Study on Electrochemical Degradation of Nicosulfuron by IrO2-Based DSA Electrodes: Performance, Kinetics, and Degradation Mechanism
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Analytical Methods
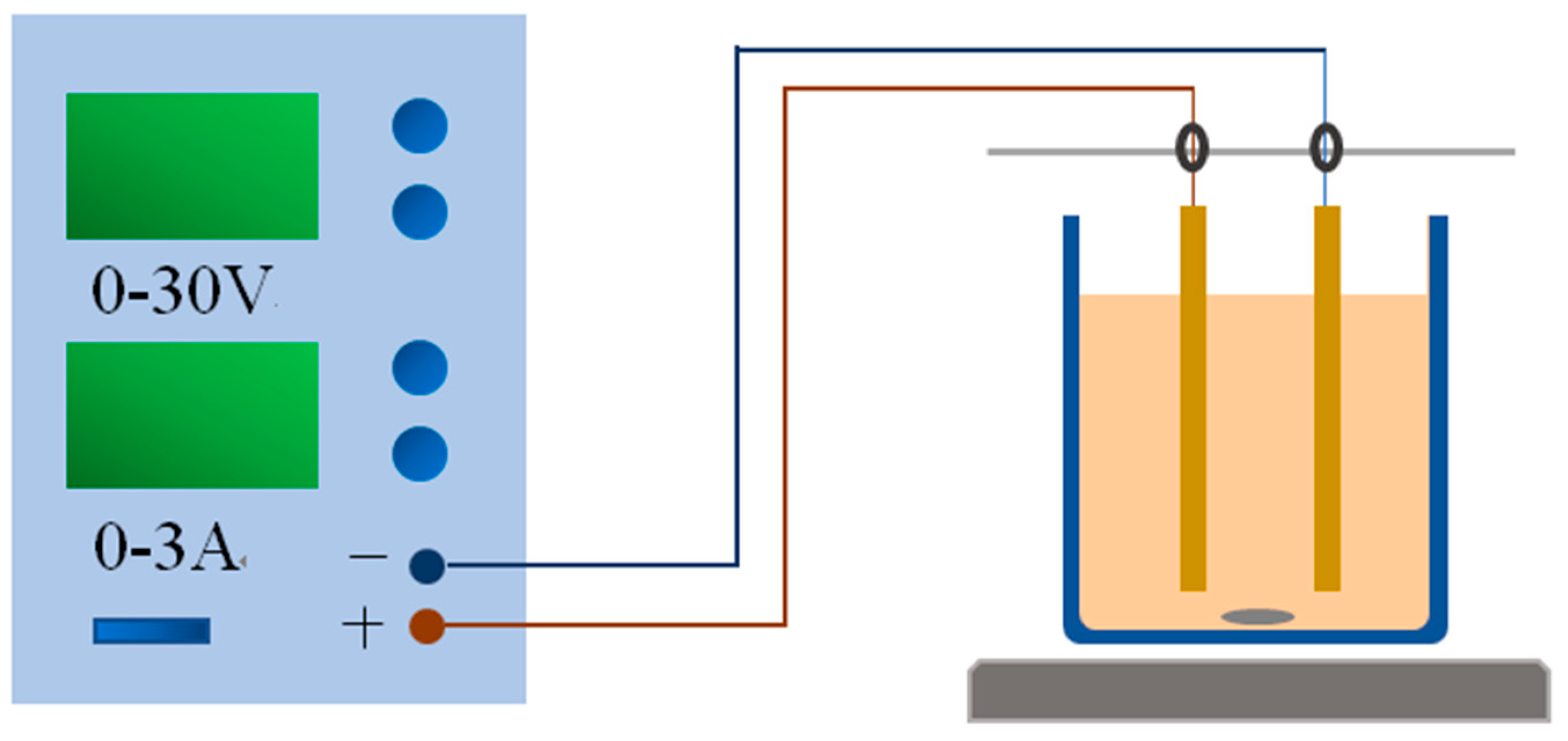
2.3. Electrolysis Experiment
3. Results and Discussion
