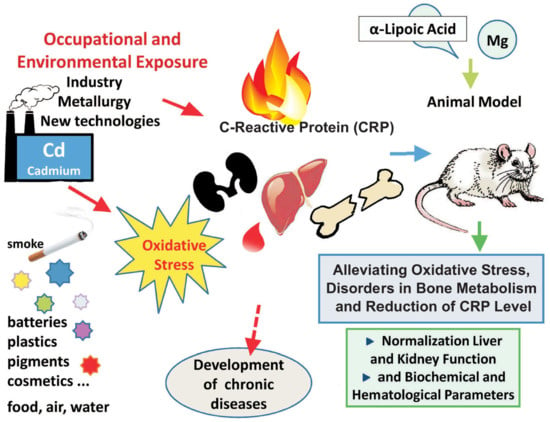
Alleviating Effect of α-Lipoic Acid and Magnesium on Cadmium-Induced Inflammatory Processes, Oxidative Stress and Bone Metabolism Disorders in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Experimental Design
2.3. Biochemical and Hematological Analysis
2.3.1. Determination of Hematological Parameters
2.3.2. Serum Biochemical Analyses
Serum Lipids and Markers of Liver and Kidney Dysfunction
Measurement of C-Reactive Protein, Bone Turnover Markers, and Vitamin D3
2.3.3. Measurement of Oxidative Stress and Antioxidant Defense in Serum, Kidney, and Liver
Thiobarbituric Acid Reactive Substances (TBARs)
Total Antioxidant Capacity
Reduced Glutathione
Antioxidant Enzyme Activity in Liver and Kidney
- (a)
- Glutathione peroxidase (GPx) assay
- (b)
- Superoxide dismutase (SOD) assay
- (c)
- Catalase (CAT) assay
2.3.4. Determination of Cadmium Content in the Femurs and Blood
