Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Saliva Collection
2.3. Analysis of Saliva
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pink, R.; Simek, J.; Vondrakova, J. Saliva as a diagnostic medium. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2009, 153, 103–110. [Google Scholar] [CrossRef]
- LyngePedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 68, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, B.; Persichilli, S.; De Sole, P.; Mordente, A.; Giardia, B. Effect of smoking one cigarette on antioxidant metabolites in the saliva of healthy smoker. Arch. Oral Biol. 1999, 44, 485–488. [Google Scholar] [CrossRef]
- Battino, M.; Ferreiro, M.S.; Gallardo, I.; Newman, H.N.; Bullon, P. The antioxidant capacity of saliva. J. Clin. Periodontol. 2002, 29, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.M.; Klein, I.; Zarzhersky, N.; Drigues, N.; Reznick, A.Z. Characterization of the differentiated antioxidant profile of human saliva. Free Radic. Biol. Med. 2002, 32, 268–277. [Google Scholar] [CrossRef]
- Mandel, I.D. The role of saliva in maintaining oral homeostasis. J. Am. Dent. Assoc. 1989, 119, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Dyba, J.; Lenkowski, M.; Surdacka, A. Evaluating the diagnostic potential of saliva in respect of periodontal disease as well as changes occurring within the endothelium. Dent. Forum 2017, 1, 21–25. [Google Scholar]
- Barton, J.R.; Riad, M.A.; Gaze, M.N.; Maran, A.G.; Ferguson, A. Mucosal immunodeficiency in smokers, and in patients with epithelial head and neck tumours. Gut 1990, 31, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M.; Attin, T. Enzymes in the acquired enamel pellicle. Eur. J. Oral Sci. 2005, 113, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, Y.H.; Samaranayake, L.P.; Wu, P.C.; So, M. The antifungal effect of lactoferrinand lysozyme on Candida krusei and Candida albicans. APMIS Oral Biol. 1997, 105, 875–883. [Google Scholar]
- Yeh, C.K.; Dodds, M.W.; Zuo, P.; Johnson, D.A. A population-based study of salivary lysozyme concentrations and candidal counts. Arch. Oral Biol. 2018, 42, 25–31. [Google Scholar] [CrossRef]
- Tsang, C.; Samaranayake, L. Salivary lysozyme and related parameters of a predominantly Chinese, HIV-infected cohort in Hong Kong. Oral Dis. 1999, 5, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.L.; Heremans, J.F.; Schonne, E. Major Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 1969, 130, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Reitamo, S.; Konttinen, Y.T.; Segerberg-Konttinen, M. Distribution of lactoferrin in human salivary glands. Histochemistry 1980, 66, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.; Drosos, Z.; Tiemann, M.; Jepsen, S.; Schröder, J.M. Immunolocalization of lactoferrin in healthy and inflamed gingival tissues. J. Periodontol. 2006, 77, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Samaranayake, L.P.; Tenovuo, J.; Pang, K.M.; Hamada, T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 1993, 38, 1057–1063. [Google Scholar] [CrossRef]