3.1. Characterization of IrO2-Based Electrodes
3.1.1. Structure and Morphology of the Electrode Coatings
3.1.2. Linear Sweep Voltammetry Measurement (LSV)
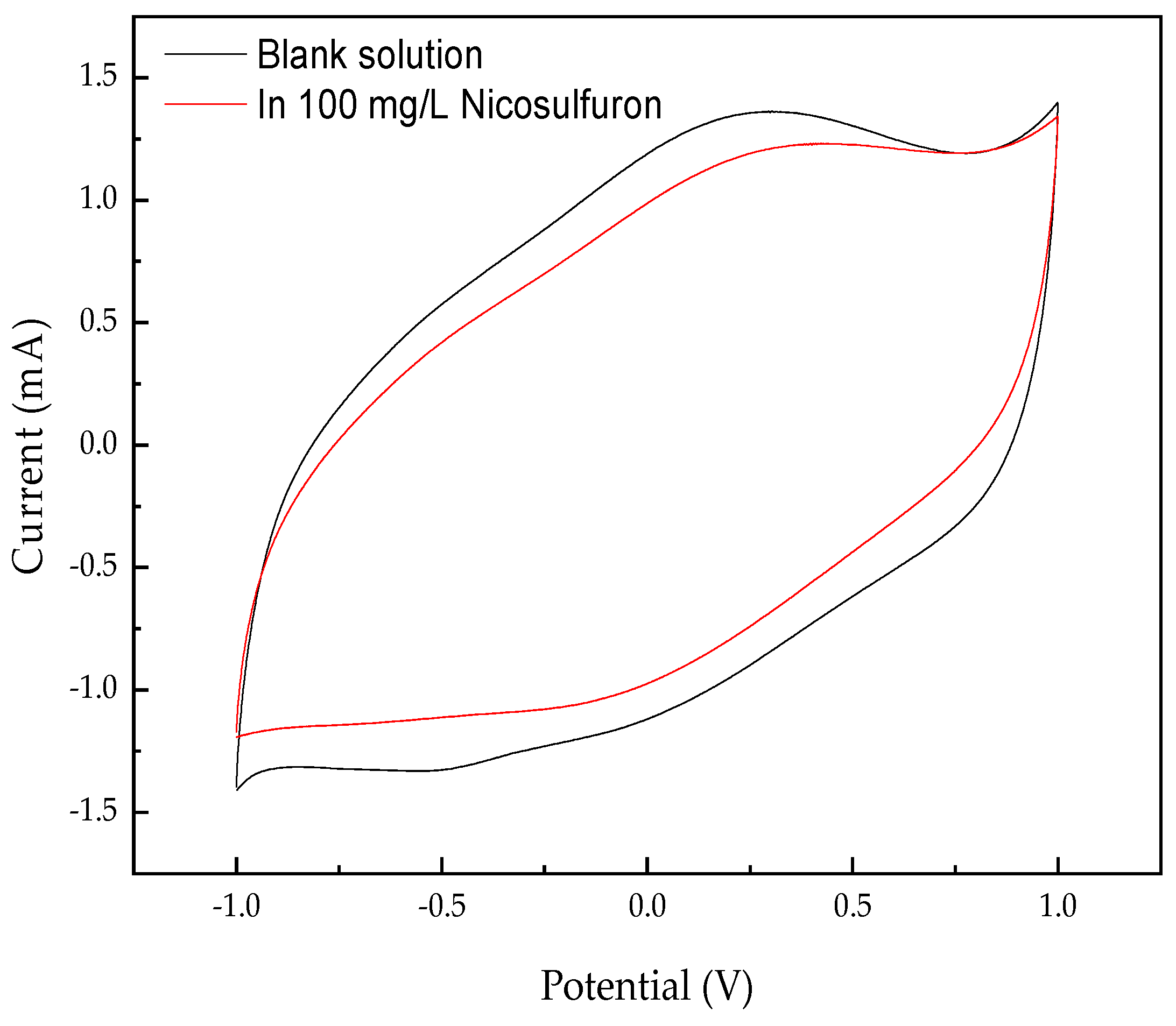
3.1.3. Cyclic Voltammetry (CV)
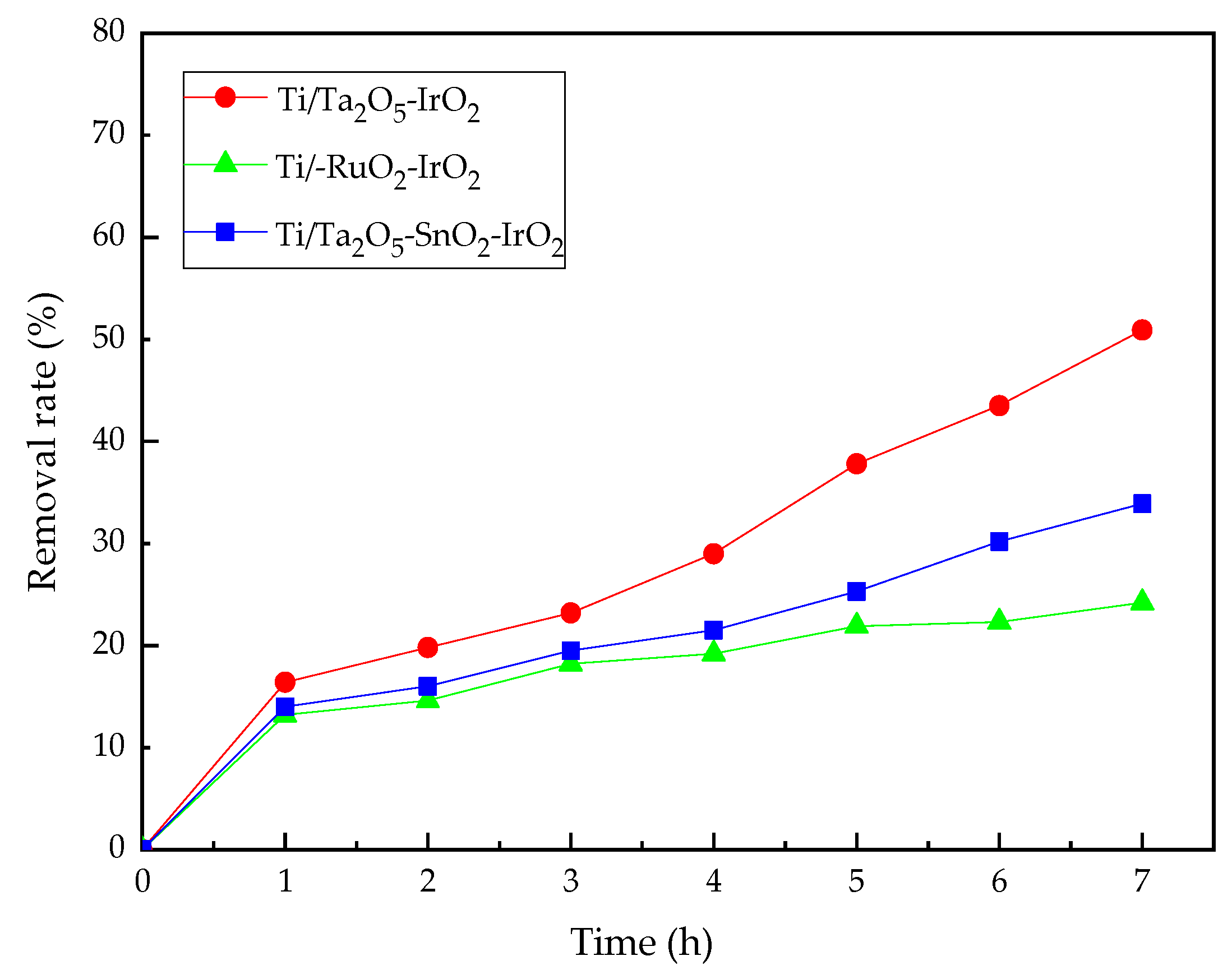