2.3.5. Determination of Serum Concentration of Calcium (Ca), Magnesium (Mg), Iron (Fe), and Zinc (Zn)
2.4. Statistical Analysis
3. Results
3.1. Body Weight Gain; Consumption of Liquid Diet; and Intake of Cd, Mg, and α-LA
3.2. Hematological Parameters
3.3. Biochemical Markers of Liver and Kidney Dysfunction
3.4. Effects on Serum Lipid Profile
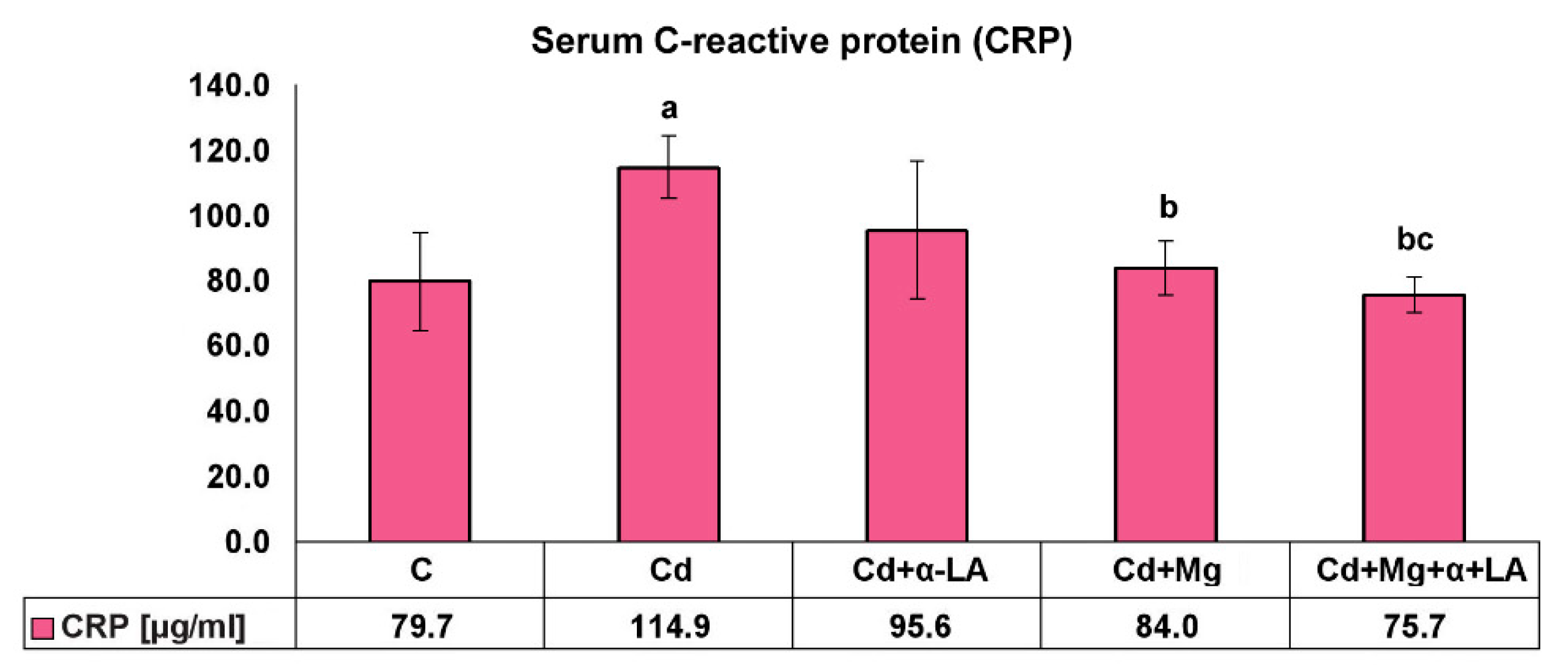
3.5. Serum C-Reactive Protein Level
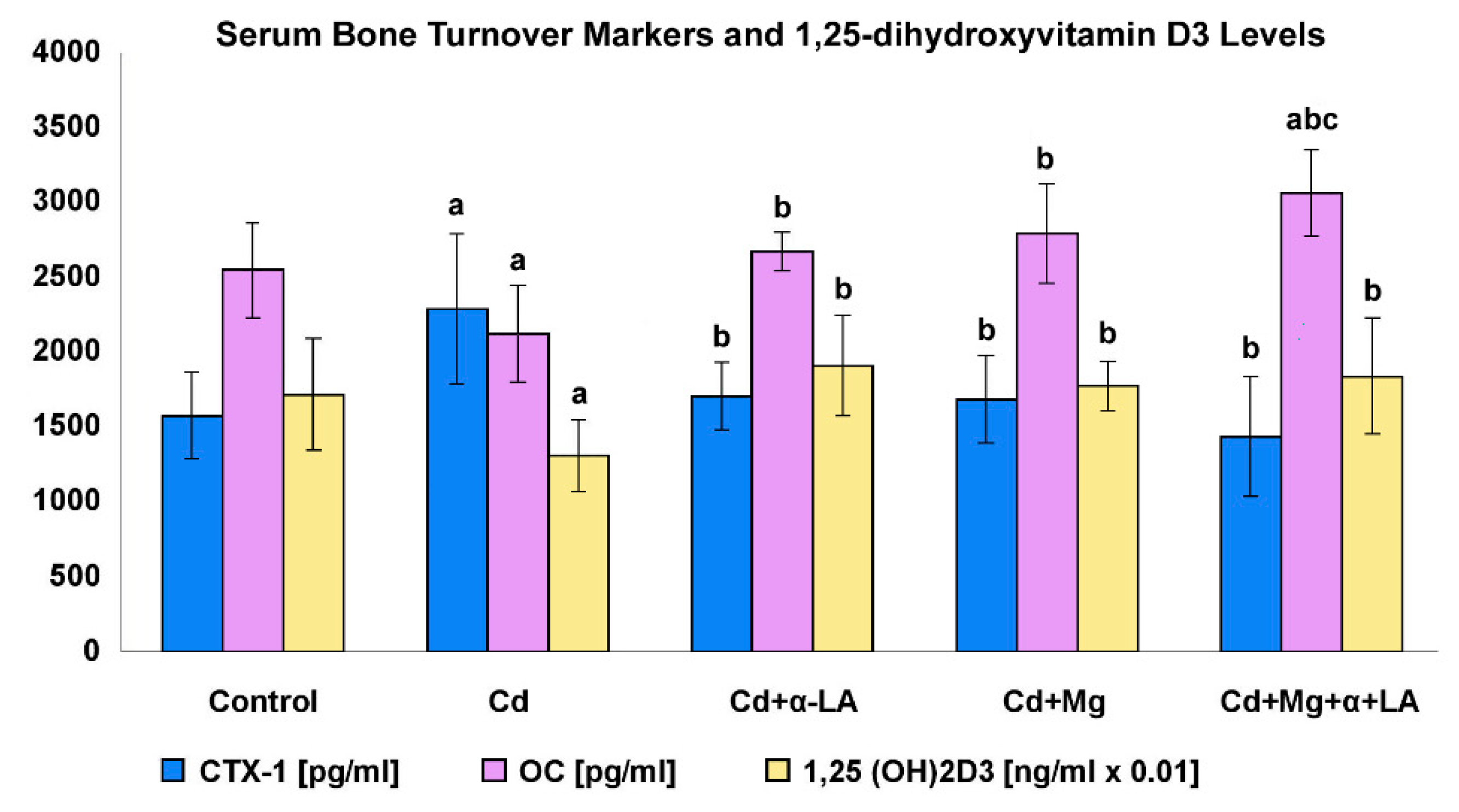
3.6. Serum Bone Turnover Markers and 1,25-dihydroxyvitamin D3 Levels
3.7. Markers of Oxidative Stress and Antioxidant Potential
3.7.1. Thiobarbituric Acid Reactive Substances (TBARs) Concentration and Antioxidant Potential in Serum
- (a)
- The level of the lipid peroxidation marker (TBARs) was significantly higher in the serum of rats exposed to cadmium alone (Cd) compared with the C group (41.1 µM/L vs. 19.4 µM/L) and with the Cd + α-LA and Cd + α-LA + Mg groups (by 22% and 45%, respectively; p < 0.05).
