A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment
Abstract
:1. Introduction
2. Physicochemical Method
2.1. Activated Carbon Adsorption
2.2. Biochar Adsorption
2.3. Other Functional Materials
3. Chemical Method
3.1. Fenton/Fenton-Like Method
3.2. Ozone Oxidation Method
3.3. Sulfate Radical (SO4−·) Oxidation Method
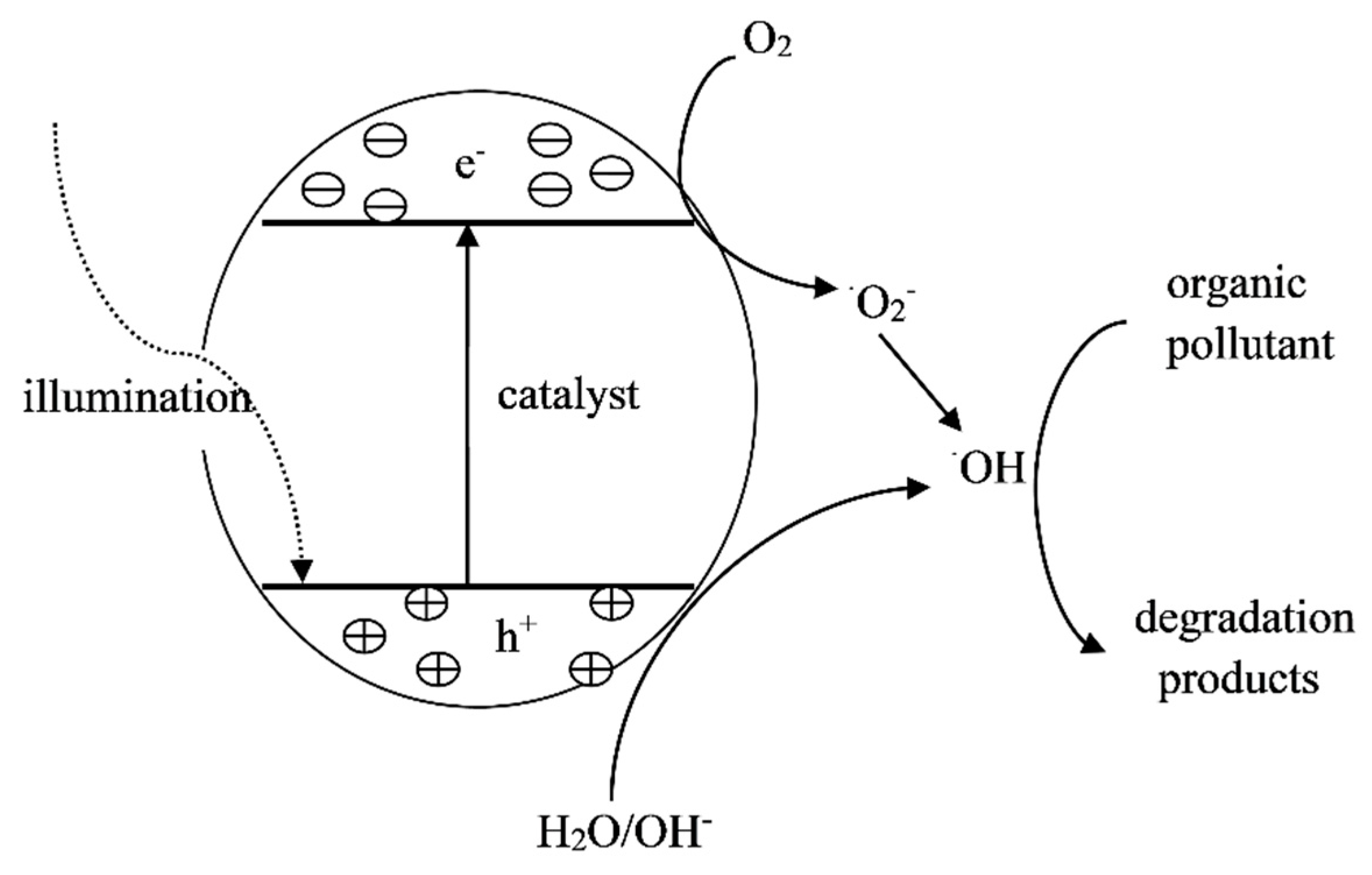
3.4. Photocatalytic Method
4. Biological Method
4.1. Microbial Remediation
4.2. Phytoremediation
4.3. Plant-Microbial Remediation
5. Material-Microbial Combined Technology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martins, E.C.; de Freitas Melo, V.; Bohone, J.B.; Abate, G. Sorption and desorption of atrazine on soils: The effect of different soil fractions. Geoderma 2018, 322, 131–139. [Google Scholar] [CrossRef]
- Taverna, M.E.; Busatto, C.A.; Lescano, M.R.; Nicolau, V.V.; Zalazar, C.S.; Meira, G.R.; Estenoz, D.A. Microparticles based on ionic and organosolv lignins for the controlled release of atrazine. J. Hazard. Mater. 2018, 359, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mac Loughlin, C.; Canosa, I.S.; Silveyra, G.R.; López Greco, L.S.; Rodríguez, E.M. Effects of atrazine on growth and sex differentiation, in juveniles of the freshwater crayfish Cherax quadricarinatus. Ecotoxicol. Environ. Saf. 2016, 131, 96–103. [Google Scholar] [CrossRef]
- Tao, Q.H.; Tang, H.X. Effect of dye compounds on the adsorption of atrazine by natural sediment. Chemosphere 2004, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, L.; Dou, D.C.; Li, X.N.; Ge, J.; Li, J.L. Atrazine induced oxidative stress and mitochondrial dysfunction in quail (Coturnix C. coturnix) kidney via modulating Nrf2 signaling pathway. Chemosphere 2018, 212, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.M.; Boydston, R.A.; Peachey, R.E.; Robinson, D. Performance consistency of reduced atrazine use in sweet corn. Field Crop. Res. 2011, 121, 96–104. [Google Scholar] [CrossRef]
- Cleary, J.A.; Tillitt, D.E.; vom Saal, F.S.; Nicks, D.K.; Claunch, R.A.; Bhandari, R.K. Atrazine induced transgenerational reproductive effects in medaka (Oryzias latipes). Environ. Pollut. 2019, 251, 639–650. [Google Scholar] [CrossRef]
- Yue, L.; Ge, C.J.; Feng, D.; Yu, H.M.; Deng, H.; Fu, B.M. Adsorption–desorption behavior of atrazine on agricultural soils in China. J. Environ. Sci. 2017, 57, 180–189. [Google Scholar] [CrossRef]
- Lin, Z.; Zhen, Z.; Liang, Y.Q.; Li, J.; Yang, J.W.; Zhong, L.Y.; Zhao, L.R.; Li, Y.T.; Luo, C.L.; Ren, L.; et al. Changes in atrazine speciation and the degradation pathway in red soil during the vermiremediation process. J. Hazard. Mater. 2019, 364, 710–719. [Google Scholar] [CrossRef]
- Wang, Q.F.; Xie, S.G. Isolation and characterization of a high-efficiency soil atrazine-degrading Arthrobacter sp. strain. Int. Biodeter. Biodegr. 2012, 71, 61–66. [Google Scholar] [CrossRef]
- Fan, X.X.; Song, F.Q. Bioremediation of atrazine: Recent advances and promises. J. Soil. Sediments 2014, 14, 1727–1737. [Google Scholar] [CrossRef]
- Bohn, T.; Cocco, E.; Gourdol, L.; Guignard, C.; Hoffmann, L. Determination of atrazine and degradation products in Luxembourgish drinking water: Origin and fate of potential endocrine-disrupting pesticides. Food Addit. Contam. Part A 2011, 28, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Wujcik, E.K.; Londoño, N.J.; Duirk, S.E.; Monty, C.N.; Masel, R.I. An acetylcholinesterase-inspired biomimetic toxicity sensor. Chemosphere 2013, 91, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Casa-Resino, I.d.l.; Valdehita, A.; Soler, F.; Navas, J.M.; Pérez-López, M. Endocrine disruption caused by oral administration of atrazine in European quail (Coturnix coturnix coturnix). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.J.; Wang, Z.L.; Gao, X.J.; Chen, D.C.; Wang, L.L.; Li, S.; Xu, S.W. Atrazine and chlorpyrifos exposure induces liver autophagic response in common carp. Ecotoxicol. Environ. Saf. 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Kar, S.; Mandal, A.; Ghosh, S.; Majumdar, S. Immobilization of tannery industrial sludge in ceramic membrane preparation and hydrophobic surface modification for application in atrazine remediation from water. J. Eur. Ceram. Soc. 2019, 39, 3235–3246. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Tataraki, D.; Rassias, G. Degradation of atrazine in soil by dielectric barrier discharge plasma—Potential singlet oxygen mediation. Chem. Eng. J. 2018, 347, 682–694. [Google Scholar] [CrossRef]
