Effects of Salinity on Earthworms and the Product During Vermicomposting of Kitchen Wastes
Abstract
:1. Introduction
2. Material and Methods
2.1. Organic Waste Collection and Earthworm Culture
2.2. The Vermicomposting of Feedstocks
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth of Earthworms
3.2. TOC and C/N Ratio
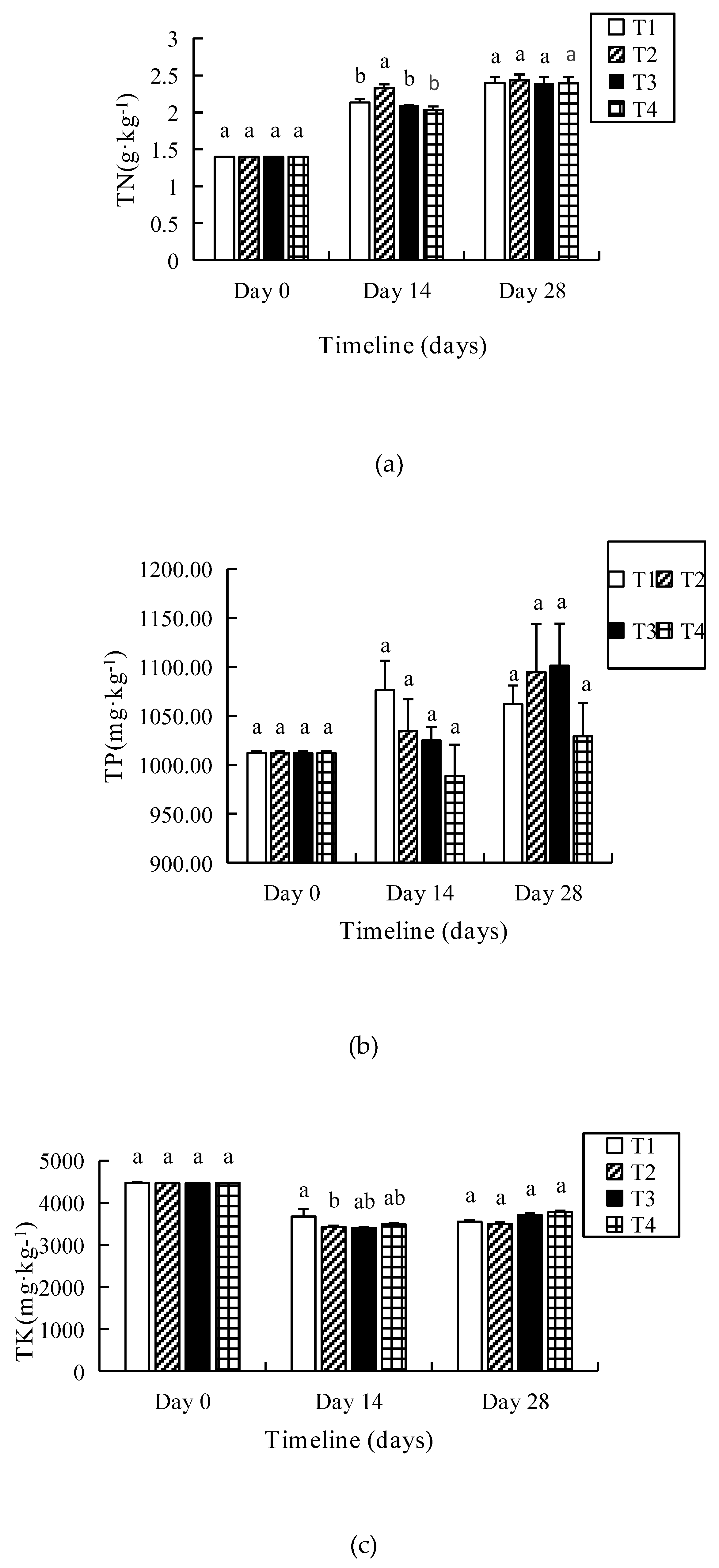
3.3. TN, TP and TK
3.4. NH4+–N, NO3−–N, AP and AK
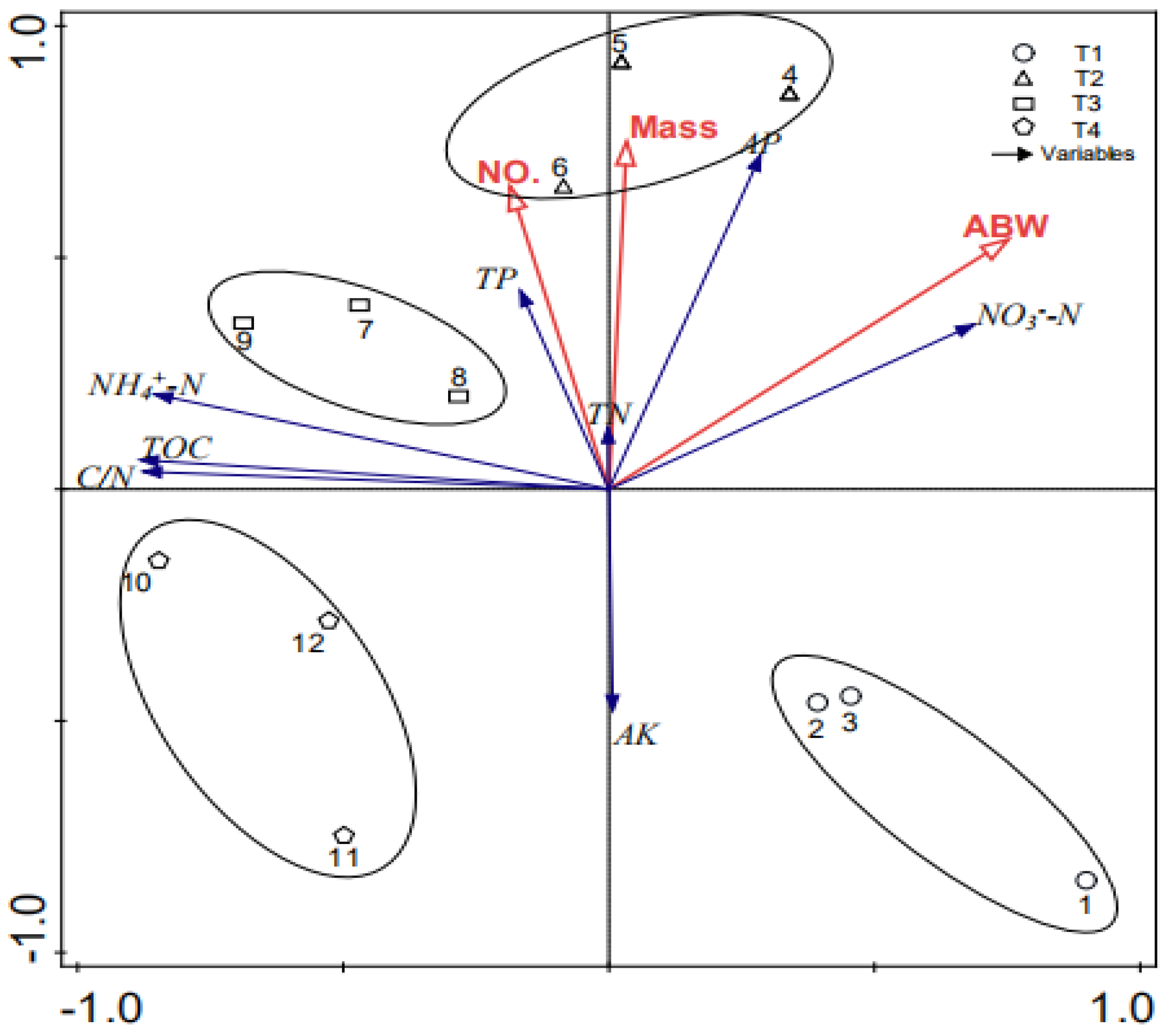
3.5. Effects of Chemical Characteristics on the Growth of Earthworms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Wang, Q.; Jiang, J.; Zhang, H. Effects of salt and oil concentrations on volatile fatty acid generation in food waste fermentation. Renew. Energy 2017, 113, 1523–1528. [Google Scholar] [CrossRef]
- Hao, X.; Zhou, P.; Cao, D. Analyses of disposal methods and carbon emissions of food wastes. Chin. J. Environ. Eng. 2017, 2, 673–682. [Google Scholar]
- Yue, Y.; Yue, Y.; Li, B.; Wang, W.F.; Xu, C.; Dong, Z.Y. Study on hydrogen production by anaerobic co-digestion of kitchen waste and sewage sludge. J. Ningbo Univ. (Nsee) 2018, 31, 110–114. [Google Scholar]
- Nair, J.; Sekiozoic, V.; Anda, M. Effect of pre-composting on vermicomposting of kitchen waste. Bioresour. Technol. 2006, 97, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lu, Y.F.; Walder, R.; Xu, H. Municipal Solid Waste Management and Treatment Technology; China Architecture & Building Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Li, R.P.; Ge, Y.J.; Wang, K.S.; Li, X.; Pang, J.Y.Z. Characteristics and anaerbic digestion performances of kitchen Wastes. Renew. Energy Resour. 2010, 28, 76–80. [Google Scholar]