- (b)
- We also noticed that the antioxidant potential of serum from cadmium rats (Cd group) dropped and equaled approximately 80% of that in control rats. However, this decline was reversed as a result of α-lipoic acid supplementation. The serum collected from the Cd + α-LA group showed a significant increase in antioxidant activity, by about 45%, when compared with the Cd group and group supplemented with magnesium alone (Cd + Mg), p < 0.05.
3.7.2. TBARs, Reduced Glutathione, and Antioxidant Enzymes in Rats’ Liver and Kidney
- (a)
- Thiobarbituric acid reactive substances (TBARs)
- (b)
- Reduced glutathione (GSH) and antioxidant enzymes
- (c)
- GSH
- (d)
- SOD
- (e)
- CAT
- (f)
- GPx
3.8. Cadmium Content in Bone and in the Blood and Concentration of Elements (Cd, Mg, Zn, P, Fe, and Ca) in the Serum of Rats
3.8.1. Cadmium Concentration in Blood and Bone
3.8.2. Concentration of Elements (Mg, Zn, P, Fe, and Ca) in the Serum of Rats
3.9. Correlations between Cd in Blood and Bone, and Other Measured Parameters
4. Discussion
4.1. Effects of Cadmium Poisoning
4.2. Attenuating Effects of Magnesium and α-Lipoic Acid against Cd toxicity
4.2.1. Influence on Cadmium Body Burden, Body Mass Gain, and Hematological Parameters
4.2.2. Influence on Level of Oxidative Stress, Inflammatory Markers, and Antioxidant Potential
4.2.3. Influence on Bone Metabolism
4.2.4. Influence on Markers of Liver and Kidney Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Honey, S.; Neetu, R.; Blessy, B.M. The characteristics, toxicity and effects of cadmium. Int. J. Nanosci. 2015, 3, 1–9. [Google Scholar]
- Mrowiec, B. Nanomaterials—New risk of the environment. Ecol. Eng. 2017, 18, 105–110. [Google Scholar]
- Tahir, M.H.; Nauman, M.; Bangash, N. Chapter 7—Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants from Physiology to Remediation, 1st ed.; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Elsevier: London, UK, 2019; pp. 163–183. [Google Scholar]
- Nair, A.R.; Degheselle, O.; Smeets, K.; Van Kerkhove, E.; Cuypers, A. 2013 Cadmium-induced pathologies: Where is the oxidative balance lost (or not)? Int. J. Mol. Sci. 2013, 14, 6116–6143. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2013, 24, 378–399. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Imafidon, C.E.; Akomolafe, R.O.; Abubakar, S.A.; Ogundipe, O.J.; Olukiran, O.S.; Ayowole, O.A. Amelioration of Cadmium-Induced Nephropathy using Polyphenol-rich Extract of Vernonia amygdalina (Del.) Leaves in Rat Model. Open Access Maced. J. Med. Sci. 2015, 3, 567–577. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, L.; Bao, J.; Sun, H.; Bi, Y.; Liu, H.; Li, J. Selenium supplementation can protect from enhanced risk of keel bone damage in laying hens exposed to cadmium. RSC Adv. 2017, 7, 7170–7178. [Google Scholar] [CrossRef]
- Elkhadragy, M.F.; Kassab, R.B.; Metwally, D.M.; Almeer, R.; Abdel-Gaber, R.; Al-Olayan, E.M.; Essawy, E.A.; Amin, H.K.; Abdel Moneim, A.E. Protective effects of fragaria ananassa methanolic extract in a rat model of cadmium chloride-induced neurotoxicity. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Matovic, V.; Bulat, P.Z.; Djukić-Ćosić, D.; Soldatović, D. Antagonism between cadmium and magnesium: A possible role of magnesium in therapy of cadmium intoxication. Magnes. Res. 2010, 23, 19–26. [Google Scholar] [CrossRef]
