Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene
Abstract
:1. Introduction
2. Research Method and Material
2.1. Sample Preparation
2.2. HS Size Fraction Separation
2.3. UV/Vis Measurements
2.4. Fluorescence Spectroscopy
2.5. Fluorescence Quenching
2.6. Statistical Analysis and Calculation of Fluorescence Data
3. Results and Discussion
3.1. DOC Concentration and Carbon Mass Fraction of Size-Fractioned HS
3.2. Optical Indicators
3.3. Sorption Constants between HS and Phe
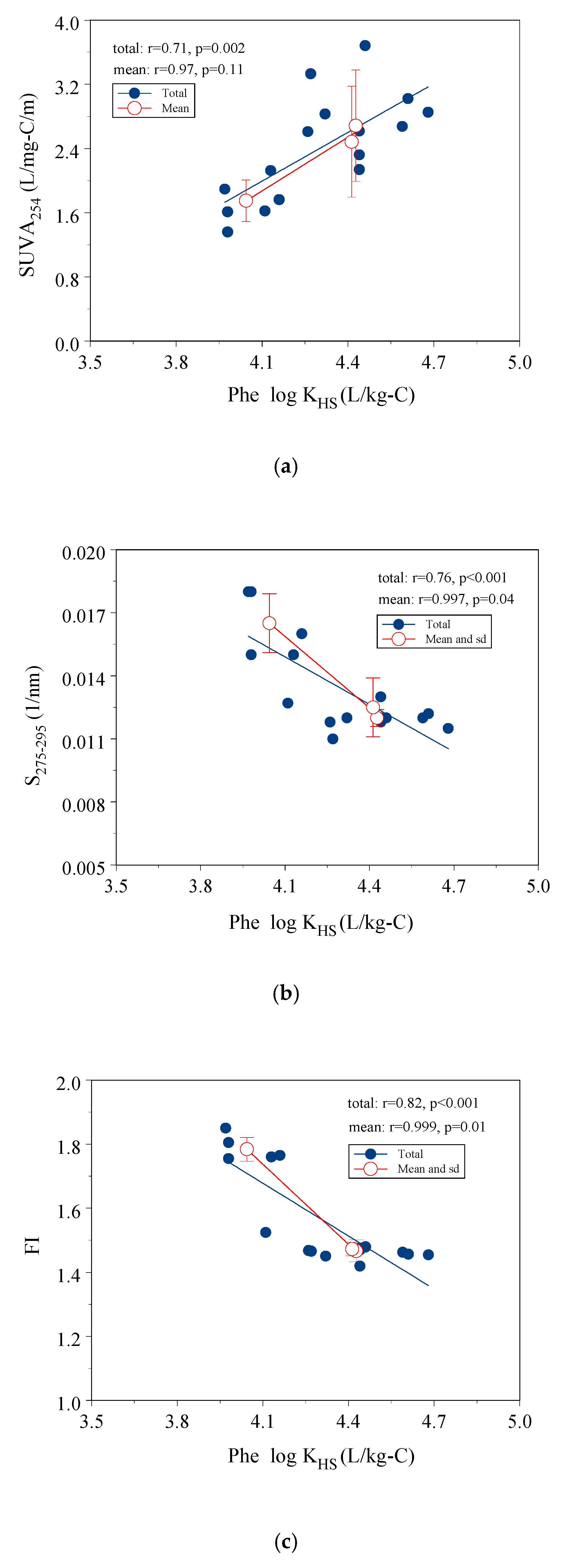
3.4. Correlation of log KHS with Indicators
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.-C.; Zhang, Y.-X.; Chen, R.F. Distribution and partitioning of polycyclic aromatic hydrocarbons (PAHs) in different size fractions in sediments from Boston Harbor, United States. Mar. Pollut. Bull. 2001, 42, 1139–1149. [Google Scholar] [CrossRef]
- Shang, J.; Chen, J.; Shen, Z.; Wang, Y.; Ruan, A. Effects of varying estuarine conditions on the sorption of phenanthrene to sediment particles of Yangtze Estuary. Mar. Pollut. Bull. 2013, 76, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jin, J.; Gao, B.; Zhang, Z.; Wang, Z.; Pan, Z.; Xu, D.; Zhao, Y. Sorption of 17α-ethinyl estradiol, bisphenol A and phenanthrene to different size fractions of soil and sediment. Chemosphere 2012, 88, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ni, J.; Xu, N.; Sun, L. Fluorescence of sediment humic substance and its effect on the sorption of selected endocrine disruptors. Chemosphere 2007, 66, 700–707. [Google Scholar] [CrossRef]
- Hur, J.; Lee, D.-H.; Shin, H.-S. Comparison of the structural, spectroscopic and phenanthrene binding characteristics of humic acids from soils and lake sediments. Org. Geochem. 2009, 40, 1091–1099. [Google Scholar] [CrossRef]
- Hur, J.; Lee, B.-M.; Shin, K.-H. Spectroscopic characterization of dissolved organic matter isolates from sediments and the association with phenanthrene binding affinity. Chemosphere 2014, 111, 450–457. [Google Scholar] [CrossRef]
- Hur, J.; Kim, G. Comparison of the heterogeneity within bulk sediment humic substances from a stream and reservoir via selected operational descriptors. Chemosphere 2009, 75, 483–490. [Google Scholar] [CrossRef]
- Vitale, C.M.; Di Guardo, A. A review of the predictive models estimating association of neutral and ionizable organic chemicals with dissolved organic carbon. Sci. Total Environ. 2019, 666, 1022–1032. [Google Scholar] [CrossRef]
- Hur, J.; Schlautman, M.A. Influence of humic substance adsorptive fractionation on pyrene partitioning to dissolved and mineral-associated humic substances. Environ. Sci. Technol. 2004, 38, 5871–5877. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, M.-H.; Hur, J. A new molecular weight (MW) descriptor of dissolved organic matter to represent the MW-dependent distribution of aromatic condensation: Insights from biodegradation and pyrene binding experiments. Sci. Total Environ. 2019, 660, 169–176. [Google Scholar] [CrossRef]