- Sun, Y.J.; Zhao, J.; Sun, W.; Yu, H.P. Screening and Identification of High-efficiency Oil Degrading Bacteria and Its Application in Catering Wastewater Treatment. Environ. Energy 2019, 01, 73–74, 76. [Google Scholar]
- Chen, Y.; Zhu, R.; Jiang, Q.; Sun, T.; Li, M.; Shi, J.; Chai, H.; Gu, L.; Ai, H.; He, Q. Effects of green waste participation on the co-digestion of residual sludge and kitchen waste: A preliminary study. Sci. Total. Environ. 2019, 671, 838–849. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Nogales, R.; Insam, H.; Romero, E.; Goberna, M. Role of vermicompost chemical composition, microbial functional diversity, and fungal community structure in their microbial respiratory response to three pesticides. Bioresour. Technol. 2011, 102, 9638–9645. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Hanc, A.; Vasak, F. Processing separated digestate by vermicomposting technology using earthworms of the genus Eisenia. Int. J. Environ. Sci. Technol. 2015, 12, 1183–1190. [Google Scholar] [CrossRef]
- Hanc, A.; Dreslova, M. Effect of composting and vermicomposting on properties of particle size fractions. Bioresour. Technol. 2016, 217, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Shak, K.P.; Wu, T.Y.; Lim, S.L.; Lee, C.A. Sustainable reuse of rice residues as feedstocks in vermicomposting for organic fertilizer production. Environ. Sci. Pollut. Res. 2014, 21, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Faraji, M.; Janjani, H. Recycling of three different types of rural wastes employing vermicomposting technology by Eisenia fetida at low temperature. Glob. Nest J. 2017, 19, 601–606. [Google Scholar]
- Fang, Z.H. Effects of Salinity on the Growth of Juvenile Tongue Sole and Its Eco-Physiological Mechanisms 2013; Ocaen University of China: Qingdao, China, 2013. [Google Scholar]
- Elefsiniotis, P.; Wareham, D. Utilization patterns of volatile fatty acids in the denitrification reaction. Enzym. Microb. Technol. 2007, 41, 92–97. [Google Scholar] [CrossRef]
- Hu, X.C.; Zhao, Y.L. Changes of lipid content and fatty acid composition in the development of larvae during the development of larvae. Freshw. Fish. 2008, 38, 46–50. [Google Scholar]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Li, Y.N.; Wu, X.; Xiao, C.Z.; Zheng, L.Y.; Liang, A.P. Earthworm Processing of Food Waste with Different Fat Contents. J. Anhui Agric. 2015, 43, 252–253. [Google Scholar]
- Tiessen, H.; Moir, J.O. Total and Organic Carbon. Soil Sampling and Methods of Analysis. 1993, pp. 187–199. Available online: http://www.niordc.ir/uploads%5C86_106_Binder1.pdf (accessed on 15 November 2019).
- McGill, W.B.; Figueiredo, C.T. Total Nitrogen. Soil Sampling and Methods of Analysis. 1993, pp. 201–211. Available online: http://www.niordc.ir/uploads%5C86_106_Binder1.pdf (accessed on 15 November 2019).