- Rude, R.K. Chapter 9—Magnesium. In Modern Nutrition in Health and Disease, 11th ed.; Ross, C., Caballero, B., Cousins, R.J., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; pp. 59–175. [Google Scholar]
- Lyn, P. Mercury Toxicity and Antioxidants: Part I: Role of Glutathione and alpha-Lipoic Acid in the Treatment of Mercury Toxicity. Altern. Med. Rev. 2002, 7, 456–471. [Google Scholar]
- Arivazhagan, P.; Shila, S.; Narchonai, E.; Panneerselvam, C. α-Lipoic Acid Enhances Reduced Glutathione, Ascorbic Acid, and α-Tocopherol in Aged Rats. J. Anti Aging Med. 2004, 5, 265–269. [Google Scholar] [CrossRef]
- Markiewicz-Górka, I.; Kuropka, P.; Januszewska, L.; Jaremków, A.; Pawłowski, P.; Pawlas, N.; Prokopowicz, A.; Gonzalez, E.; Nikodem, A.; Pawlas, K. Influence of physical training on markers of bone turnover, mechanical properties, morphological alterations, density and mineral contents in the femur of rats exposed to cadmium and/or alcohol. TIH 2019, 35, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Galażyn-Sidorczuk, M.; Dźwilewska, I. Ethanol consumption modifies the body turnover of cadmium: A study in a rat model of human exposure. J. Appl. Toxicol. 2013, 33, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Youness, E.R.; Mohammed, N.A.; Morsy, F.A. Cadmium impact and osteoporosis: Mechanism of action. Toxicol. Mech. Methods 2012, 22, 560–567. [Google Scholar] [CrossRef]
- Drobnik, M.; Latour, T. Fizjologiczne znaczenie składników mineralnych dostarczanych z wodą naturalną na podstawie badań farmakodynamicznych niektórych wód leczniczych. (Physiological importance of mineral components in natural mineral water according pharmacodynamic investigation of some therapeutic mineral waters.). J. Elem. 2006, 11, 259–270. [Google Scholar]
- Guerrera, M.P.; Volpe, S.L.; Mao, J.J. Therapeutic uses of magnesium. Am. Fam. Physician 2009, 80, 157–162. [Google Scholar]
- Stoeppler, M.; Brandt, K. Contributions to automated trace analysis. Part, V. Determination of cadmium in whole blood and urine by electrotermal atomic absorption spectrophotometry. Fresenius Z. Anal. Chem. 1980, 300, 372–380. [Google Scholar] [CrossRef]
- Gałecka, E.; Jacewicz, R.; Mrowicka, M.; Florkowski, A.; Gałecki, P. Antioxidative enzymes--structure, properties, functions. Pol. Merkur. Lek. 2008, 25, 266–268. [Google Scholar]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Zhao, X. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014, 65, 260–268. [Google Scholar] [CrossRef]
- Mikolić, A.; Schönwald, N.; Piasek, M. Cadmium, iron and zinc interaction and hematological parameters in rat dams and their offspring. J. Trace Elem. Med. Biol. 2016, 38, 108–116. [Google Scholar] [CrossRef]
- Donmez, H.H.; Donmez, N.; Kısadere, I.; Undag, I. Protective effect of quercetin on some hematological parameters in rats exposed to cadmium. Biotech. Histochem. 2019, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Malara, P.; Fischer, A.; Malara, B. Selected toxic and essential heavy metals in impacted teeth and the surrounding mandibular bones of people exposed to heavy metals in the environment. J. Occup. Med. Toxicol. 