- Hur, J.; Park, S.-W.; Kim, M.C.; Kim, H.S. Enhanced binding of hydrophobic organic contaminants by microwave-assisted humification of soil organic matter. Chemosphere 2013, 93, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Ö.; Nilsson, N.; Bucheli, T.D. Dynamic colloid− water partitioning of pyrene through a coastal Baltic spring bloom. Environ. Sci. Technol. 2001, 35, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.D.; Love, N.G.; Novak, J.T. Investigation of sorption behavior between pyrene and colloidal organic carbon from activated sludge processes. Environ. Sci. Technol. 2004, 38, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef] [Green Version]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B.; Liu, W.; Tao, S.; Lin, X.; Zhang, X.; Zhang, Y.; Xiao, Y.; Dai, H.; Yuan, H. Distribution of sorbed phenanthrene and pyrene in different humic fractions of soils and importance of humin. Environ. Pollut. 2006, 143, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-N.; Lin, T.-F.; Chiu, C.-H.; Chiou, C.T. On the use of a freeze-dried versus an air-dried soil humic acid as a surrogate of soil organic matter for contaminant sorption. Environ. Pollut. 2012, 160, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Laor, Y.; Rebhun, M. Evidence for nonlinear binding of PAHs to dissolved humic acids. Environ. Sci. Technol. 2002, 36, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.D.; Breidenich, J.; DeRose, P.C. Impact of reclaimed water on select organic matter properties of a receiving stream fluorescence and perylene sorption behavior. Environ. Sci. Technol. 2005, 39, 6453–6460. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.R.; Danielsen, K.M. Binding of pyrene to aquatic and commercial humic substances: The role of molecular weight and aromaticity. Environ. Sci. Technol. 1997, 31, 1630–1635. [Google Scholar] [CrossRef]
- Pan, B.; Ghosh, S.; Xing, B. Nonideal binding between dissolved humic acids and polyaromatic hydrocarbons. Environ. Sci. Technol. 2007, 41, 6472–6478. [Google Scholar] [CrossRef]
- Yeh, Y.-L.; Yeh, K.-J.; Hsu, L.-F.; Yu, W.-C.; Lee, M.-H.; Chen, T.-C. Use of fluorescence quenching method to measure sorption constants of phenolic xenoestrogens onto humic fractions from sediment. J. Hazard. Mater. 2014, 277, 27–33. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Shao, L.-M.; He, P.-J. Fluorescent characteristics and metal binding properties of individual molecular weight fractions in municipal solid waste leachate. Environ. Pollut. 2012, 162, 63–71. [Google Scholar] [CrossRef]
- McPhedran, K.N.; Seth, R.; Drouillard, K.G. Investigation of Hydrophobic Organic Carbon (HOC) partitioning to 1 kDa fractionated municipal wastewater colloids. Environ. Sci. Technol. 2013, 47, 2548–2553. [Google Scholar] [CrossRef]
- Chen, G.; Lin, C.; Chen, L.; Yang, H. Effect of size-fractionation dissolved organic matter on the mobility of prometryne in soil. Chemosphere 2010, 79, 1046–1055. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Sun, H.-W.; Wang, C.-P.; Li, Y.-H. Binding of pyrene to different molecular weight fractions of dissolved organic matter: Effects of chemical composition and steric conformation. Chem. Res. Chin. Univ. 2012, 28, 624–630. [Google Scholar]