- Hou, X.J.; Huang, X.P.; Ai, Z.H.; Zhao, J.C.; Zhang, L.Z. Ascorbic acid induced atrazine degradation. J. Hazard. Mater. 2017, 327, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Buser, H.R. Atrazine and other s-triazine herbicides in lakes and in rain in Switzerland. Environ. Sci. Technol. 1990, 24, 1049–1058. [Google Scholar] [CrossRef]
- Mandelbaum, R.T.; Wackett, L.P.; Allan, D.L. Mineralization of the s-triazine ring of atrazine by stable bacterial mixed cultures. Appl. Environ. Microb. 1993, 59, 1695–1701. [Google Scholar]
- Wirbisky-Hershberger, S.E.; Sanchez, O.F.; Horzmann, K.A.; Thanki, D.; Yuan, C.; Freeman, J.L. Atrazine exposure decreases the activity of DNMTs, global DNA methylation levels, and dnmt expression. Food Chem. Toxicol. 2017, 109, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Özgür, E.; Bereli, N.; Türkmen, D.; Denizli, A. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater. Sci. Eng. Part C Mater. Biol. Appl. 2017, 73, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Locoro, G.; Comero, S.; Contini, S.; Schwesig, D.; Werres, F.; Balsaa, P.; Gans, O.; Weiss, S.; Blaha, L.; et al. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 2010, 44, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Ma, J.; Jia, R.; Xue, L.Q.; Tao, C.J.; Li, C.J.; Ma, X.D.; Lin, Y. Impact of Long-Term Atrazine Use on Groundwater Safety in Jilin Province, China. J. Integr. Agric. 2013, 12, 305–313. [Google Scholar] [CrossRef]
- Farooq, M.; Bell, A.H.; Almustapha, M.N.; Andresen, J.M. Bio-methane from an-aerobic digestion using activated carbon adsorption. Anaerobe 2017, 46, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Martı́n-Gullón, I.; Font, R. Dynamic pesticide removal with activated carbon fibers. Water Res. 2001, 35, 516–520. [Google Scholar] [CrossRef]
- Shao, S.L.; Feng, Y.J.; Yu, H.R.; Li, J.Y.; Li, G.B.; Liang, H. Presence of an adsorbent cake layer improves the performance of gravity-driven membrane (GDM) filtration system. Water Res. 2017, 108, 240–249. [Google Scholar] [CrossRef]
- Amaral, P.; Partlan, E.; Li, M.; Lapolli, F.; Mefford, O.T.; Karanfil, T.; Ladner, D.A. Superfine powdered activated carbon (S-PAC) coatings on microfiltration membranes: Effects of milling time on contaminant removal and flux. Water Res. 2016, 100, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.H.; He, H.J.; Inthapanya, X.; Yang, C.P.; Lu, L.; Zeng, G.M.; Han, Z.F. Role of biochar on composting of organic wastes and remediation of contaminated soils-a review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, M.X.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Sun, H.W.; Yu, L.; Sun, T.H. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Ouyang, W.; Hao, F.H.; Lin, C.Y.; Wang, F.L.; Han, S.; Geng, X.J. Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour. Technol. 2013, 147, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Singh, N. Surfactant-modified bentonite clays: Preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res. 2015, 22, 3876–3885. [Google Scholar] [CrossRef]
- Jamil, T.S.; Gad-Allah, T.A.; Ibrahim, H.S.; Saleh, T.S. Adsorption and isothermal models of atrazine by zeolite prepared from Egyptian kaolin. Solid State Sci. 2011, 13, 198–203. [Google Scholar] [CrossRef]
- Gkementzoglou, C.; Kotrotsiou, O.; Koronaiou, M.; Kiparissides, C. Development of a sandwich-type filtration unit packed with MIP nanoparticles for removal of atrazine from water sources. Chem. Eng. J. 2016, 287, 233–240. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.G.; Huang, R.F.; Xie, X.W.; Guo, L.H.; Zhang, M.Y. Synthesis of a molecularly imprinted polymer on mSiO2@Fe3O4 for the selective adsorption of atrazine. J. Sep. Sci. 2018, 41, 2837–2845. [Google Scholar] [CrossRef]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.P.; Dong, C.D.; Tang, Z.H. Advanced chemical oxidation: Its present role and potential future in hazardous waste treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Chu, W.; Chan, K.H.; Kwan, C.Y.; Choi, K.Y. Degradation of atrazine by modified stepwise-Fenton’s processes. Chemosphere 2007, 67, 755–761. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Du, Y.X.; Liu, D.Q.; Bian, W.J. The role of dissolved oxygen in the Ta(O)N-driven visible Fenton-like degradation of atrazine. J. Environ. Chem. Eng. 2014, 2, 1691–1698. [Google Scholar] [CrossRef]
