Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Personalized Exercise Training Program
2.3. Statistical Analysis
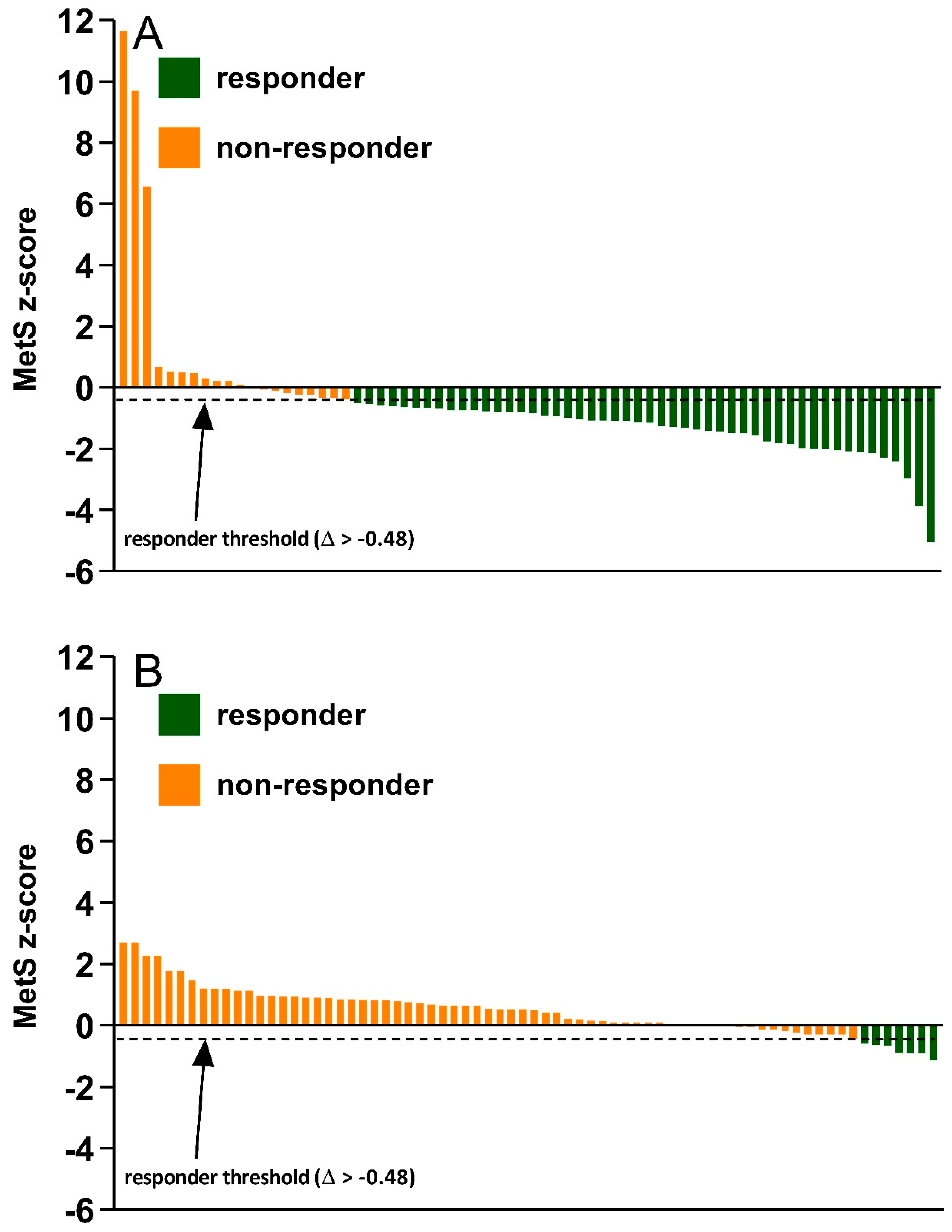
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larsson, S.C.; Wallin, A.; Håkansson, N.; Stackelberg, O.; Bäck, M.; Wolk, A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int. J. Cardiol. 2018, 262, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Global Report on Diabetes. Available online: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=C1C77775CDDBC4FF4568A581E746088A?sequence=1 (accessed on 26 November 2019).
