar] [CrossRef] [PubMed]
- Munch, Z.; Van Lill, S.; Booysen, C.; Zietsman, H.; Enarson, D.; Beyers, N. Tuberculosis transmission patterns in a high- incidence area: A spatial analysis. Int. J. Tuberc. Lung Dis. 2003, 7, 271–277. [Google Scholar] [PubMed]
- Wei, W.; Yuan-Yuan, J.; Ci, Y.; Ahan, A.; Ming-Qin, C. Local spatial variations analysis of smear-positive tuberculosis in Xinjiang using Geographically Weighted Regression model. BMC Public Health 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. USA 2004, 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure of polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar]
- Li, Y.; Juhasz, A.L.; Ma, L.Q.; Cui, X. Inhalation bioaccessibility of PAHs in PM2.5: Implications for risk assessment and toxicity prediction. Sci. Total Environ. 2019, 650, 56–64. [Google Scholar] [CrossRef]
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnquist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002, 110, 451–488. [Google Scholar]
- Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int. 2011, 37, 383–392. [Google Scholar] [CrossRef]
- Shrivastava, M.; Lou, S.; Zelenyuk, A.; Easter, R.C.; Corley, R.A.; Thrall, B.D.; Rasch, P.J.; Fast, J.D.; Simonich, S.L.M.; Shen, H.; et al. Global long-range transport and lung cancer risk from polycyclic aromatic hydrocarbons shielded by coating of organic aerosol. Proc. Natl. Acad. Sci. USA 2017, 114, 1246–1251. [Google Scholar] [CrossRef]