- Xu, H.; Zou, L.; Guan, D.; Li, W.; Jiang, H. Molecular weight-dependent spectral and metal binding properties of sediment dissolved organic matter from different origins. Sci. Total Environ. 2019, 665, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Xing, B.; Liu, W.; Xing, G.; Tao, S. Investigating interactions of phenanthrene with dissolved organic matter: Limitations of Stern–Volmer plot. Chemosphere 2007, 69, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Fengchang, W.; Liying, W.; Yingchen, B.; Wen, L.; Haiqing, L. Binding characteristics of perylene, phenanthrene and anthracene to different DOM fractions from lake water. J. Environ. Sci. 2009, 21, 414–423. [Google Scholar]
- Backhus, D.A.; Golini, C.; Castellanos, E. Evaluation of fluorescence quenching for assessing the importance of interactions between nonpolar organic pollutants and dissolved organic matter. Environ. Sci. Technol. 2003, 37, 4717–4723. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.-Y.; Yu, H.-Q. Temperature–dependent conformational variation of chromophoric dissolved organic matter and its consequent interaction with phenanthrene. Environ. Pollut. 2017, 222, 23–31. [Google Scholar] [CrossRef]
- Chen, X.-M.; Zhao, Y.; Ma, Y.-Y.; Zhu, L.-J.; Yang, T.-X.; Wei, Z.-M.; Dong, Y.-L.; Wei, Q.-B. Assessing the environmental impact of phenanthrene in different types of land use based on the binding characteristics with dissolved organic matter. Ecotoxicol. Environ. Saf. 2018, 147, 394–400. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Kinniburgh, D. An R script for visualising and analysing fluorescence excitation–emission matrices (EEMs). Comput. Geosci. 2009, 35, 2160–2163. [Google Scholar] [CrossRef] [Green Version]
- Fei, Y.-H.; Li, X.-D.; Li, X.-Y. Organic diagenesis in sediment and its impact on the adsorption of bisphenol A and nonylphenol onto marine sediment. Mar. Pollut. Bull. 2011, 63, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Burdige, D.J.; Komada, T. Sediment pore waters. In Biogeochemistry of Marine Dissolved Organic Matter; Elsevier: Amsterdam, The Netherlands, 2015; pp. 535–577. [Google Scholar]
- Batchelli, S.; Muller, F.L.; Baalousha, M.; Lead, J.R. Size fractionation and optical properties of colloids in an organic-rich estuary (Thurso, UK). Mar. Chem. 2009, 113, 227–237. [Google Scholar] [CrossRef]
- Maizel, A.C.; Remucal, C.K. Molecular composition and photochemical reactivity of size-fractionated dissolved organic matter. Environ. Sci. Technol. 2017, 51, 2113–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Tanoue, E. Molecular mass distribution and fluorescence characteristics of dissolved organic ligands for copper (II) in Lake Biwa, Japan. Org. Geochem. 2001, 32, 11–20. [Google Scholar] [CrossRef]
- Xu, H.; Houghton, E.M.; Houghton, C.J.; Guo, L. Variations in size and composition of colloidal organic matter in a negative freshwater estuary. Sci. Total Environ. 2018, 615, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, L. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef]
- Chen, M.; Hur, J. Pre-treatments, characteristics, and biogeochemical dynamics of dissolved organic matter in sediments: A review. Water Res. 2015, 79, 10–25. [Google Scholar] [CrossRef]
- Luo, X.-J.; Mai, B.-X.; Yang, Q.-S.; Chen, S.-J.; Zeng, E.Y. Distribution and partition of polycyclic aromatic hydrocarbon in surface water of the Pearl River Estuary, South China. Environ. Monit. Assess. 2008, 145, 427–436. [Google Scholar] [CrossRef]
