Utilization of Magnetic Resonance Imaging by Comorbidity of Patients with Dementia
Abstract
1. Introduction
2. Subjects and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Lin, S.K.; Tsai, Y.T.; Lai, J.N.; Wu, C.T. Demographic and medication characteristics of traditional Chinese medicine users among dementia patients in Taiwan: A nationwide database study. J. Ethnopharmacol. 2015, 161, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.; Lim, L.L.; Heller, R.F. Accuracy of administrative data to assess comorbidity in patients with heart disease: An Australian perspective. J. Clin. Epidemiol. 2001, 54, 687–693. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Samsa, G.P.; Matchar, D.B.; Homer, R.D. Charlson index comorbidity adjustment for ischemic stroke outcome studies. Stroke 2004, 35, 1941–1945. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef]
- Youn, K.I. The Characteristics and Length of Stay Determinants of the Patients with Dementia: A Comparison among Hospital Types. J. Health Info. Stat. 2015, 40, 1–14. [Google Scholar]
- Teel, C.; Carson, P. Family experiences in the journey through dementia diagnosis and care. J. Fam. Nurs. 2003, 9, 38–58. [Google Scholar] [CrossRef]
- Jung, Y.H. Analysis of contributions to risk factors for dementia and management for dementia patients. Health Welf. Issue Focus 2017, 9, 338. [Google Scholar]
- DeKosky, S. Early intervention is key to successful management of Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2003, 17, S99–S104. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Longstreth, W.T., Jr.; Koudstaal, P.J. Silent brain infarcts: A systematic review. Lancet Neurol. 2007, 6, 611–619. [Google Scholar] [CrossRef]
- Matsui, T.; Nemoto, M.; Maruyama, M.; Yuzuriha, T.; Yao, H.; Tanji, H.; Ootsuki, M.; Tomita, N.; Matsushita, S.; Higuchi, S.; et al. Plasma homocysteine and risk of coexisting silent brain infarction in Alzheimer’s disease. Neurodegener. Dis. 2005, 2, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Rasero, J.; Amoroso, N.; La Rocca, M.; Tangaro, S.; Bellotti, R.; Stramaglia, S. Multivariate regression analysis of structural MRI connectivity matrices in Alzheimer’s disease. PLoS ONE 2017, 12, e0187281. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.A.M.; Damanti, S.; Scortichini, V.; Galli, A.; Rossi, P.D.; Abbate, C.; Arosio, B.; Mari, D.; Arighi, A.; Fumagalli, G.G.; et al. Alzheimer’s Disease Diagnosis: Discrepancy between Clinical, Neuroimaging, and Cerebrospinal Fluid Biomarkers Criteria in an Italian Cohort of Geriatric Outpatients: A Retrospective Cross-sectional Study. Front. Med. Lausanne. 