2016, 11, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Omelka, R.; Jančová, A.; Stawarz, R.; Formicki, G. Concentrations of Selected Heavy Metals in Bones and Femoral Bone Structure of Bank (Myodes glareolus) and Common (Microtus arvalis) Voles from Different Polluted Biotopes in Slovakia. Arch. Environ. Contam. Toxicol. 2011, 60, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Caciari, T.; Sancini, A.; Fioravanti, M.; Casale, T.; Montuori, L.; Fiaschetti, M.; Schifano, M.P.; Andreozzi, G.; Nardone, N.; Tomei, G.; et al. Cadmium and hypertension in exposed workers: A meta-analysis. Int. J. Occup. Med. Environ. Health 2013, 26, 440–456. [Google Scholar] [CrossRef]
- Orisakwe, O.E. Lead and Cadmium in Public Health in Nigeria: Physicians Neglect and Pitfall in Patient Management. N. Am. J. Med. Sci. 2014, 6, 61–70. [Google Scholar] [CrossRef]
- Alli, L.A. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip. Toxicol. 2015, 8, 146–150. [Google Scholar] [CrossRef]
- Olszowski, T.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Pro-inflammatory properties of cadmium. Acta. Biochim. Pol. 2012, 59, 475–482. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Mumford, S.L.; Sjaarda, L.; Perkins, N.J.; Malik, F.; Wactawski-Wende, J. Blood lead, cadmium and mercury in relation to homocysteine and C-reactive protein in women of reproductive age: A panel study. Environ. Health 2017, 16, 84–92. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Fagerberg, B.; Borné, Y.; Persson, M.; Hedblad, B.; Engström, G. Blood Cadmium Levels and Incident Cardiovascular Events during Follow-up in a Population-Based Cohort of Swedish Adults: The Malmö Diet and Cancer Study. Environ. Health Perspect. 2016, 124, 594–600. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Kedwany, F.S.; Baghdadi, H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2004, 42, 1563–1571. [Google Scholar] [CrossRef]
- Nemmiche, S.; Chabane-Sari, D.; Kadri, M.; Guiraud, P. Cadmium chloride-induced oxidative stress and DNA damage in the human Jurkat T cell line is not linked to intracellular trace elements depletion. Toxicol. In Vitro 2011, 25, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; De Ponte, C.; D’andrea, P.; et al. Cadmium-induced oxidative stress impairs glycemic control in adolescents. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liaw, F.Y.; Chen, W.L.; Kao, T.W.; Chang, Y.W.; Huang, C.F. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci. Rep. 2017, 7, 1723. [Google Scholar] [CrossRef]
- Hartwig, A. Cadmium and cancer. Met. Ions Life Sci. 2013, 11, 491–507. [Google Scholar] [PubMed]
- Akesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mishra, R.; Biswas, S.; Bhadra, R.K.; Mukhopadhyay, P.K. α-Lipoic Acid Mitigates Arsenic-Induced Hematological Abnormalities in Adult Male Rats. Iran. J. Med. Sci. 2017, 42, 242–250. [Google Scholar]
- Nikolić, R.; Krstić, N.; Jovanović, J.; Kocić, G.; Cvetković, T.P.; Stevanović, N.R. Monitoring the toxic effects of Pb, Cd and Cu on hematological parameters of Wistar rats and potential protective role of lipoic acid and glutathione. Toxicol. Ind. Health 2015, 31, 239–246. [Google Scholar]
- Mbarki, S.; Dhibi, S.; Bouzenna, H.; Abdelfettah Elfeki, A.; Hfaiedh, N. Effects of MgCl2 supplementation on blood parameters and kidney injury of rats exposed to CCl4. Open Life Sci. 2016, 11, 250–258. [Google Scholar] [CrossRef]