- Baldassarre, P.A.; Andersen, A.; Consoli, A.; Knop, F.K.; Vilsboll, T. Cardiovascular biomarkers in clinical studies of type 2 diabetes. Diabetes Obes. Metab. 2008, 20, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: An analysis of the diabetes prevention program. Diabetes Care 2018, 41, 2421–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, V.W.; Baruth, M.; Beets, M.W.; Durstine, J.L.; Liu, J.; Blair, S.N. Fitness vs. fatness on all-cause mortality: A meta-analysis. Prog. Cardiovasc. Dis. 2014, 56, 382–390. [Google Scholar] [CrossRef]
- Johnson, J.L.; Slentz, C.A.; Houmard, J.A.; Samsa, G.P.; Duscha, B.D.; Aiken, L.B.; McCartney, J.S.; Tanner, C.J.; Kraus, W.E. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am. J. Cardiol. 2007, 100, 1759–1766. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.S.; Dalleck, L.C.; Borrani, F.; Beetham, K.S.; Wallen, M.P.; Mallard, A.R.; Clark, B.; Gomersall, S.; Keating, S.E.; Fassett, R.G.; et al. Low-volume high intensity interval training is sufficient to ameliorate the severity of metabolic syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 319–328. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. Incidence of VO2max responders to personalized versus standardized exercise prescription. Med. Sci. Sports Exerc. 2019, 51, 681–691. [Google Scholar] [CrossRef]
- Byrd, B.R.; Keith, J.; Keeling, S.M.; Weatherwax, R.M.; Nolan, P.B.; Ramos, J.S.; Dalleck, L.C. Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness. Int. J. Environ. Res. Public Health 2019, 16, 2088. [Google Scholar] [CrossRef] [Green Version]
- Dalleck, L.C.; Van Guilder, G.P.; Quinn, E.M.; Bredle, D.L. Primary prevention of metabolic syndrome in the community using an evidence-based exercise program. Prev. Med. 2013, 57, 392–395. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. The incidence of training responsiveness to cardiorespiratory fitness and cardiometabolic measurements following individualized and standardized exercise prescription: Study protocol for a randomized controlled trial. Trials 2016, 17, 601. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2018. [Google Scholar]
- Bryant, C.X.; Green, D.J. ACE Personal Trainer Manual; American Council on Exercise: San Diego, CA, USA, 2010. [Google Scholar]
- Dalleck, L.C.; Haney, D.E.; Buchanan, C.A.; Weatherwax, R.M. Does a personalized exercise prescription enhance training efficacy and limit training unresponsiveness? A randomized controlled trial. J. Fit. Res. 2016, 5, 15–27. [Google Scholar]
- Gurka, M.J.; Guo, Y.; Filipp, S.L.; DeBoer, M.D. Metabolic syndrome severity is significantly associated with future coronary heart disease in type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Unal, B.; Critchley, J.A.; Capewell, S. Modelling the decline in coronary heart disease deaths in England and wales, 1981–2000: Comparing contributions from primary prevention and secondary prevention. BMJ 2005, 331, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Capewell, S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation 2009, 120, 1181–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzati, M.; Riboli, E. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science 2012, 337, 1482–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, D.M.; Yanek, L.R.; Johnson, W.R., Jr.; Garrett, D.; Moy, T.F.; Reynolds, S.S.; Blumenthal, R.S.; Vaidya, D.; Becker, L.C. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation 2005, 111, 1298–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, S.N.; Kampert, J.B.; Kohl, H.W.; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.A., Jr.; Gibbons, L.A. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205–210. [Google Scholar] [CrossRef]
- Meyer, T.; Scharhag, J.; Kindermann, W. Peak oxygen uptake: Myth and truth about an internationally accepted reference value. Z. Kardiol. 2005, 94, 255–264. [Google Scholar] [CrossRef]
- Williams, C.J.; Gurd, B.J.; Bonafiglia, J.T.; Voisin, S.; Li, Z.X.; Harvey, N.; Croci, I.; Taylor, J.L.; Gajanand, T.; Ramos, J.S.; et al. A multi-center comparison of VO2peak trainability between interval training and moderate intensity continuous training. Front. Physiol. 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Earnest, C.P.; Lupo, M.; Thibodaux, J.; Hollier, C.; Bututta, B.; Lejeune, E.; Johannsen, N.M.; Gibala, M.J.; Church, T.S. Interval training in men at risk for insulin resistance. Int. J. Sports Med. 2013, 34, 355–363. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.F.; Carrington, M.J. A metabolic syndrome severity score: A tool to quantify cardio-metabolic risk factors. Prev. Med. 2016, 88, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.D. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 ; Submaximal VO2max test (talk test)
; Submaximal VO2max test (talk test)  ; Balance test
; Balance test  ; 8 feet up and go
; 8 feet up and go  ; Shoulder rotation, sit and reach, trunk rotation
; Shoulder rotation, sit and reach, trunk rotation  ; Blood pressure
; Blood pressure  ; Resting heart rate
; Resting heart rate  ; BMI
; BMI  ; Waist circumference
; Waist circumference  .