- Yang, Z.C.; Yu, A.Q.; Shan, C.; Gao, G.D.; Pan, B.C. Enhanced Fe (III)-mediated Fenton oxidation of atrazine in the presence of functionalized multi-walled carbon nanotubes. Water Res. 2018, 137, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Quan, X.; Chen, S.; Yu, H.T.; Zhang, Y.B.; Zhao, H.M. Enhanced electro-Fenton performance by fluorine-doped porous carbon for removal of organic pollutants in wastewater. Chem. Eng. J. 2018, 354, 606–615. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Wan, J.; Gong, X.M.; Zhu, Y. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Romero, R.; Contreras, D.; Sepúlveda, M.; Moreno, N.; Segura, C.; Melin, V. Assessment of a Fenton reaction driven by insoluble tannins from pine bark in treating an emergent contaminant. J. Hazard. Mater. 2020, 382, 120982. [Google Scholar] [CrossRef]
- Khandarkhaeva, M.; Batoeva, A.; Aseev, D.; Sizykh, M.; Tsydenova, O. Oxidation of atrazine in aqueous media by solar-enhanced Fenton-like process involving persulfate and ferrous ion. Ecotoxicol. Environ. Saf. 2017, 137, 35–41. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Zhao, D.Y. Effects of oil dispersant on ozone oxidation of phenanthrene and pyrene in marine water. Chemosphere 2017, 172, 468–475. [Google Scholar] [CrossRef]
- Zhu, S.M.; Dong, B.Z.; Yu, Y.H.; Bu, L.J.; Deng, J.; Zhou, S.Q. Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]
- Yuan, X.J.; Xie, R.L.; Zhang, Q.; Sun, L.; Long, X.J.; Xia, D.S. Oxygen functionalized graphitic carbon nitride as an efficient metal-free ozonation catalyst for atrazine removal: Performance and mechanism. Sep. Purif. Technol. 2019, 211, 823–831. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.D.; Ma, J.; Lu, X.H.; Qi, J.Y.; Song, S. Strong promoted catalytic ozonation of atrazine at low temperature using tourmaline as catalyst: Influencing factors, reaction mechanisms and pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Saylor, G.L.; Zhao, C.; Kupferle, M.J. Synergistic enhancement of oxidative degradation of atrazine using combined electrolysis and ozonation. J. Water Process Eng. 2018, 21, 154–162. [Google Scholar] [CrossRef]
- Yuan, X.J.; Qin, W.L.; Lei, X.M.; Sun, L.; Li, Q.; Li, D.Y.; Xu, H.M.; Xia, D.S. Efficient enhancement of ozonation performance via ZVZ immobilized g-C3N4 towards superior oxidation of micropollutants. Chemosphere 2018, 205, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Li, J.; Dong, W.Y.; Ma, J.; Cao, J.; Li, T.T.; Li, J.Y.; Gu, J.; Liu, P.X. Study on enhanced degradation of atrazine by ozonation in the presence of hydroxylamine. J. Hazard. Mater. 2016, 316, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Q.; Bu, L.J.; Shi, Z.; Bi, C.; Yi, Q.H. A novel advanced oxidation process using iron electrodes and ozone in atrazine degradation: Performance and mechanism. Chem. Eng. J. 2016, 306, 719–725. [Google Scholar] [CrossRef]
- Yang, Y.X.; Cao, H.B.; Peng, P.; Bo, H.M. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J. Hazard. Mater. 2014, 279, 444–451. [Google Scholar] [CrossRef]
- Yuan, X.J.; Yan, X.; Xu, H.M.; Li, D.Y.; Sun, L.; Cao, G.; Xia, D.S. Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: Performance, kinetics and effects. J. Environ. Sci. 2017, 61, 3–13. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Garcia-Cañibano, C.; Sarro, M.; Encinas, Á.; Medana, C.; Fabbri, D.; Calza, P.; Marugán, J. Evaluation of transformation products from chemical oxidation of micropollutants in wastewater by photoassisted generation of sulfate radicals. Chemosphere 2019, 226, 509–519. [Google Scholar] [CrossRef]