- De Franceschi, L.; Bachir, D.; Galacteros, F.; Tchernia, G.; Cynober, T.; Alper, S.; Platt, O.; Beuzard, Y.; Brugnara, C. Oral magnesium supplements reduce erythrocyte dehydration in patients with sickle cell disease. J. Clin. Investig. 1997, 100, 1847–1852. [Google Scholar] [CrossRef] [Green Version]
- Cinar, V.; Nizamlioglu, M.; Mogulkoc, R.; Baltaci, A.K. Effects of magnesium supplementation on blood parameters of athletes at rest and after exercise. Biol. Trace Elem. Res. 2007, 115, 205–212. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Guo, Y.; Wang, Z.; Nie, W. Effects of source and level of magnesium on catalase activity and its gene expression in livers of broiler chickens. Arch. Anim. Nutr. 2007, 61, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Xun, P.; He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2014, 68, 510–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Rezaie, P.; Banach, M. Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. 2018, 14, 707–716. [Google Scholar] [CrossRef]
- Saboori, S.; Falahi, E.; Eslampour, E.; Zeinali Khosroshahi, M.; Yousefi Rad, E. Effects of alpha-lipoic acid supplementation on C-reactive protein level: A systematic review and meta-analysis of randomized controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 779–786. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hariri, M. The effect of alpha-lipoic acid on inflammatory mediators: A systematic review and meta-analysis on randomized clinical trials. Eur. J. Pharmacol. 2019, 849, 115–123. [Google Scholar] [CrossRef]
- Nemmiche, S. Oxidative Signaling Response to Cadmium Exposure. Toxicol. Sci. 2017, 156, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Park, T.J. Magnesium metabolism. Electrolyte Blood Press. 2008, 6, 86–95. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, 194–198. [Google Scholar]
- Rodríguez, J.; Mandalunis, P.M. Effect of cadmium on bone tissue in growing animals. Exp. Toxicol. Pathol. 2016, 68, 391–397. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, H.; Katsumata, S.; Kajita, Y.; Miwa, M. Magnesium deficiency regulates vitamin D metabolizing enzymes and type II sodium-phosphate cotransporter mRNA expression in rats. Magnes. Res. 2013, 26, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Moreau, R. Emerging role of alpha-lipoic acid in the prevention and treatment of bone loss. Nutr. Rev. 2015, 73, 116–125. [Google Scholar] [CrossRef]


| Group of Rats | Treatment |
|---|---|
| C (control) | Rats were fed with liquid diet (LD101) (without any additives) 1 |
| Cd (exposed to cadmium) | Rats were fed with liquid diet (LD 101) with the addition of cadmium dosed 30 mg Cd/kg (as CdCl2 × 2.5H20) of feed 2 |
| Cd + α-LA (exposed to cadmium and supplemented with α-lipoic acid) | Rats were fed with liquid diet (LD 101) with the addition of cadmium dosed 30 mg Cd (as CdCl2 × 2.5H20)/kg of feed 2, and were supplemented with α-lipoic acid (100 mg/kg body weight, four times a week) 3 |
| Cd + Mg (exposed to cadmium and supplemented with magnesium) | Rats were fed with liquid diet (LD 101) with the addition of cadmium dosed 30 mg Cd (as CdCl2 × 2.5H20)/kg of feed and magnesium dosed 150 mg Mg (as C3H7MgO6P × H2O)/kg of feed 4 |