2017, 4, 203. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, D.; Park, H. Disability-Adjusted Life Years (DALYs) for Injuries Using Death Certificates and Hospital Discharge Survey by the Korean Burden of Disease Study 2012. J. Korean Med. Sci. 2016, 31, S200–S207. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quach, C.; Hommet, C.; Mondon, K.; Lauvin, M.A.; Cazals, X.; Cottier, J.P. Early-onset dementias: Specific etiologies and contribution of MRI. Diagn. Interv. Imaging 2014, 95, 377–398. [Google Scholar] [CrossRef][Green Version]
- Verkaik, R.; Francke, A.L.; Van Meijel, B.; Ribbe, M.W.; Bensing, J.M. Comorbid depression in dementia on psychogeriatric nursing home wards: Which symptoms are prominent? Am. J. Geriatr. Psychiatry. 2009, 17, 565–573. [Google Scholar] [CrossRef]
- Rius, C.; Perez, G.; Martinez, J.M.; Bares, M.; Schiaffino, A.; Gispert, R.; Fernandez, E. An adaptation of Charlson comorbidity index predicted subsequent mortality in a health survey. J. Clin. Epidemiol. 2004, 57, 403–408. [Google Scholar] [CrossRef]
- Pimenta, F.A.; Bicalho, M.A.; Romano-Silva, M.A.; Moraes, E.N.; Rezende, N.A. Chronic diseases, cognition, functional decline, and the Charlson index in elderly people with dementia. Rev. Assoc. Med. Bras. (1992) 2013, 59, 326–334. [Google Scholar] [CrossRef]
- Choi, D.; Kim, S.H.; Lim, J.H.; Cho, J.M.; Lee, W.J.; Lee, S.J.; Lim, H.K. Detection of hepatocellular carcinoma: Combined T2-weighted and dynamic gadolinium-enhanced MRI versus combined CT during arterial portography and CT hepatic arteriography. J. Comput Assist. Tomogr. 2001, 25, 777–785. [Google Scholar] [CrossRef] [PubMed]

| Comorbidity | CCI Weight | ICD-10 Code |
|---|---|---|
| Myocardial infarction | 1 | I21.x, I22.x, I25.2 |
| Congestive heart failure | 1 | I09.9, I11.0, I13.0, I42.0, I42.5–I42.9, I43.x, I50.x, P29.0 |
| Peripheral vascular disease | 1 | I70.x, I71.x, I73.1, I73.8, I73.9, I77.1, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 |
| Cerebrovascular disease | 1 | G45.x, G46.x, H34.0, I60.x-I69.x |
| Chronic pulmonary disease | 1 | I27.8, I27.9, J40.x–J47.x, J60.x–J67.x, J68.4, J70.1, J70.3 |
| Connective tissue disease | 1 | M05.x, M06.x, M31.5, M32.x–M34.x, M35.1, M35.3, M36.0 |
| Ulcer disease | 1 | K25.x–K28.x |
| Mild liver disease | 1 | B18.x, K70.0–K70.3, K70.9, K71.3–K71.5, K71.7, K73.x, K74.x, K76.0, K76.2–K76.4, K76.8, K76.9, Z94.4 |