- Bansal, S.; Kapoor, K.K. Vermicomposting of crop residues and cattle dung with Eisenia foetida. Bioresour. Technol. 2000, 73, 95–98. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yin, B.Y.; Song, X.; Zhao, Q. Effects of different lipid contents on growth of earthworms and the products during vermicomposting. Waste Manag. Res. 2019, 37, 934–940. [Google Scholar] [CrossRef]
- Suthar, S.; Kumar, K.; Mutiyar, P.K. Nutrient recovery from compostable fractions of municipal solid wastes using vermitechnology. J. Mater. Cycles Waste Manag. 2015, 17, 174–184. [Google Scholar] [CrossRef]
- Kilambi, R.V. Food consumption, growth and survival of grass carp Ctenopharyngodon idella Val at four salinities. J. Fish Biol. 1980, 17, 613–618. [Google Scholar] [CrossRef]
- Chaudhuri, P.S.; Pal, T.K.; Bhattacharjee, G.; Dey, S.K. Chemical changes during vermicomposting (Perionyx excavatus) of kitchen wastes. Trop. Ecol. 2000, 41, 107–110. [Google Scholar]
- Tripathi, G.; Bhardwaj, P. Comparative studies on biomass production, life cycles and composting efficiency of Eisenia fetida (Savigny) and Lampito mauritii (Kinberg). Bioresour. Technol. 2004, 92, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Sun, Q. Diversity of free-living nitrogen-fixing microorganisms in wastelands of copper mine tailings during the process of natural ecological restoration. J. Environ. Sci. 2011, 23, 476–487. [Google Scholar] [CrossRef]
- Gajalakshmi, S.A.; Abbasi, S. Effect of the application of water hyacinth compost/vermicompost on the growth and flowering of Crossandra undulaefolia, and on several vegetables. Bioresour. Technol. 2002, 85, 197–199. [Google Scholar] [CrossRef]
- Luna-Guido, M.; Beltrán-Hernández, R.; Dendooven, L. Dynamics of 14 C-labelled glucose in alkaline saline soil. Soil Boil. Biochem. 2001, 33, 707–719. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Ceccanti, B.; Garcia, C. Coupled chemical and biochemical methodologies to characterize a composting process and the humic substances. In Humic Substances in the Global Environment and Its Implication on Human Health; Elsevier: New York, NY, USA, 1994; pp. 1279–1285. [Google Scholar]
- Komilis, D.P.; Ham, R.K. Carbon dioxide and ammonia emissions during composting of mixed paper, yard waste and food waste. Waste Manag. 2006, 26, 62–70. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Bell, T.H.; Martin, V.J.J.; Gélinas, Y. Dynamics of Physicochemical Variables and Cultivable Bacteria in Vermicompost during Steady Food Waste Addition and Upon Feed Interruption. Compos. Sci. Util. 2016, 24, 117–135. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Domínguez, J.; Subler, S.; Edwards, C.A. Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei, Bouché) and the effects on seedling growth. Pedobiologia 2000, 44, 709–724. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S. Vermicomposting of vegetable-market solid waste using Eisenia fetida: Impact of bulking material on earthworm growth and decomposition rate. Ecol. Eng. 2009, 35, 914–920. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V. Nutrient recycling from industrial solid wastes and weeds by vermiprocessing using earthworms. Pedosphere 2013, 23, 668–677. [Google Scholar] [CrossRef]
- Sharma, S. Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour. Technol. 2003, 90, 169–173. [Google Scholar]