| Cd + Mg + α-LA (exposed to cadmium and supplemented with magnesium and α-lipoic acid) | Rats were fed with liquid diet (LD 101) with the addition of cadmium dosed 30 mg Cd (as CdCl2x2.5H20)/kg of feed and magnesium dosed 150 mg Mg (as C3H7MgO6P × H2O)/kg of feed 4, and were supplemented with α-lipoic acid (100 mg/kg body weight, four times a week) 3 |
| Element | Wavelength (nm) | Gap (mm) | Background Correction | Flame Type |
|---|---|---|---|---|
| Ca | 422.7 | 0.5 | off | stoichiometric |
| Mg | 285.2 | 0.5 | on | stoichiometric |
| Zn | 213.9 | 0.2 | on | oxidizing |
| Fe | 248.3 | 0.2 | on | oxidizing |
| Group of Rats | Body Weight Gain (g/rat) 2 | Consumption of Liquid Diet (mL) 3 | Cadmium Intake (mg) 4 | α-Lipoic Acid Intake (mg) 4,5 | Magnesium Intake (mg) 4 |
|---|---|---|---|---|---|
| Control | 226.2 (±31.4) | 214.3 (±10.99) | – | – | – |
| Cd | 137.3 (±24.8) a | 189.4 (±9.03) a | 6.87 (±0.33) | – | – |
| Cd + α-LA | 149.3 (±28.6) a | 210.0 (±7.06) b | 7.63 (±0.26) b | 100 | – |
| Cd + Mg | 151.2 (±30.7) a | 210.0 (±12.95) b | 7.63 (±0.51) b | – | 38.1 (±2.5) |
| Cd + Mg + α-LA | 166.3 (±10.5) a | 208.0 (±7.98) b | 7.58 (±0.3) b | 100 | 37.9 (±1.5) |
| Control | Cd | Cd + α-LA | Cd + Mg | Cd + Mg + α-LA | |
|---|---|---|---|---|---|
| WBC (count × 103/μL) | 4.97 (±1.05) | 9.45 (±1.57) a | 5.28 (±1.07) b | 8.07 (±3.15) ac | 8.18 (±2.39) ac |
| HGB (g/dL) | 15.8 (±1.2) | 10.52 (±2.7) a | 13.3 (±0.8) ab | 11.5 (±1.2) a | 11.6 (±1.1) a |
| RBC (count × 103/μL) | 9.2 (±0.7) | 7.5 (±2.0) | 8.7 (±0.5) | 6.2 (±2.2) ac | 7.1 (±2.3) a |
| HCT (%) | 48.3 (±4.5) | 32.5 (±10.1) a | 38.8 (±4.0) | 27.2 (±10.2) a | 30.4 (±9.5) a |
| MCV (fl) | 52.7 (±1.8) | 42.5 (±3.2) a | 44.4 (±2.2) a | 43.8 (±1.5) | 42.8 (±0.9) a |
| MCH (pg) | 17.2 (±0.50) | 14.1 (±0.27) a | 15.2 (±0.14) a | 20.5 (±5.67) bc | 17.9 (±5.72) |
| MCHC (g/dL) | 32.7 (±0.89) | 33.4 (±3.20) | 34.4 (±1.27) a | 46.9 (±13.66) abc | 41.6 (±1.8) abc |
| RDW (fl) | 18.8 (±1.19) | 25.3 (±1.41) a | 25.5 (±2.12) a | 24.5 (±1.00) a | 25.5 (±0.88) abc |
| PLT (count × 103/μL) | 870.3 (±78.2) | 652.5 (±268.5) a | 582.7 (±207.2) a | 822.6 (±132.3) bc | 888.5 (±134.8) bc |
| Control | Cd | Cd + α-LA | Cd + Mg | Cd + Mg + α-LA | |
|---|---|---|---|---|---|
| AST (U/L) | 73.5 (±5.3) | 356.8 (±357.3) a | 285.0 (±245.6) | 155.3 (±77.6) | 124.8 (±12.9) |
| ALT (U/L) | 28.8 (±3.1) | 137.8 (±85.3) a | 122.5 (±26.5) a | 96.2 (±16.7) a | 116.3 (±23.6) a |
| GGTP (U/L) | 4.0 (±0) | 5.2 (±1.8) a | 4.0 (±0) b | 4.0 (±0) b | 4.0 (±0) b |
| LDH (U/L) | 290.3 (±114.2) | 2404.8 (±1281.9) a | 1431.7 (±1132.8) | 594.7 (±253.7) b | 400.7 (±155.6) bc |
| CRE (mg/dL) | 0.362 (±0.157) | 0.327 (±0.039) | 0.292 (±0.047) | 0.308 (±0.024) | 0.293 (±0.031) |
| Urea (mg/dL) | 34.0 (±11.0) | 25.7 (±3.7) a | 22.5 (±0.8) a | 26.2 (±2.8) a | 29.3 (±2.7) |
| BUN (mg/dL)] | 15.9 (±5.1) | 12.0 (±1.7) a | 10.5 (±0.4) a | 12.2 (±1.3) a | 13.7 (±1.3) c |
| BUN/CRE | 45.3 (±6.6) | 36.7 (±2.8) a | 37.2 (±8.4) a | 39.8 (±4.1) | 47.0 (±5.3) bcd |
| Uric acid (mg/dL) | 2.73 (±0.76) | 3.32 (±2.95) | 2.77 (±1.30) | 3.13 (±0.87) | 2.88 (±0.83) |
| Control | Cd | Cd + α-LA | Cd + Mg | Cd + Mg + α-LA | |
|---|---|---|---|---|---|
| CHOL (mg/dL) | 67.5 (±10.8) | 56.5 (±10.7) a | 48.5 (±8.0) a | 53.2 (±6.0) a | 58.2 (±7.7) |