- Chen, L.W.; Hu, X.X.; Yang, Y.; Jiang, C.L.; Bian, C.; Liu, C.; Zhang, M.Y.; Cai, T.M. Degradation of atrazine and structurally related s-triazine herbicides in soils by ferrous-activated persulfate: Kinetics, mechanisms and soil-types effects. Chem. Eng. J. 2018, 351, 523–531. [Google Scholar] [CrossRef]
- Wu, S.H.; He, H.J.; Li, X.; Yang, C.P.; Zeng, G.M.; Wu, B.; He, S.Y.; Lu, L. Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: Performances and mechanisms. Chem. Eng. J. 2018, 341, 126–136. [Google Scholar] [CrossRef]
- Wu, S.H.; Li, H.R.; Li, X.; He, H.J.; Yang, C.P. Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants. Chem. Eng. J. 2018, 353, 533–541. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Fu, C.X.; Wang, Z.Y.; Zhang, X.L.; Yang, J.X.; Hogland, W.; Gao, L. Kinetics and pathway of atrazine degradation by a novel method: Persulfate coupled with dithionite. Chem. Eng. J. 2019, 373, 803–813. [Google Scholar] [CrossRef]
- Peng, J.L.; Lu, X.H.; Jiang, X.; Zhang, Y.H.; Chen, Q.X.; Lai, B.; Yao, G. Degradation of atrazine by persulfate activation with copper sulfide (CuS): Kinetics study, degradation pathways and mechanism. Chem. Eng. J. 2018, 354, 740–752. [Google Scholar] [CrossRef]
- Xu, X.M.; Chen, W.M.; Zong, S.Y.; Ren, X.; Liu, D. Atrazine degradation using Fe3O4-sepiolite catalyzed persulfate: Reactivity, mechanism and stability. J. Hazard. Mater. 2019, 377, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.J.; Zhan, G.M.; Huang, X.P.; Wang, N.; Ai, Z.H.; Zhang, L.Z. Persulfate Activation Induced by Ascorbic Acid for Efficient Organic Pollutants Oxidation. Chem. Eng. J. 2019, 382, 122355. [Google Scholar] [CrossRef]
- Li, J.; Wan, Y.J.; Li, Y.J.; Yao, G.; Lai, B. Surface Fe (III)/Fe (II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Appl. Catal. Part B Environ. 2019, 256, 117782. [Google Scholar] [CrossRef]
- Dangwang, D.J.M.; Gong, Y.; Noumi, G.B.; Sieliechi, J.M.; Zhao, X.; Ma, N.; Yang, M.; Tchatchueng, J.B. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere 2019, 217, 833–842. [Google Scholar] [CrossRef]
- He, H.J.; Cheng, Y.; Yang, C.P.; Zeng, G.M.; Zhu, C.Y.; Yan, Z. Influences of anion concentration and valence on dispersion and aggregation of titanium dioxide nanoparticles in aqueous solutions. J. Environ. Sci. 2017, 54, 135–141. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. Part B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- He, H.J.; Wu, B.; Yang, C.P. Effects of fulvic acids and electrolytes on colloidal stability and photocatalysis of nano-TiO2 for atrazine removal. Int. J. Environ. Sci. Technol. 2018, 16, 7275–7284. [Google Scholar] [CrossRef]
- Sun, S.W.; He, H.J.; Yang, C.P.; Cheng, Y.; Liu, Y.P. Effects of Ca2+ and fulvic acids on atrazine degradation by nano-TiO2: Performances and mechanisms. Sci. Rep. 2019, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Yola, M.L.; Eren, T.; Atar, N. A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for photocatalytic degradation of atrazine. Chem. Eng. J. 2014, 250, 288–294. [Google Scholar] [CrossRef]
- Belver, C.; Han, C.; Rodriguez, J.J.; Dionysiou, D.D. Innovative W-doped titanium dioxide anchored on clay for photocatalytic removal of atrazine. Catal. Today 2017, 280, 21–28. [Google Scholar] [CrossRef]
- Sudrajat, H.; Sujaridworakun, P. Correlation between particle size of Bi2O3 nanoparticles and their photocatalytic activity for degradation and mineralization of atrazine. J. Mol. Liq. 2017, 242, 433–440. [Google Scholar] [CrossRef]
- Sudrajat, H. Reducing agent-free formation of Cu (I) nanoclusters on gC3N4 for enhanced photocatalysis. J. Alloys Compd. 2017, 716, 119–127. [Google Scholar] [CrossRef]
- Li, K.X.; Huang, Y.; Yan, L.S.; Dai, Y.H.; Xue, K.P.; Guo, H.Q.; Huang, Z.M.; Xiong, J.J. Simulated sunlight photodegradation of aqueous atrazine and rhodamine B catalyzed by the ordered mesoporous graphene–titania/silica composite material. Catal. Commun. 2012, 18, 16–20. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Han, C.; Nadagouda, M.N.; Dionysiou, D.D. The fabrication of innovative single crystal N, F-codoped titanium dioxide nanowires with enhanced photocatalytic activity for degradation of atrazine. Appl. Catal. Part B Environ. 2015, 168–169, 550–558. [Google Scholar] [CrossRef]