- Adi, A.; Noor, Z. Waste recycling: Utilization of coffee grounds and kitchen waste in vermicomposting. Bioresour. Technol. 2009, 100, 1027–1030. [Google Scholar] [CrossRef]
- Negi, R.; Suthar, S. Degradation of paper mill wastewater sludge and cow dung by brown-rot fungi Oligoporus placenta and earthworm (Eisenia fetida) during vermicomposting. J. Clean. Prod. 2018, 201, 842–852. [Google Scholar] [CrossRef]
- Rietz, D.; Haynes, R. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Tietjen, T.; Wetzel, R.G. Extracellular enzyme-clay mineral complexes: Enzyme adsorption, alteration of enzyme activity, and protection from photodegradation. Aquat. Ecol. 2003, 37, 331–339. [Google Scholar] [CrossRef]




| Index (g·kg−1) | TOC | TN | NH4+-N | NO3−–N | TP | TK | AP | AK | pH | |
|---|---|---|---|---|---|---|---|---|---|---|
| substrates | ||||||||||
| Soil | 37.4 | 1.40 | 0.01 | 0.03 | 1.01 | 4.40 | 0.067 | 0.270 | 7.60 | |
| Kitchen wastes | 506 | 60.0 | 0.01 | 0.17 | 5.50 | 16.2 | 3.30 | 15.5 | 8.50 |
| Treatments | Setting Value (%) | Measured Value (%) |
|---|---|---|
| T1 | 0 | 0.1 |
| T2 | 0.1 | 0.2 |
| T3 | 0.2 | 0.27 |
| T4 | 0.3 | 0.39 |
| Treatments | Total Organic Carbon (g·kg−1) | Biodegradation Rate % | C/N | ||||
|---|---|---|---|---|---|---|---|
| Day 0 (Soil) | Day 14 | Day 28 | Day 0 (Soil) | Day 14 | Day 28 | ||
| T1 | 37.42 ± 0.44 | 33.27 ± 0.15 a | 19.70 ± 1.30 b | 47% | 26.73 ± 0.6 | 14.27 ± 0.35 c | 8.10 ± 0.56 b |
| T2 | 37.42 ± 0.44 | 28.23 ± 1.46 b | 16.52 ± 1.62 b | 56% | 26.73 ± 0.6 | 13.25 ± 0.91 c | 6.89 ± 0.66 b |
| T3 | 37.42 ± 0.44 | 34.53 ± 0.97 a | 24.36 ± 1.73 a | 35% | 26.73 ± 0.6 | 16.44 ± 0.46 b | 10.14 ± 0.49 a |
| T4 | 37.42 ± 0.44 | 36.42 ± 0.15 a | 26.48 ± 0.75 a | 29% | 26.73 ± 0.6 | 17.92 ± 0.38 a | 11.05 ± 0.66 a |
| TOC | TN | TP | AP | AK | NH4+–N | NO3−–N | C/N | No. | Biomass | Abw | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TOC | 1 | ||||||||||
| TN | 0.02 | 1 | |||||||||
| TP | −0.04 | 0.09 | 1 | ||||||||
| AP | −0.4 | 0.19 | 0.54 | 1 | |||||||
| AK | −0.3 | 0.22 | 0.31 | −0.07 | 1 | ||||||
| NH4+–N | 0.97 ** | −0.01 | 0.01 | −0.3 | −0.33 | 1 | |||||
| NO3−–N | −0.81 ** | 0.15 | 0.47 | 0.74 ** | 0.33 | −0.80 ** | 1 | ||||
| C/N | 0.99 ** | −0.14 | −0.05 | −0.4 | −0.31 | 0.96 ** | −0.82 ** | 1 | |||
| a No. | 0.2 | 0.2 | 0.66 * | 0.28 | −0.18 | 0.23 | 0.13 | 0.15 | 1 | ||
| Biomass | 0.39 | 0.20 | 0.63 * | 0.43 | 0.28 | 0.04 | 0.30 | −0.04 | 0.97 ** | 1 | |
| b Abw | −0.66 * | 0.12 | 0.30 | 0.53 | −0.13 | −0.61 * | 0.70 * | −0.67 * | 0.50 | 0.69 * | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Yin, B.; Song, X.; Qiu, J.; Cao, L.; Zhao, Q. Effects of Salinity on Earthworms and the Product During Vermicomposting of Kitchen Wastes. Int. J. Environ. Res. Public Health 2019, 16, 4737. https://doi.org/10.3390/ijerph16234737
Wu Z, Yin B, Song X, Qiu J, Cao L, Zhao Q. Effects of Salinity on Earthworms and the Product During Vermicomposting of Kitchen Wastes. International Journal of Environmental Research and Public Health. 2019; 16(23):4737. https://doi.org/10.3390/ijerph16234737
Chicago/Turabian StyleWu, Zexuan, Bangyi Yin, Xu Song, Jiangping Qiu, Linkui Cao, and Qi Zhao. 2019. "Effects of Salinity on Earthworms and the Product During Vermicomposting of Kitchen Wastes" International Journal of Environmental Research and Public Health 16, no. 23: 4737. https://doi.org/10.3390/ijerph16234737