| Diabetes | 1 | E10.0, E10.1, E10.6, E10.8, E10.9, E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9 |
| Diabetes with end-organ damage | 2 | E10.2–E10.5, E10.7, E11.2–E11.5, E11.7, E12.2–E12.5, E12.7, E13.2–E13.5, E13.7, E14.2–E14.5, E14.7 |
| Hemiplegia | 2 | G04.1, G11.4, G81.x, G82.x, G83.0–G83.4, G83.9 |
| Moderate or severe renal disease | 2 | I12.0, I13.1, N03.2–N03.7, N05.2–N05.7, N18.x, N19.x, N25.0, Z49.0–Z49.2, Z94.0, Z99.2 |
| Leukemia, Lymphoma, any tumor | 2 | C00.x–C26.x, C30.x–C34.x, C37.x–C41.x, C43.x, C45.x–C58.x, C60.x–C76.x, C81.x–C85.x, C88.x, C90.x–C97.x |
| Moderate or severe liver disease | 3 | I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K72.1, K72.9, K76.5, K76.6, K76.7 |
| Metastatic solid tumor | 6 | C77.x–C80.x |
| Acquired immune deficiency syndrome | 6 | B20.x–B22.x, B24.x |
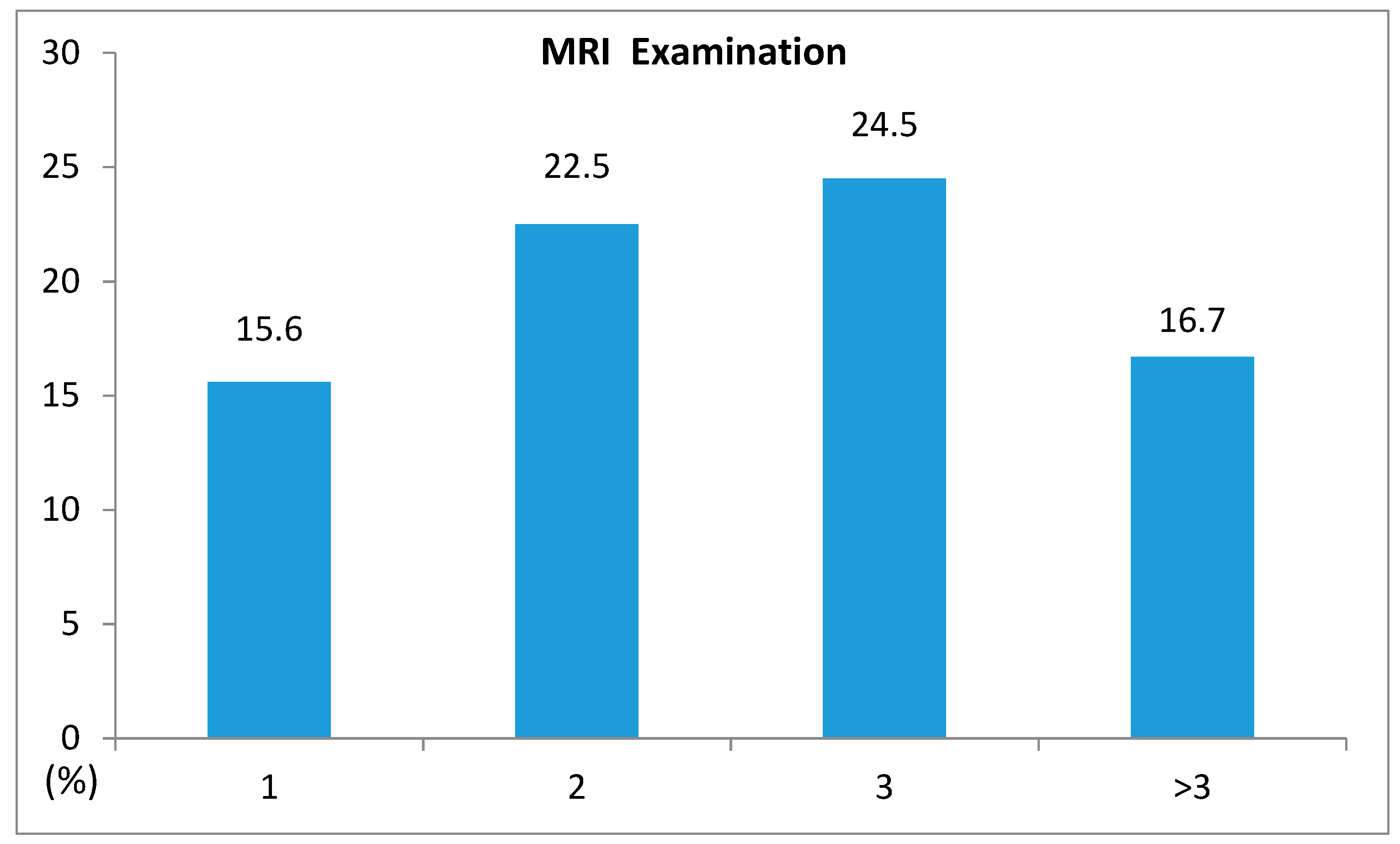
| CCI = O (n = 405) | CCI = 1 (n = 160) | CCI = 2 (n = 53) | CCI ≥ 3 (n = 24) | Total (n = 642) | |
|---|---|---|---|---|---|
| MRI Yes n (%) | 63 (15.6) | 36 (22.5) | 13 (24.5) | 4 (16.7) | 116 (18.1) |
| Variables | MRI Examination | Total (n = 642) | X2 | p-Value | |
|---|---|---|---|---|---|
| Yes (n = 116) | |||||
| Sex | Male | 42 (16.7) | 252 (39.3) | 0.551 | 0.458 |
| Female | 74 (19.0) | 390 (60.7) | |||
| Age | <60 | 11 (27.5) | 40 (6.2) | 7.592 | 0.108 |
| 60–69 | 13 (21.7) | 60 (9.3) | |||
| 70–79 | 50 (20.1) | 249 (38.8) | |||
| 80–89 | 39 (15.3) | 255 (39.7) | |||
| ≥90 | 3 (7.9) | 38 (5.9) | |||
| Insurance type | National health | 97 (18.4) | 527 (82.1) | 2.782 | 0.249 |
| Medicare | 16 (15.0) | 107 (16.7) | |||
| Others | 3 (37.5) | 8 (1.2) | |||
| Admission route | Emergency | 23 (15.5) | 148 (23.1) | 0.830 | 0.362 |
| Ambulatory | 93 (18.8) | 494 (76.9) | |||
| Length of stay | 1–4 | 31 (18.6) | 167 (26.0) | 16.106 | 0.001 * |
| 5–9 | 41 (26.5) | 155 (24.1) | |||
| 10–25 | 30 (18.3) | 164 (25.5) | |||
| ≥26 | 14 (9.0) | 156 (24.3) | |||
| Result of treatment | Improved | 109 (20.0) | 545 (84.9) | 12.218 | 0.007 * |
| Not improved | 5 (7.9) | 63 (9.8) | |||
| Diagnosis only | 2 (28.6) | 7 (1.1) | |||
| Death | 0 (0.0) | 27 (4.2) | |||
| Number of hospital beds | 100–299 | 47 (14.4) | 326 (50.8) | 20.637 | 0.000 * |
| 300–499 | 4 (9.1) | 44 (6.9) | |||
| 500–999 | 55 (28.4) | 194 (30.2) | |||
| ≥1000 | 10 (12.8) | 78 (12.1) | |||
| Hospital location | Seoul | 19 (14.3) | 133 (30.6) | 27.636 | 0.000 * |
| Metropolitan | 22 (11.5) | 191 (43.9) | |||
| Gyeonggi | 34 (36.2) | 94 (21.6) | |||