- Shamsedini, N.; Dehghani, M.; Nasseri, S.; Baghapour, M.A. Photocatalytic degradation of atrazine herbicide with Illuminated Fe+3-TiO2 Nanoparticles. J. Environ. Health Sci. Eng. 2017, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Samsudin, E.M.; Abd Hamid, S.B.; Juan, J.C.; Basirun, W.J.; Kandjani, A.E.; Bhargava, S.K. Controlled nitrogen insertion in titanium dioxide for optimal photocatalytic degradation of atrazine. RSC Adv. 2015, 5, 44041–44052. [Google Scholar] [CrossRef]
- Xu, L.; Zang, H.M.; Zhang, Q.; Chen, Y.; Wei, Y.G.; Yan, J.H.; Zhao, Y.H. Photocatalytic degradation of atrazine by H3PW12O40/Ag–TiO2: Kinetics, mechanism and degradation pathways. Chem. Eng. J. 2013, 232, 174–182. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ma, F.; Feng, C.J.; Bai, S.W.; Yang, J.X.; Wang, L. Complete genome sequence of Arthrobacter sp. ZXY-2 associated with effective atrazine degradation and salt adaptation. J. Biotechnol. 2017, 248, 43–47. [Google Scholar] [CrossRef]
- El Sebaï, T.; Devers-Lamrani, M.; Changey, F.; Rouard, N.; Martin-Laurent, F. Evidence of atrazine mineralization in a soil from the Nile Delta: Isolation of Arthrobacter sp. TES6, an atrazine-degrading strain. Int. Biodeterior. Biodegrad. 2011, 65, 1249–1255. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhu, L.S.; Wang, Q.; Wang, J.; Xie, H. Isolation and Characterization of Atrazine Mineralizing Bacillus subtilis Strain HB-6. PLoS ONE 2014, 9, e107270. [Google Scholar] [CrossRef] [PubMed]
- Tonelli Fernandes, A.F.; Braz, V.S.; Bauermeister, A.; Rizzato Paschoal, J.A.; Lopes, N.P.; Stehling, E.G. Degradation of atrazine by Pseudomonas sp. and Achromobacter sp. isolated from Brazilian agricultural soil. Int. Biodeterior. Biodegrad. 2018, 130, 17–22. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, L.; Ma, F.; Bai, S.W.; Yang, J.X.; Qi, S.S. Pseudomonas sp. ZXY-1, a newly isolated and highly efficient atrazine-degrading bacterium, and optimization of biodegradation using response surface methodology. J. Environ. Sci. 2017, 54, 152–159. [Google Scholar] [CrossRef]
- Ma, L.M.; Chen, S.S.; Yuan, J.; Yang, P.P.; Liu, Y.P.; Stewart, K. Rapid biodegradation of atrazine by Ensifer sp. strain and its degradation genes. Int. Biodeterior. Biodegrad. 2017, 116, 133–140. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Sharma, A.; Sagarkar, S.; Kapley, A. Mapping atrazine and phenol degradation genes in Pseudomonas sp. EGD-AKN5. Biochem. Eng. J. 2015, 102, 125–134. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhu, L.S.; Liu, A.J.; Ma, T.T.; Wang, Q.; Xie, H.; Wang, J.; Jiang, T.; Zhao, R.S. Isolation and characterization of an Arthrobacter sp. strain HB-5 that transforms atrazine. Environ. Geochem. Health 2011, 33, 259–266. [Google Scholar] [CrossRef]
- Bastos, A.C.; Magan, N. Trametes versicolor: Potential for atrazine bioremediation in calcareous clay soil, under low water availability conditions. Int. Biodeterior. Biodegrad. 2009, 63, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Mandelbaum, R.T.; Allan, D.L.; Wackett, L.P. Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl. Environ. Microb. 1995, 61, 1451–1457. [Google Scholar]
- Yang, X.Y.; Wei, H.Y.; Zhu, C.X.; Geng, B. Biodegradation of atrazine by the novel Citricoccus sp. strain TT3. Ecotoxicol. Environ. Saf. 2018, 147, 144–150. [Google Scholar] [CrossRef]
- Getenga, Z.; Dörfler, U.; Iwobi, A.; Schmid, M.; Schroll, R. Atrazine and terbuthylazine mineralization by an Arthrobacter sp. isolated from a sugarcane-cultivated soil in Kenya. Chemosphere 2009, 77, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, X.Y.; Wang, Z.Y.; Cao, B.; Deng, S.J.; Bi, M.C.; Zhang, Y. Enhanced biodegradation of atrazine by Arthrobacter sp. DNS10 during co-culture with a phosphorus solubilizing bacteria: Enterobacter sp. P1. Ecotoxicol. Environ. Saf. 2019, 172, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.M.; Wang, L.; Ma, F.; Yang, J.X.; Bai, S.S.; You, J.Y. Self-immobilized biomixture with pellets of Aspergillus niger Y3 and Arthrobacter. sp. ZXY-2 to remove atrazine in water: A bio-functions integration system. Sci. Total Environ. 2019, 689, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Modin, O.; Yamamoto, K.; Fukushi, K.; Nakajima, F.; Nghiem, L.D. Pesticide removal by a mixed culture of bacteria and white-rot fungi. J. Taiwan Inst. Chem. Eng. 2012, 43, 459–462. [Google Scholar] [CrossRef] [Green Version]