3.2. The Effect of Electrode Material
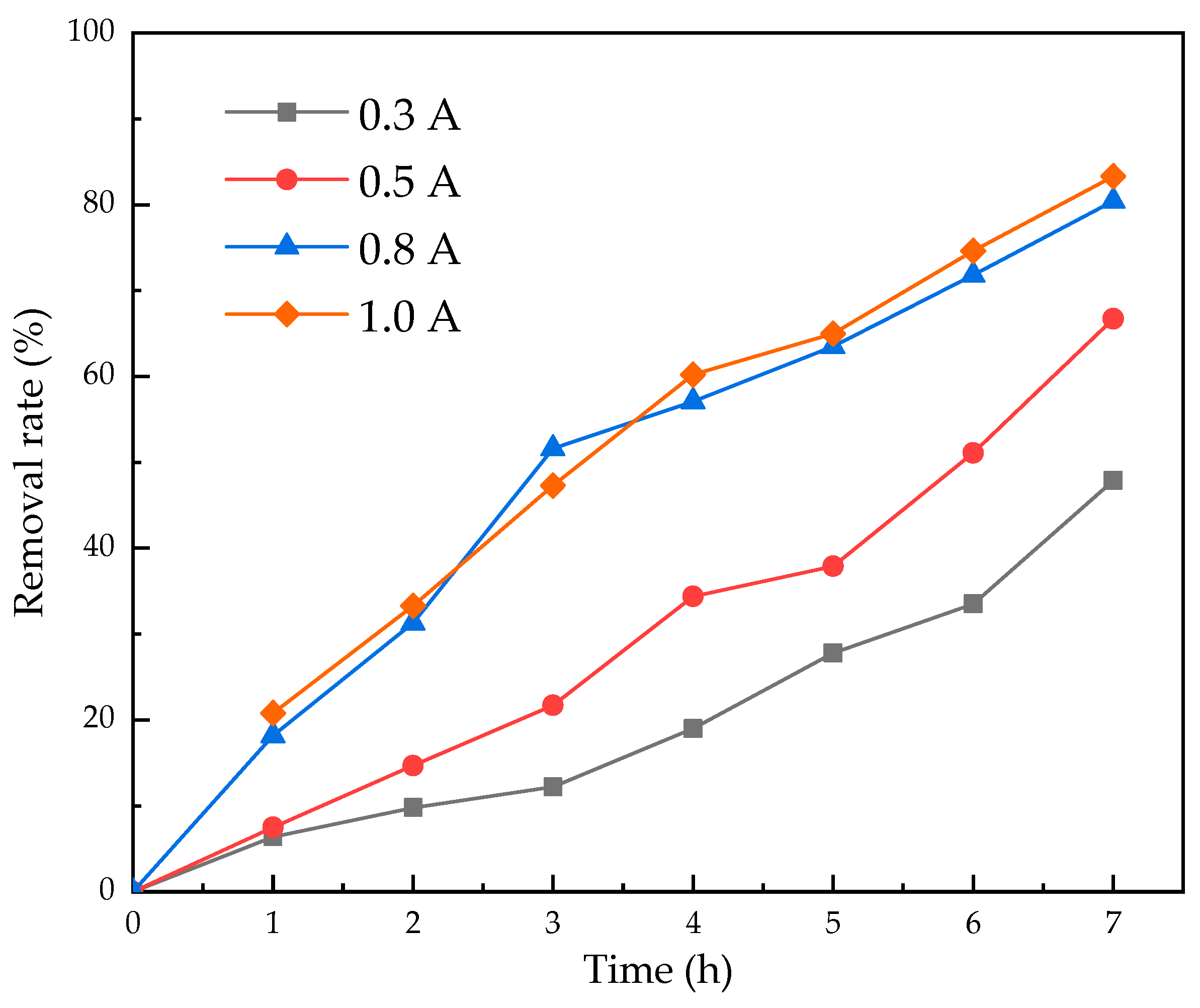
3.3. The Effect of Current Intensity
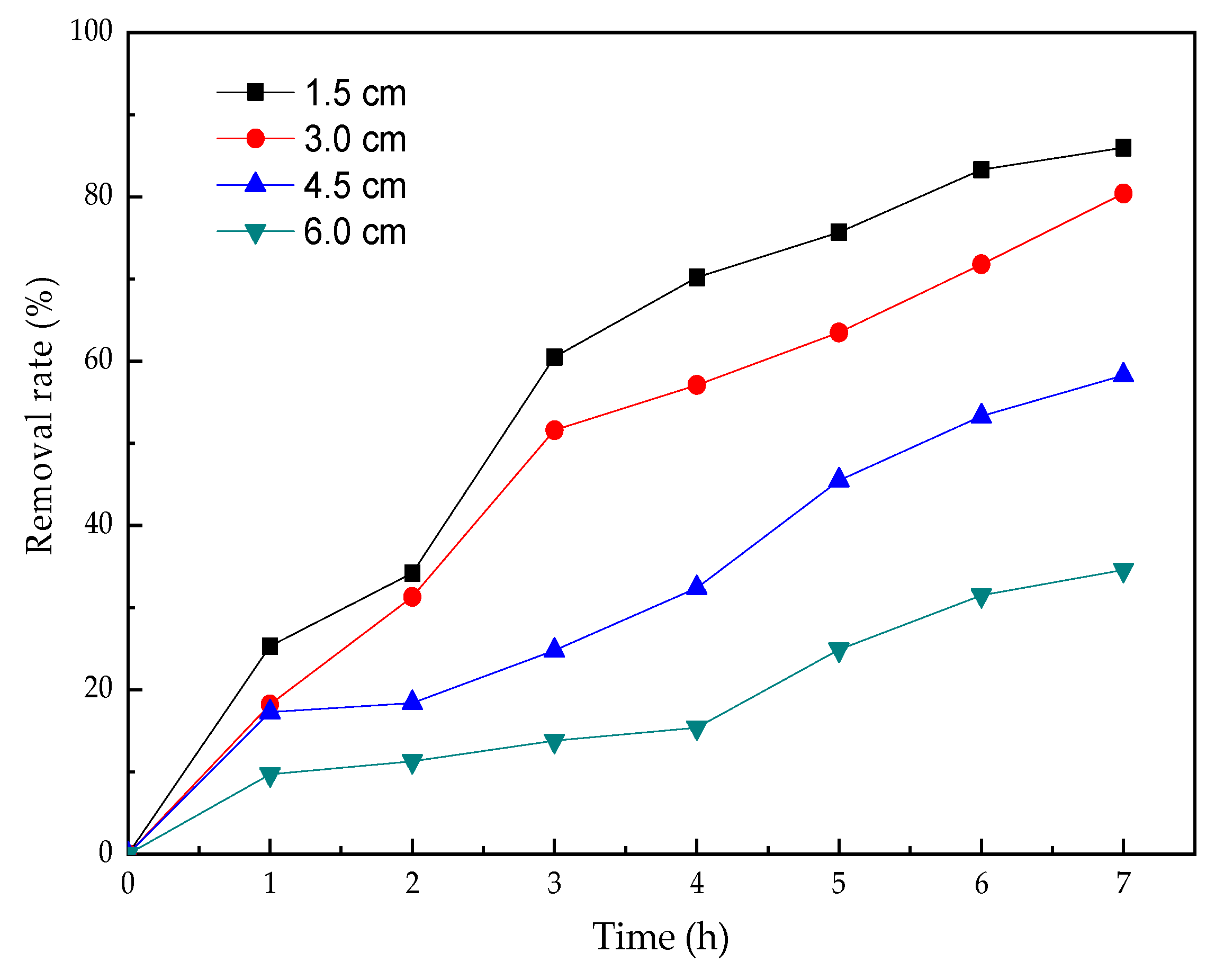