- ECDC/WHO European Centre for Disease Prevention and Control; Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2018. 2016 Data; ECDC: Stockholm, Sweden; WHO: Geneva, Switzerland, 2018; p. 195. [Google Scholar]
- ÚZIS CR (Institute of Health Information and Statistics of the Czech Republic). Basic Overview of Tuberculosis Epidemiology in the Czech Republic in 2016; ÚZIS CR: Prague, Czech Republic, 2017; p. 13. [Google Scholar]

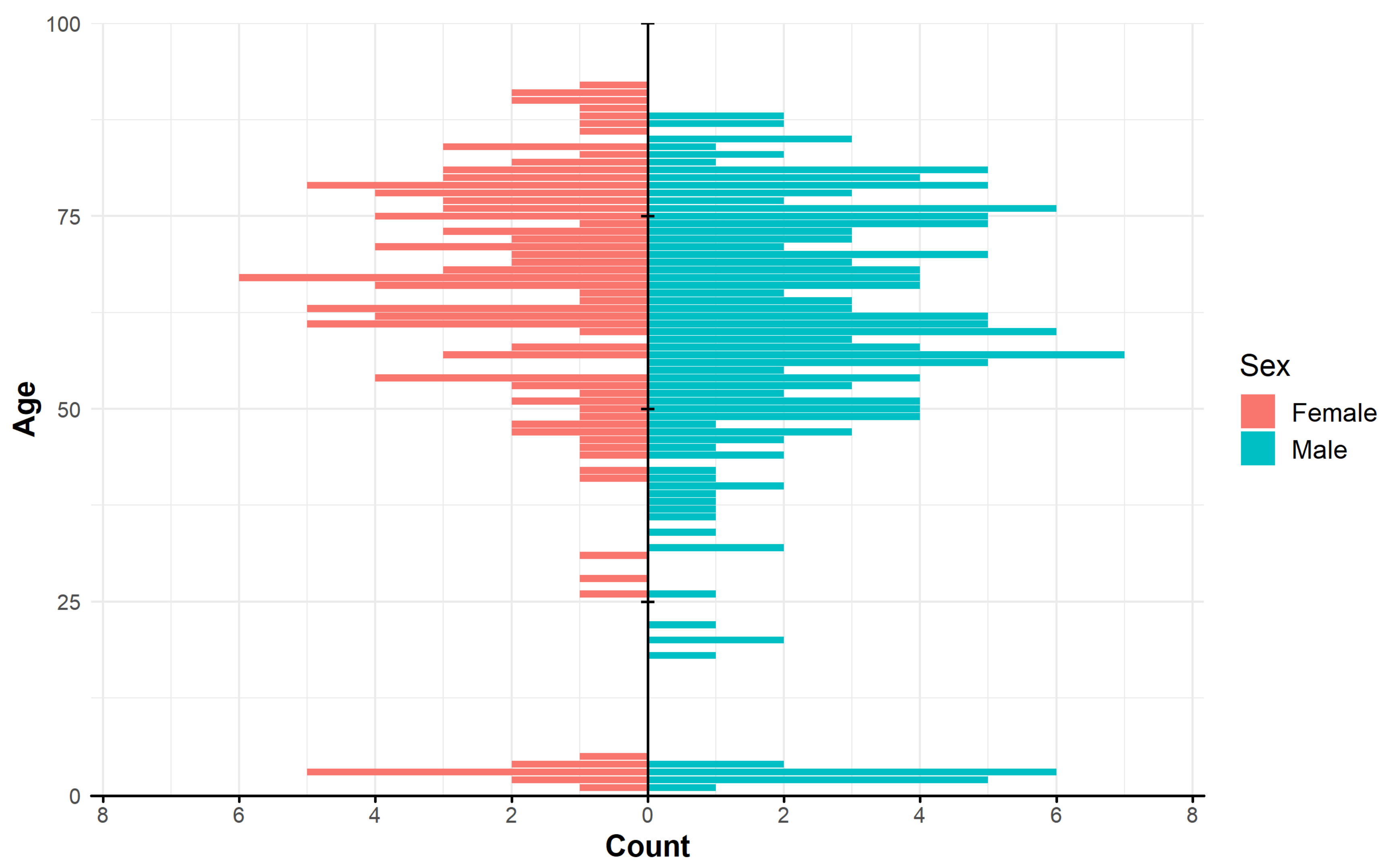
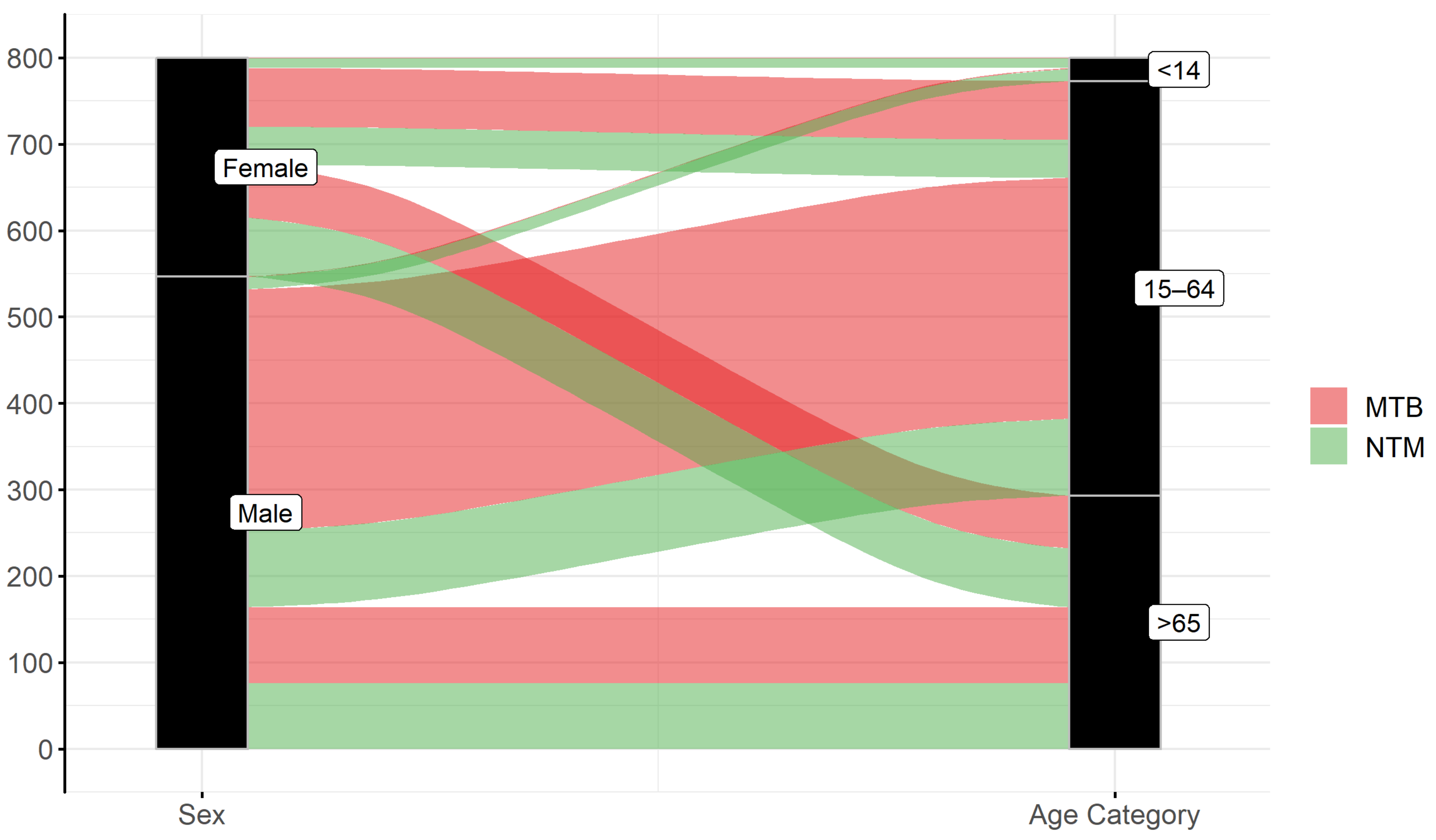
| No. | NTM Species | Total Individual Patient Isolates n (%) | Number of Isolates from Patients that Met ATS Criteria † n (%) |
|---|---|---|---|
| 1. | MAIC * | 386 (17.74) | 143 (47.19) |
| 2. | Mycobacterium kansasii | 134 (6.16) | 72 (23.76) |
| 3. | M. xenopi | 792 (36.40) | 55 (18.15) |
| 4. | M. chelonae ** | 41 (1.88) | 7 (2.31) |
| 5. | M. malmoense | 10 (0.46) | 6 (1.98) |
| 6. | M. fortuitum ** | 210 (9.65) | 6 (1.98) |
| 7. | M. abscessus ** | 12 (0.55) | 4 (1.32) |
| 8. | M. mucogenicum ** | 45 (2.07) | 3 (0.99) |
| 9. | M. marinum | 2 (0.09) | 2 (0.66) |
| 10. | M. chimaera | 3 (0.14) | 2 (0.66) |
| 11. | M. vulneris | 1 (0.05) | 1 (0.33) |
| M. sp. | 66 (3.03) | 2 (0.66) | |
| Subtotal | 1702 (78.22) | 303 (100) | |
| M. gordonae | 341 (15.67) | 0 | |
| M. smegmatis ** | 55 (2.53) | 0 | |
| M. celatum | 22 (1.01) | 0 | |
| M. goodie ** | 12 (0.55) | 0 | |