- Merini, L.J.; Bobillo, C.; Cuadrado, V.; Corach, D.; Giulietti, A.M. Phytoremediation potential of the novel atrazine tolerant Lolium multiflorum and studies on the mechanisms involved. Environ. Pollut. 2009, 157, 3059–3063. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, F.J.; Cañizares, P.; Rodríguez, L. Assessing the phytoremediation potential of crop and grass plants for atrazine-spiked soils. Chemosphere 2017, 185, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.T.; Tyler, H.L.; Locke, M.A. Aqueous pesticide mitigation efficiency of Typha latifolia (L.), Leersia oryzoides (L.) Sw., and Sparganium americanum Nutt. Chemosphere 2013, 92, 1307–1313. [Google Scholar] [CrossRef]
- Zhang, J.J.; Gao, S.; Xu, J.Y.; Lu, Y.C.; Lu, F.F.; Ma, L.Y.; Su, X.N.; Yang, H. Degrading and Phytoextracting Atrazine Residues in Rice (Oryza sativa) and Growth Media Intensified by a Phase II Mechanism Modulator. Environ. Sci. Technol. 2017, 51, 11258–11268. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, F.J.; Rodrigo, M.A.; Rodríguez, L. Electrokinetic-assisted phytoremediation of atrazine: Differences between electrode and interelectrode soil sections. Sep. Purif. Technol. 2019, 211, 19–27. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, J.; Rodrigo, M.A.; Rodríguez, L. Enhancing the removal of atrazine from soils by electrokinetic-assisted phytoremediation using ryegrass (Lolium perenne L.). Chemosphere 2019, 232, 204–212. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhong, D.X.; Du, Y.Z.; Lu, S.Y.; Fu, D.Q.; Li, Z.; Li, X.D.; Chi, Y.; Luo, Y.M.; Yan, J.H. Emission and Control Characteristics for Incineration of Sedum Plumbizincicola Biomass in a Laboratory-Scale Entrained Flow Tube Furnace. Int. J. Phytoremediat. 2013, 15, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, L.; Ma, F.; Yang, J.X.; Qi, S.S.; Zhao, T. The effect of Funnelliformis mosseae inoculation on the phytoremediation of atrazine by the aquatic plant Canna indica L. var. flava Roxb. RSC Adv. 2016, 6, 22538–22549. [Google Scholar] [CrossRef]
- Bazhanov, D.P.; Yang, K.; Li, H.M.; Li, C.Y.; Li, J.S.; Chen, X.F.; Yang, H.T. Colonization of plant roots and enhanced atrazine degradation by a strain of Arthrobacter ureafaciens. Appl. Microbiol. Biotechnol. 2017, 101, 6809–6820. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Singh, D.K.; Khankhane, P.J. Enhanced atrazine removal by hydrophyte-bacterium associations and in vitro screening of the isolates for their plant growth-promoting potential. Int. J. Phytoremediat. 2018, 20, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.T.; Wong, Y.S.; Tam, N.F.Y. Removal and biodegradation of nonylphenol by immobilized Chlorella vulgaris. Bioresour. Technol. 2011, 102, 10230–10238. [Google Scholar] [CrossRef]
- Shin, D.C.; Kim, J.S.; Park, C.H. Study on physical and chemical characteristics of microorganism immobilized media for advanced wastewater treatment. J. Water Process Eng. 2019, 29, 100784. [Google Scholar] [CrossRef]
- Wu, X.; He, H.J.; Yang, W.L.; Yu, J.P.; Yang, C.P. Efficient removal of atrazine from aqueous solutions using magnetic Saccharomyces cerevisiae bionanomaterial. Appl. Microbiol. Biotechnol. 2018, 102, 7597–7610. [Google Scholar] [CrossRef]
- Yu, J.P.; He, H.J.; Yang, W.L.; Yang, C.P.; Zeng, G.M.; Wu, X. Magnetic bionanoparticles of Penicillium sp. yz11-22N2 doped with Fe3O4 and encapsulated within PVA-SA gel beads for atrazine removal. Bioresour. Technol. 2018, 260, 196–203. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Yang, W.L.; He, H.J.; Yang, C.P.; Yu, J.P.; Wu, X.; Zeng, G.M.; Tarre, S.; Green, M. Preparation, performances and mechanisms of magnetic Saccharomyces cerevisiae bionanocomposites for atrazine removal. Chemosphere 2018, 200, 380–387. [Google Scholar] [CrossRef]