- Fu, H.; Wei, C.; Qu, X.; Li, H.; Zhu, D. Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: A mechanism of pseudomicelle partition and environmental implications. Environ. Pollut. 2018, 232, 402–410. [Google Scholar] [CrossRef]
- Mott, H.V. Association of hydrophobic organic contaminants with soluble organic matter: Evaluation of the database of Kdoc values. Adv. Environ. Res. 2002, 6, 577–593. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Björklund, K.; Strömvall, A.-M.; Blom, L. Partitioning of polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachates and stormwater. Water Res. 2013, 47, 1317–1328. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wilding, A.; Hibberd, A.; Zhou, J.L. Partition of endocrine-disrupting chemicals between colloids and dissolved phase as determined by cross-flow ultrafiltration. Environ. Sci. Technol. 2005, 39, 2753–2761. [Google Scholar] [CrossRef]
- Yamamoto, H.; Liljestrand, H.M.; Shimizu, Y.; Morita, M. Effects of physical−chemical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environ. Sci. Technol. 2003, 37, 2646–2657. [Google Scholar] [CrossRef] [PubMed]

| Samples | pH | OM (%) | TOC (%) |
|---|---|---|---|
| AD | 7.14 ± 0.12 | 6.60 ± 1.03 | 2.51 ± 0.69 |
| FD | 7.22 ± 0.03 | 7.06 ± 1.36 | 2.71 ± 0.70 |
| Samples | BHS mg/L | HHS mg/L | MHS mg/L | LHS mg/L |
|---|---|---|---|---|
| AD | 276–329 | 1451–1833 | 638–752 | 59–78 |
| FD | 279–370 | 1108–2220 | 728–946 | 52–71 |
| Samples (MW) | SUVA254 (L/mg-C/m) | S275–295 | FI |
|---|---|---|---|
| AD_HHS (10 kDa to 0.45 μm) | 2.26 ± 0.62 a | 0.0121 ± 0.0006 a | 1.47 ± 0.05 a |
| FD_HHS (10 kDa to 0.45 μm) | 3.11 ± 0.54 a | 0.0120 ± 0.0002 a | 1.47 ± 0.01 a |
| AD_MHS (1–10 kDa) | 2.95 ± 0.34 a | 0.0117 ± 0.0006 a | 1.46 ± 0.01 a |
| FD_MHS (1–10 kDa) | 2.02 ± 0.66 a | 0.0133 ± 0.0016 a | 1.49 ± 0.02 a |
| AD_LHS (<1 kDa) | 1.75 ± 0.38 b | 0.0153 ± 0.0006 *,b | 1.76 ± 0.01 b |
| FD_LHS (<1 kDa) | 1.75 ± 0.14 b | 0.0177 ± 0.0006 b | 1.81 ± 0.04 b |
| Samples | HHS | MHS | LHS |
|---|---|---|---|
| AD | 4.41 ± 0.29 (3) * | 4.39 ± 0.17 (3) | 4.09 ± 0.10 (3) |
| FD | 4.44 ± 0.17 (3) | 4.44 ± 0.00 (2) | 3.97 ± 0.01 (2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.-S.; Huang, W.-S.; Hsu, L.-F.; Yeh, Y.-L.; Chen, T.-C. Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene. Int. J. Environ. Res. Public Health 2019, 16, 5087. https://doi.org/10.3390/ijerph16245087
Shi M-S, Huang W-S, Hsu L-F, Yeh Y-L, Chen T-C. Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene. International Journal of Environmental Research and Public Health. 2019; 16(24):5087. https://doi.org/10.3390/ijerph16245087
Chicago/Turabian StyleShi, Mei-Sheu, Wei-Shiang Huang, Liang-Fong Hsu, Yi-Lung Yeh, and Ting-Chien Chen. 2019. "Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene" International Journal of Environmental Research and Public Health 16, no. 24: 5087. https://doi.org/10.3390/ijerph16245087
APA StyleShi, M.-S., Huang, W.-S., Hsu, L.-F., Yeh, Y.-L., & Chen, T.-C. (2019). Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene. International Journal of Environmental Research and Public Health, 16(24), 5087. https://doi.org/10.3390/ijerph16245087