| M. lentiflavum | 10 (0.46) | 0 | |
| M. peregrinum ** | 8 (0.37) | 0 | |
| M. neoaurum ** | 5 (0.23) | 0 | |
| M. arupense | 3 (0.14) | 0 | |
| M. scrofulaceum | 3 (0.14) | 0 | |
| Others # | 15 (0.69) | 0 | |
| Subtotal | 474 (21.78) | 0 (0) | |
| Total number | 2176 (100) | 303 (100) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrá, H.; Ulmann, V.; Caha, J.; Hübelová, D.; Konečný, O.; Svobodová, J.; Weston, R.T.; Pavlík, I. Socio-Economic and Environmental Factors Related to Spatial Differences in Human Non-Tuberculous Mycobacterial Diseases in the Czech Republic. Int. J. Environ. Res. Public Health 2019, 16, 3969. https://doi.org/10.3390/ijerph16203969
Modrá H, Ulmann V, Caha J, Hübelová D, Konečný O, Svobodová J, Weston RT, Pavlík I. Socio-Economic and Environmental Factors Related to Spatial Differences in Human Non-Tuberculous Mycobacterial Diseases in the Czech Republic. International Journal of Environmental Research and Public Health. 2019; 16(20):3969. https://doi.org/10.3390/ijerph16203969
Chicago/Turabian StyleModrá, Helena, Vít Ulmann, Jan Caha, Dana Hübelová, Ondřej Konečný, Jana Svobodová, Ross Tim Weston, and Ivo Pavlík. 2019. "Socio-Economic and Environmental Factors Related to Spatial Differences in Human Non-Tuberculous Mycobacterial Diseases in the Czech Republic" International Journal of Environmental Research and Public Health 16, no. 20: 3969. https://doi.org/10.3390/ijerph16203969