- Liu, J.W.; Pan, D.D.; Wu, X.W.; Chen, H.Y.; Cao, H.Q.; Li, Q.X.; Hua, R.M. Enhanced degradation of prometryn and other s-triazine herbicides in pure cultures and wastewater by polyvinyl alcohol-sodium alginate immobilized Leucobacter sp. JW-1. Sci. Total Environ. 2018, 615, 78–86. [Google Scholar] [CrossRef]
- Ying, Z.; Zhang, Q.Y.; Chao, N.; Ge, S.J.; Zhao, J.; Miao, H.; Bo, C. Biodegradation of atrazine by free and immobilized cells of Arthrobacter sp. strain DNS10. Environ. Eng. Manag. J. 2015, 14, 819–826. [Google Scholar] [CrossRef]
- Desitti, C.; Beliavski, M.; Tarre, S.; Avrahami, R.; Zussman, E.; Green, M. Durable electrospun microtubes for encapsulation of bacteria in atrazine bioremediation. J. Water Process Eng. 2017, 19, 205–211. [Google Scholar] [CrossRef]
- Abigail M, E.A.; Das, N. Removal of atrazine from aqueous environment using immobilized Pichia kudriavzevii Atz-EN-01 by two different methods. Int. Biodeterior. Biodegrad. 2015, 104, 53–58. [Google Scholar] [CrossRef]
- Pannier, A.; Lehrer, T.; Vogel, M.; Soltmann, U.; Bottcher, H.; Tarre, S.; Green, M.; Raff, J.; Pollmann, K. Long-term activity of biohybrid coatings of atrazine-degrading bacteria Pseudomonas sp. ADP. RSC Adv. 2014, 4, 19970–19979. [Google Scholar] [CrossRef] [Green Version]
| Reaction system | Removal Effect |
|---|---|
| Fe2+/H2O2 | The kinetic constant of atrazine degradation achieved, and the Fenton system could effectively remove atrazine [43]. |
| Steel converter slag (SCS)/H2O2 | The degradation rate of atrazine was 93.7% under the optimal conditions [44]. |
| Fe3+/ tannins /H2O2 | Under the optimal conditions, the degradation efficiency of atrazine reached 98% after 30 min. reaction [45]. |
| UV/ S2O82−/Fe2+/H2O2 | The system had obvious synergistic effects and could completely degrade atrazine after 30 min. of reaction [46]. |
| Technical Method | Removal Effect |
|---|---|
| Zn0 immobilized g-C3N4 catalyzed ozonation | The composite exhibited superior degradation activity with an improvement of 61.2% on atrazine in 1.5 min reaction [52]. |
| Hydroxylamine catalyzed ozonation | Approximately 80% of atrazine was degraded by ozonation in the presence of hydroxylamine, while only 20% of atrazine was degraded by ozonation alone [53]. |
| Iron electrode catalyzed ozonation | When the applied current increased to 20 mA, the removal rate of atrazine increased to 89.8% which the rate was significantly improved compared with ozonation alone [54]. |
| TiO2 catalyzed ozonation | Compared with the separate ozonation system, the TiO2-ozone system could produce more ·OH, and the degradation rate of atrazine reached 93% after 30 minutes of reaction [55]. |
| Nano-ZnO catalyzed ozonation | The system showed obvious synergistic effect, the degradation efficiency of the system to atrazine was increased by 41.8%, and the degradation reaction was accorded with the pseudo-first-order kinetics [56]. |
| Activation Method | Removal Effect |
|---|---|
| Dithionite activated PS | The system could completely degrade atrazine within 90 min. and the degradation reaction followed the pseudo-first-order kinetics [61]. |
| Copper sulfide (CuS) activated PS | The removal efficiency of atrazine by the system was 91.5% after 40 min. reaction when the concentrations of PS and CuS were 4.0 and 25 mmol/L, respectively [62]. |
| Fe3O4−sepiolite composite activated PS | As the PS concentration of 92 mmol/L, the system could remove 71.6% of atrazine after 60 min. reaction [63]. |
| Ascorbic acid (AA) activated PS | When added AA to the reaction system, the degradation rate of atrazine was increased by 29 times [64]. |
| Fe3O4−hydroxylamine activated PMS | The degradation rate of the system to atrazine was 38 times comparing to the Fe3O4/PMS system [65]. |
| Graphitic-carbon nitride composites activated PMS | Under the irradiation of xenon lamp, the system could achieve the removal of 78.76% atrazine in 120 min. reaction [66]. |
| Photocatalyst | Preparation Method | Removal Effect |
|---|---|---|
| Ordered mesoporous graphene–TiO2/SiO2 composite material | Used a direct sol–gel co-condensation method | The degradation efficiency of atrazine by the composite reached 93.1% after 180 minutes of xenon lamp irradiation [75]. |