| Others | 41 (18.3) | 224 (34.9) | |||
| Variables | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Sex | Male | 1 | ||
| Female | 1.236 | (0.781–1.956) | 0.365 | |
| Age | <60 | 1 | ||
| 60–69 | 1.068 | (0.383–2.977) | 0.899 | |
| 70–79 | 0.733 | (0.314–1.712) | 0.472 | |
| 80–89 | 0.578 | (0.243–1.373) | 0.214 | |
| ≥90 | 0.323 | (0.075–1.394) | 0.130 | |
| Insurance type | National health | 1 | ||
| Medicare | 1.068 | (0.567–2.014) | 0.838 | |
| Others | 1.668 | (0.336–8.288) | 0.532 | |
| Admission route | Emergency | 1 | ||
| Ambulatory | 1.329 | (0.772–2.289) | 0.305 | |
| Length of stay | 1–4 | 1 | ||
| 5–9 | 1.245 | (0.705–2.198) | 0.451 | |
| 10–25 | 0.683 | (0.374–1.248) | 0.214 | |
| ≥26 | 0.432 | (0.209–0.896) | 0.024 * | |
| Result of treatment | Improved | 1 | ||
| Not improved | 0.467 | (0.174–1.255) | 0.131 | |
| Diagnosis only | 1.236 | (0.200–7.639) | 0.819 | |
| Death | 0.000 | (0.000) | 0.998 | |
| CCI | 0 | 1 | ||
| 1 | 1.757 | (1.070–2.884) | 0.026 * | |
| 2 | 1.706 | (0.796–3.656) | 0.169 | |
| ≥3 | 1.343 | (0.388–4.653) | 0.642 | |
| Number of hospital beds | 100–299 | 1 | ||
| 300–499 | 0.458 | (0.149–1.407) | 0.173 | |
| 500–999 | 1.537 | (0.911–2.591) | 0.107 | |
| ≥1000 | 0.754 | (0.308–1.844) | 0.536 | |
| Hospital location | Seoul | 1 | ||
| Metropolitan | 0.932 | (0.440–1.975) | 0.853 | |
| Gyeonggi | 2.645 | (1.278–5.474) | 0.009 * | |
| Others | 1.472 | (0.722–3.001) | 0.288 | |
| Model Chi-square (df.) −2 log likelihood Nagelkerke R square | 70.520 (23) 536.081 (0.000) 0.170 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Cheon, S. Utilization of Magnetic Resonance Imaging by Comorbidity of Patients with Dementia. Int. J. Environ. Res. Public Health 2019, 16, 4741. https://doi.org/10.3390/ijerph16234741
Lim J, Cheon S. Utilization of Magnetic Resonance Imaging by Comorbidity of Patients with Dementia. International Journal of Environmental Research and Public Health. 2019; 16(23):4741. https://doi.org/10.3390/ijerph16234741
Chicago/Turabian StyleLim, Jihye, and Songhee Cheon. 2019. "Utilization of Magnetic Resonance Imaging by Comorbidity of Patients with Dementia" International Journal of Environmental Research and Public Health 16, no. 23: 4741. https://doi.org/10.3390/ijerph16234741
APA StyleLim, J., & Cheon, S. (2019). Utilization of Magnetic Resonance Imaging by Comorbidity of Patients with Dementia. International Journal of Environmental Research and Public Health, 16(23), 4741. https://doi.org/10.3390/ijerph16234741