.
 ; Submaximal VO2max test (talk test)
; Submaximal VO2max test (talk test)  ; Balance test
; Balance test  ; 8 feet up and go
; 8 feet up and go  ; Shoulder rotation, sit and reach, trunk rotation
; Shoulder rotation, sit and reach, trunk rotation  ; Blood pressure
; Blood pressure  ; Resting heart rate
; Resting heart rate  ; BMI
; BMI  ; Waist circumference
; Waist circumference  .
.

| Outcome Variable | Control Group (N = 72) | Treatment Group (N = 70) | ||
|---|---|---|---|---|
| Baseline | Post-Program | Baseline | Post-Program | |
| Age (yr) | 45.6 ± 12.5 | ------- | 46.6 ± 16.7 | ------- |
| Body mass (kg) | 75.5 ± 12.3 | 75.7 ± 12.0 * | 77.3 ± 18.7 | 76.7 ± 18.4 *, † |
| Waist circumference (cm) | 82.4 ± 8.8 | 82.7 ± 8.6 | 84.0 ± 14.2 | 83.1 ± 12.9 *, † |
| Systolic BP (mm Hg) | 119.0 ± 11.0 | 121.2 ± 9.6 * | 122.6 ± 14.1 | 117.4 ± 13.1 *, † |
| Diastolic BP (mm Hg) | 79.4 ± 8.4 | 81.4 ± 6.6 * | 79.7 ± 9.7 | 77.3 ± 7.7 *, † |
| Total cholesterol (mg·dL−1) | 201.3 ± 40.0 | 204.4 ± 37.5 | 187.5 ± 39.1 | 185.1 ± 37.7 |
| HDL cholesterol (mg·dL−1) | 50.7 ± 18.2 | 49.4 ± 16.5 * | 54.2 ± 17.9 | 57.8 ± 15.9 *, † |
| LDL cholesterol (mg·dL−1) | 119.9 ± 37.7 | 122.0 ± 36.3 | 107.2 ± 32.9 | 100.6 ± 31.1 |
| Triglycerides (mg·dL−1) | 130.0 ± 64.3 | 136.1 ± 67.2 | 110.8 ± 54.4 | 104.5 ± 45.7 † |
| Blood glucose (mg·dL−1) | 93.1 ± 9.0 | 94.8 ± 9.1 | 92.5 ± 8.6 | 89.7 ± 7.0 *, † |
| VO2max (mL·kg−1·min−1) | 29.0 ± 6.1 | 28.4 ± 5.8 * | 31.4 ± 7.9 | 35.0 ± 8.0 *, † |
| MetS z-score | −4.15 ± 4.01 | −3.68 ± 4.07 * | −3.52 ± 3.82 | −4.12 ± 3.24 *, † |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seward, S.; Ramos, J.; Drummond, C.; Dalleck, A.; Byrd, B.; Kehmeier, M.; Dalleck, L. Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming. Int. J. Environ. Res. Public Health 2019, 16, 4855. https://doi.org/10.3390/ijerph16234855
Seward S, Ramos J, Drummond C, Dalleck A, Byrd B, Kehmeier M, Dalleck L. Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming. International Journal of Environmental Research and Public Health. 2019; 16(23):4855. https://doi.org/10.3390/ijerph16234855
Chicago/Turabian StyleSeward, Sophie, Joyce Ramos, Claire Drummond, Angela Dalleck, Bryant Byrd, Mackenzie Kehmeier, and Lance Dalleck. 2019. "Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming" International Journal of Environmental Research and Public Health 16, no. 23: 4855. https://doi.org/10.3390/ijerph16234855
APA StyleSeward, S., Ramos, J., Drummond, C., Dalleck, A., Byrd, B., Kehmeier, M., & Dalleck, L. (2019). Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming. International Journal of Environmental Research and Public Health, 16(23), 4855. https://doi.org/10.3390/ijerph16234855