| N, F-codoped TiO2 nanowires | Synthesized by hydrothermal method using isopropanol as a protective capping agent | The material could effectively degrade atrazine, and the removal rate exceeded 60% after 6.0 h of visible light irradiation [76]. |
| Fe3+-TiO2 | Prepared by a cell gel method | After exposure to UV for 2.0 h, the degradation efficiency of the catalyst to atrazine was as high as 99.5% [77]. |
| N-TiO2 | A modified sol-gel method was employed to prepare the material | The removal rate of atrazine by the material reached 79% after 2.0 h of visible light irradiation [78]. |
| H3PW12O40/Ag-TiO2 | Preparation of the nanocomposite by single-step sol-gel-hydrothermal method | Under the xenon lamp, the degradation rate of atrazine by the nanocomposite was 2.4 times faster than TiO2 alone, and the degradation reaction followed the pseudo-first reaction kinetics [79]. |
| Strain Name | Strain Source | Strain Category | Removal Effect |
|---|---|---|---|
| ZXY-2 | Soil samples near a pesticide factory | Arthrobacter | Complete degradation of 100 mg/L atrazine within 15 h [80]. |
| TES6 | Corn field | Arthrobacter | 30 mg/L of atrazine was completely degraded after 3.0 h [81]. |
| HB-6 | Industrial wastewater | Bacillus subtilis | The degradation rate of 200 mg/L atrazine reached 90% after 24 h [82]. |
| A02 | Soil samples | Pseudomonas | After 24 h, the degradation rate of 100 mg/L atrazine was 99% [83]. |
| ZXY-1 | Soil samples | Pseudomonas | 100 mg/L atrazine could be completely degraded within 11 h, and the degradation rate was 9.09 mg/(L·h) [84]. |
| CX-T | Industrial soil | Ensifer | Complete degradation of 100 mg/L atrazine within 30 h [85]. |
| EGD-AKN5 | Sugarcane field | Pseudomonas | Degradation efficiency of 100 mg/L atrazine exceeded 80% within 30 h [86]. |
| HB-5 | Industrial wastewater | Arthrobacter | After 18 h, the removal rate of 100 mg/L atrazine was 100% [87]. |
| Trametes versicolor | Wet sawdust | Coriolus versicolor | The degradation rate of atrazine in soil reached 96% after 24 weeks [88]. |
| Material-Microbial Composite | Preparation Method | Removal Effect |
|---|---|---|
| Fe3O4-Saccharomyces cerevisiae (S. cerevisiae) | Nano-Fe3O4 and S. cerevisiae were encapsulated in a sodium alginate-polyvinyl alcohol matrix | The removal rate of 50 mg/L atrazine by the microspheres was 95.53% under the conditions of 28 °C, pH 7.0 and 150 rpm [107]. |
| Fe3O4-Penicillium sp. yz11-22N2 | Penicillium sp. yz11-22N2 and nano Fe3O4 were entrapped in polyvinyl alcohol-sodium alginate gel beads | Under the optimal conditions, the new biomaterial had a removal efficiency of 91.2% for 8.0 mg/L atrazine [108]. |
| Fe3O4-chitosan (CS)- S. cerevisiae | S. cerevisiae and nano Fe3O4 linked with CS through epichlorohydrin were encapsulated in calcium alginate | The removal rate of 2.0 mg/L atrazine was 88% at 25 °C and pH 7.0, and the recycled biomaterial still had a good removal capacity [109]. |
| Polyvinyl alcohol-sodium alginate (PVA-SA)-Leucobacter sp. JW-1 cells | Leucobacter sp. JW-1 cells were immobilized in PVA-SA beads by immobilized microorganism technique | The new material could completely degraded 50 mg/L of atrazine within 2 days [110]. |
| Sodium alginate (SA)- Arthrobacter sp. DNS10 | Arthrobacter sp. DNS10 was immobilized by a SA gel matrix | Under the optimal conditions, the removal rate of 100 mg/L atrazine by the material was 99.67% within 36 h [111]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. https://doi.org/10.3390/ijerph16245129
He H, Liu Y, You S, Liu J, Xiao H, Tu Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. International Journal of Environmental Research and Public Health. 2019; 16(24):5129. https://doi.org/10.3390/ijerph16245129
Chicago/Turabian StyleHe, Huijun, Yongpan Liu, Shaohong You, Jie Liu, He Xiao, and Zhihong Tu. 2019. "A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment" International Journal of Environmental Research and Public Health 16, no. 24: 5129. https://doi.org/10.3390/ijerph16245129
APA StyleHe, H., Liu, Y., You, S., Liu, J., Xiao, H., & Tu, Z. (2019). A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. International Journal of Environmental Research and Public Health, 16(24), 5129. https://doi.org/10.3390/ijerph16245129