- Wu, T.; Samaranayake, L.P.; Leung, W.K.; Sullivan, P.A. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J. Med. Microbiol. 1999, 48, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Välimaa, H.; Tenovuo, J.; Waris, M.; Hukkanen, V. Human lactoferrin but not lysozyme neutralizes HSV-1 and inhibits HSV-1 replication and cell-to-cell spread. Virol. J. 2009, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.; Kochańska, B.; Limon, J.; Ochocińska, J. The physico-chemical properties of saliva in Turner’s syndrome. Dent. Forum 2011, 39, 19–23. [Google Scholar]
- Weiner, D.; Levy, Y.; Khankin, E.V.; Reznick, A.Z. Inhibition of salivary amylase activity by cigarette smoke aldehydes. J. Physiol. Pharmacol. 2008, 59, 727–737. [Google Scholar] [PubMed]
- Salaspuro, V.; Salaspuro, M. Synergistic effect of alkohol drinking and smoking on in vitro acetaldehyde concentration in saliva. Int. J. Cancer 2004, 111, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Gawron, M.; Balwicki LSobczak, A.; Matynia, M.; Goniewicz, M.L. Exclusive versus dual use of tobacco and electronic cigarettes among adolescents in Poland, 2010–2016. Addict. Behav. 2019, 90, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.; Chomyszyn-Gajewska, M.; Pietruska, M.; Maj, A.; Szkarlat, B.; Cabała, A. Analysis of electronic cigarette use among Polish dental students. Dent. Med. Probl. 2017, 54, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.L.; Pokhrel, P. Weight Concerns and Use of Cigarettes and E-Cigarettes among Young Adults. Int. J. Environ. Res. Public Health 2018, 15, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinouani, S.; Pereira, E.; Tzourio, C. Electronic Cigarette Use in Students and Its Relation with Tobacco-Smoking: A Cross-Sectional Analysis of the i-Share Study. Int. J. Environ. Res. Public Health 2017, 14, 1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maj, A.; Kusiak, A.; Wojtaszek-Slominska, A.; Kalinowska, J.; Zdrojewski, T.; Suligowska, K. Electronic cigarettes use among 10–11-years old: Preliminary report. Dent. Forum 2018, 46, 31–34. [Google Scholar] [CrossRef]
- Bertholon, J.F.; Becquemin, M.H.; Annesi-Maesano, I.; Dautzenberg, B. Electronic Cigarettes: A Short Review. Respiration 2013, 86, 433–438. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Brose, L.S.; Calder, R.; Hitchman, S.C.; Hajek, P.; McRobbie, H. E-Cigarettes: An Evidence Update; Public Health England Wellington House: London, UK, 2015. [Google Scholar]
- Brown, C.J.; Cheng, J.M. Electronic cigarettes: Product characterisation and design considerations. Tob. Control 2014, 23, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.S.; Hall, M.G.; Parada, H.; Peebles, K.; Brodar, K.E.; Brewer, N.T. Symptoms during Adolescents’ First Use of Cigarettes and E-Cigarettes: A Pilot Study. Int. J. Environ. Res. Public Health 2017, 14, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisinger, C.; Dossing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014, 69, 248–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, V.; Rahimy, M.; Korrapati, A. Electronic cigarettes induce DNA stand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semlali, A.; Chakir, J.; Goulet, J.P.; Chmielewski, W.; Rouabhia, M. Whole cigarette smoke promotes human gingival epithelial cell apoptosis and inhibits cell repair processes. J. Periodontal Res. 2011, 46, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, R.; Kist, R.; Bauld, L. E-cigarette vapour is not inert and exposure can lead to cell damage. Evid.