| HDL (mg/dL) | 21.5 (±3.9) | 20.7 (±3.0) | 18.7 (±3.1) | 18.7 (±3.3) | 15.7 (±3.1) ab |
| TRIG (mg/dL) | 120.2 (±45.6) | 148.5 (±75.1) | 101.5 (±21.2) | 113.2 (±26.4) | 137.3 (±66.5) |
| CHOL/HDL | 3.16 (±0.19) | 2.73 (±0.27) | 2.61 (±0.28) | 2.89 (±0.39) | 3.87 (±1.16) abcd |
| Control | Cd | Cd + α-LA | Cd + Mg | Cd + Mg + α-LA | |
|---|---|---|---|---|---|
| TBARs (ng/g tissue) | |||||
| Liver | 423.3 (±96.6) | 526.7 (±83.8) a | 415.0 (±48.2) b | 425.0 (±85.3) b | 431.7 (±50.9) b |
| Kidney | 273.4 (±57.3) | 442.6 (±289.3) a | 295.9 (±24.8) | 254.4 (±26.3) b | 233.6 (±61.2) bc |
| GSH (mM/g tissue) | |||||
| Liver | 19.0 (±2.3) | 15.1 (±1.6) a | 17.2 (±3.1) | 18.2 (±5.3) | 20.3 (±4.8) b |
| Kidney | 2.61 (±0.43) | 1.82 (±0.17) a | 2.13 (±0.15) ab | 2.13 (±0.10) ab | 2.77 (±0.81) bcd |
| SOD (USOD/mg protein) | |||||
| Liver | 7.50 (±2.46) | 14.01 (±3.06) a | 8.83 (±2.06) b | 14.03 (±3.95) ac | 10.29 (±02.33) bd |
| Kidney | 2.55 (±0.54) | 3.89 (±0.60) a | 3.08 (±0.65) b | 3.21 (±0.57) b | 3.33 (±0.37) a |
| CAT (UCAT/mg protein) | |||||
| Liver | 1524.5 (±260.7) | 2050.4 (±194.3) a | 1521.7 (±164.5) b | 1993.7 (±298.5) ab | 1790.7 (±135.8) ab |
| Kidney | 492.4 (±82.2) | 482.4 (±67.7) | 535.9 (±73.7) | 510.4 (±53.7) | 498.6 (±47.0) |
| GPx (mUGPx/mg protein) | |||||
| Liver | 129.1 (±27.3) | 334.5 (±75.3) a | 218.9 (±37.0) ab | 206.7 (±24.8) ab | 129.1 (±52.0) bcd |
| Kidney | 503.1 (±114.3) | 214.1 (±118.5) a | 370.6 (±130.8) | 394.6 (±148.0) b | 412.9 (±146.1) b |
| Control | Cd | Cd + α-LA | Cd + Mg | Cd + Mg + α-LA | |
|---|---|---|---|---|---|
| Iron (mg/L) | 2.42 (±0.33) | 1.39 (±0.17) a | 1.92 (±0.36) ab | 1.77 (±0.71) a | 1.36 (±0.40) a |
| Zinc (mg/L) | 1.45 (±0.12) | 1.01 (±0.10) a | 1.35 (±0.17) b | 1.00 (±0.06) a | 1.15 (±0.12) a |
| Magnesium (mg/L) | 26.2 (±3.85) | 31.8 (±4.60) a | 30.6 (±5.90) | 32.3 (±1.85) a | 30.9 (±4.55) |
| Phosphorus (mg/dL) | 6.91 (±1.08) | 9.83 (±4.24) a | 8.57 (±1.30) | 8.17 (±0.70) | 7.86 (±0.91) |
| Calcium (mg/L) | 102.6 (±3.68) | 97.5 (±7.23) | 95.9 (±1.73) | 99.2 (±2.22) | 100.2 (±3.72) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markiewicz-Górka, I.; Pawlas, K.; Jaremków, A.; Januszewska, L.; Pawłowski, P.; Pawlas, N. Alleviating Effect of α-Lipoic Acid and Magnesium on Cadmium-Induced Inflammatory Processes, Oxidative Stress and Bone Metabolism Disorders in Wistar Rats. Int. J. Environ. Res. Public Health 2019, 16, 4483. https://doi.org/10.3390/ijerph16224483
Markiewicz-Górka I, Pawlas K, Jaremków A, Januszewska L, Pawłowski P, Pawlas N. Alleviating Effect of α-Lipoic Acid and Magnesium on Cadmium-Induced Inflammatory Processes, Oxidative Stress and Bone Metabolism Disorders in Wistar Rats. International Journal of Environmental Research and Public Health. 2019; 16(22):4483. https://doi.org/10.3390/ijerph16224483
Chicago/Turabian StyleMarkiewicz-Górka, Iwona, Krystyna Pawlas, Aleksandra Jaremków, Lidia Januszewska, Paweł Pawłowski, and Natalia Pawlas. 2019. "Alleviating Effect of α-Lipoic Acid and Magnesium on Cadmium-Induced Inflammatory Processes, Oxidative Stress and Bone Metabolism Disorders in Wistar Rats" International Journal of Environmental Research and Public Health 16, no. 22: 4483. https://doi.org/10.3390/ijerph16224483