-Based Dent. 2016, 17, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.; Lischinsky, S.; Diamond, E.; Drigues, N.; Klein, I.; Reznick, A.Z. Effect of cigarette smoke on salivary proteins and enzyme activities. Arch. Biochem. Biophys. 2000, 379, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rudney, J.D. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Crit. Rev. Oral Biol. Med. 1995, 6, 343–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennet, K.R.; Reade, P.C. Salivary immunoglobulin a levels in normal subjects, tobacco smokers and patients with minor aphthous ulceration. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 461–465. [Google Scholar] [CrossRef]
- Kibayashi, M.; Tanaka, M.; Nishida, N.; Kuboniwa, M.; Kataoka, K.; Nagata, H.; Nakayama, K.; Morimoto, K.; Shizukuishi, S. Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J. Periodontol. 2007, 78, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yamamoto, Y.; Tanaka, M.; Kataoka, K.; Kuboniwa, M.; Nakayama, K.; Morimoto, K.; Shizukuishi, S. Association between involuntary smoking and salivary markers related to periodontitis: A 2-year longitudinal study. J. Periodontol. 2008, 79, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ingle, N.A.; Kaur, N.; Yadav, P.; Ingle, E. Effect of long-term smoking on salivary flow rate and salivary pH. J. Indian Assoc. Public Health Dent. 2015, 13, 11–13. [Google Scholar] [CrossRef]
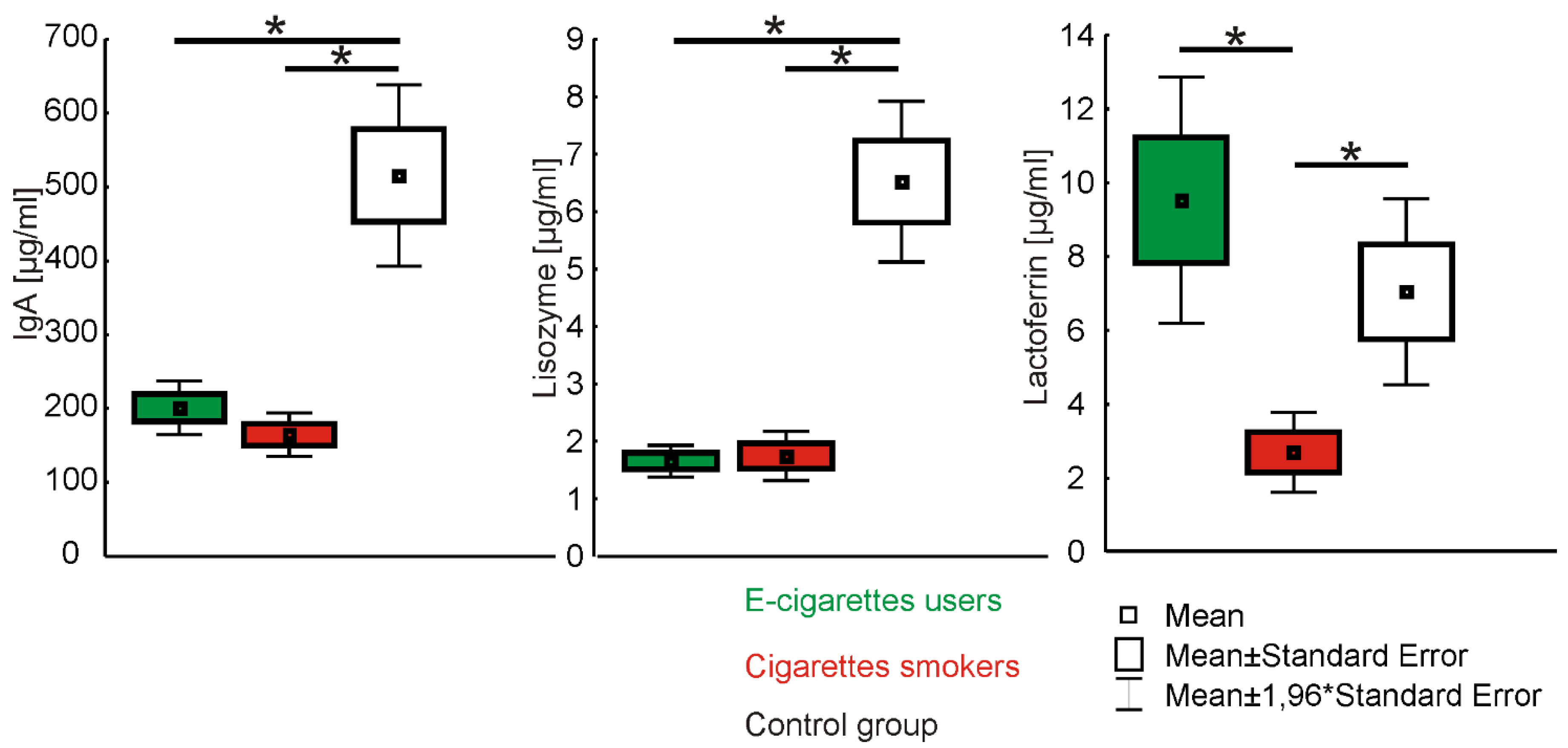
| Groups | IgA [µg/mL] | Lisozyme [µg/mL] | Lactoferrin [µg/mL] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Me | Mean ± SD | Range | Me | Mean ± SD | Range | Me | |
| E-cigarette users (n = 40) | 201.1 ± 118 | 12.0–560.0 | 169.0 a | 1.7 ± 0.9 | 0.2–3.8 | 1.6 c | 9.5 ± 10.6 | 0.1–40.4 | 7.1 e |
| Cigarette smokers (n = 40) | 164.7 ± 95 | 16.0–332.0 | 157.5 a,b | 1.8 ± 1.4 | 0.3–6.1 | 1.4 d | 2.7 ± 3.5 | 0.3–15.7 | 1.4 f |
| Control group (n = 40) | 515.8 ± 430 | 36.0–2182.0 | 399.0 b | 6.5 ± 4.8 | 1.3–22.1 | 4.8 c,d | 7.0 ± 8.8 | 1.1–61.7 | 5.6 e,f |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichońska, D.; Kusiak, A.; Kochańska, B.; Ochocińska, J.; Świetlik, D. Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. Int. J. Environ. Res. Public Health 2019, 16, 4433. https://doi.org/10.3390/ijerph16224433
Cichońska D, Kusiak A, Kochańska B, Ochocińska J, Świetlik D. Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. International Journal of Environmental Research and Public Health. 2019; 16(22):4433. https://doi.org/10.3390/ijerph16224433
Chicago/Turabian StyleCichońska, Dominika, Aida Kusiak, Barbara Kochańska, Jolanta Ochocińska, and Dariusz Świetlik. 2019. "Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva" International Journal of Environmental Research and Public Health 16, no. 22: 4433. https://doi.org/10.3390/ijerph16224433
APA StyleCichońska, D., Kusiak, A., Kochańska, B., Ochocińska, J., & Świetlik, D. (2019). Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. International Journal of Environmental Research and Public Health, 16(22), 4433. https://doi.org/10.3390/ijerph16224433