3.4. The Effect of Electrode Spacing
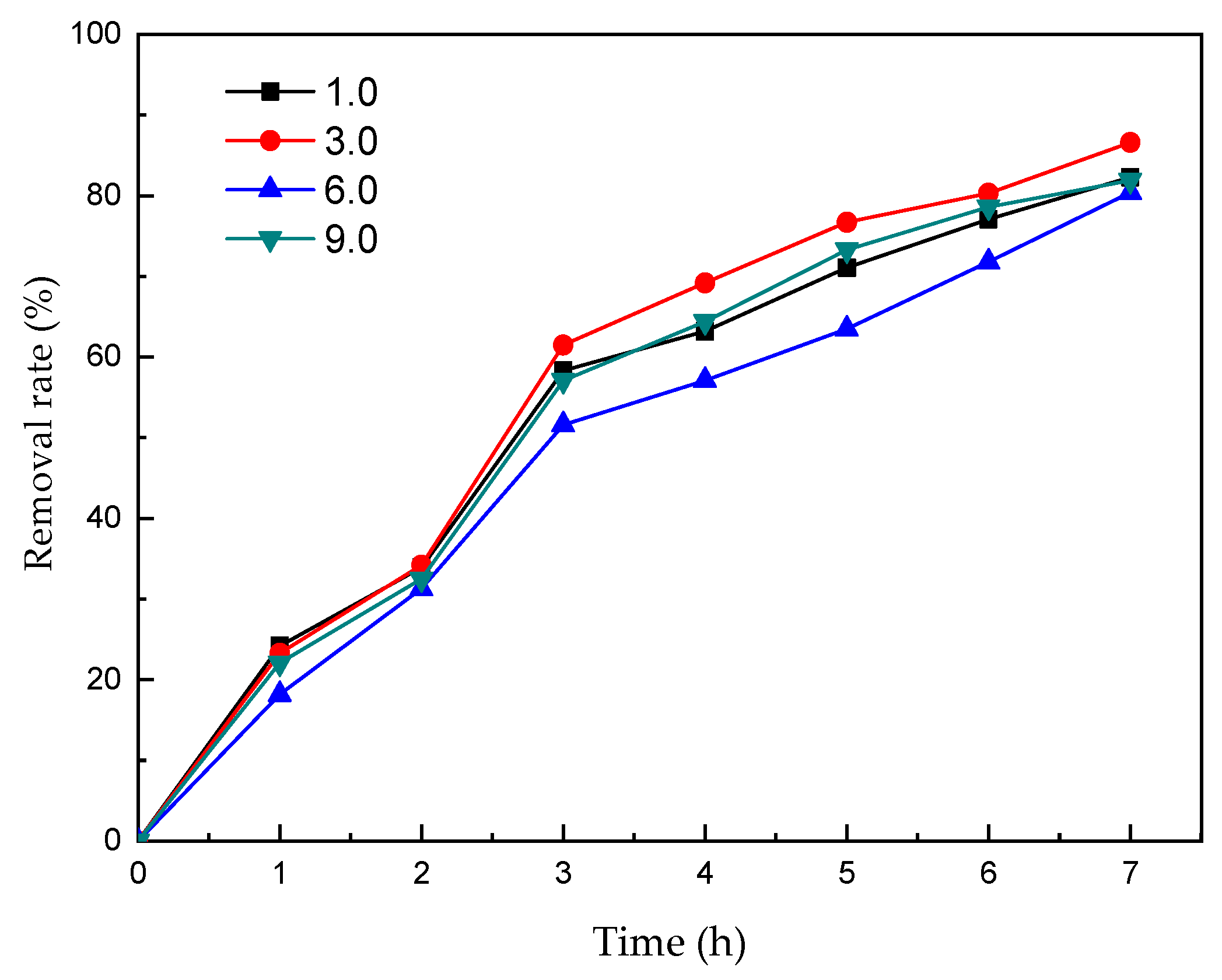
3.5. The Effect of Electrolyte pH
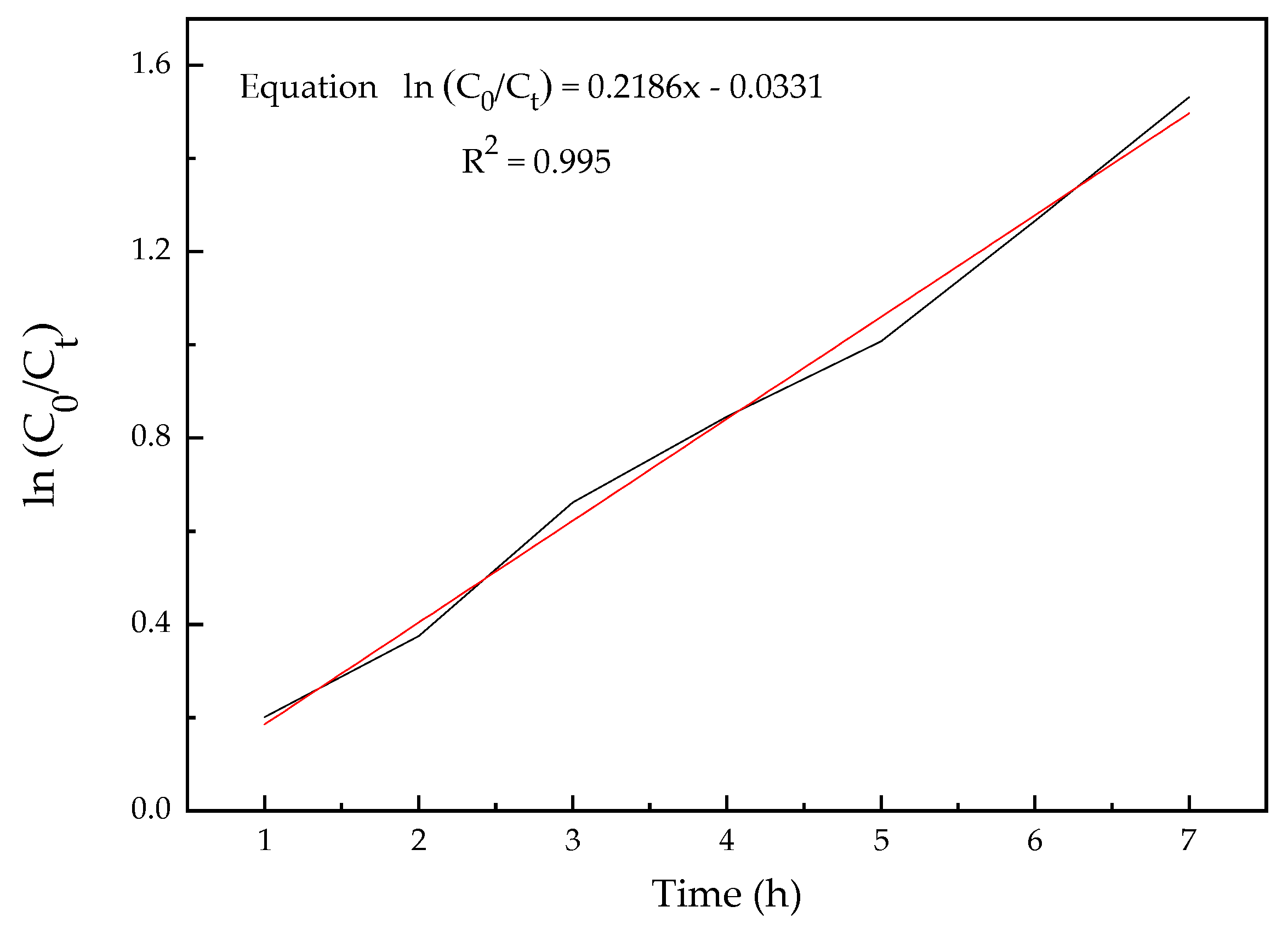
3.6. Optimization Experiment
3.7. Electrochemical Degradation Mechanism of Nicosulfuron on Ti/Ta2O5-IrO2 Electrode
3.7.1. Electrochemical Mechanism
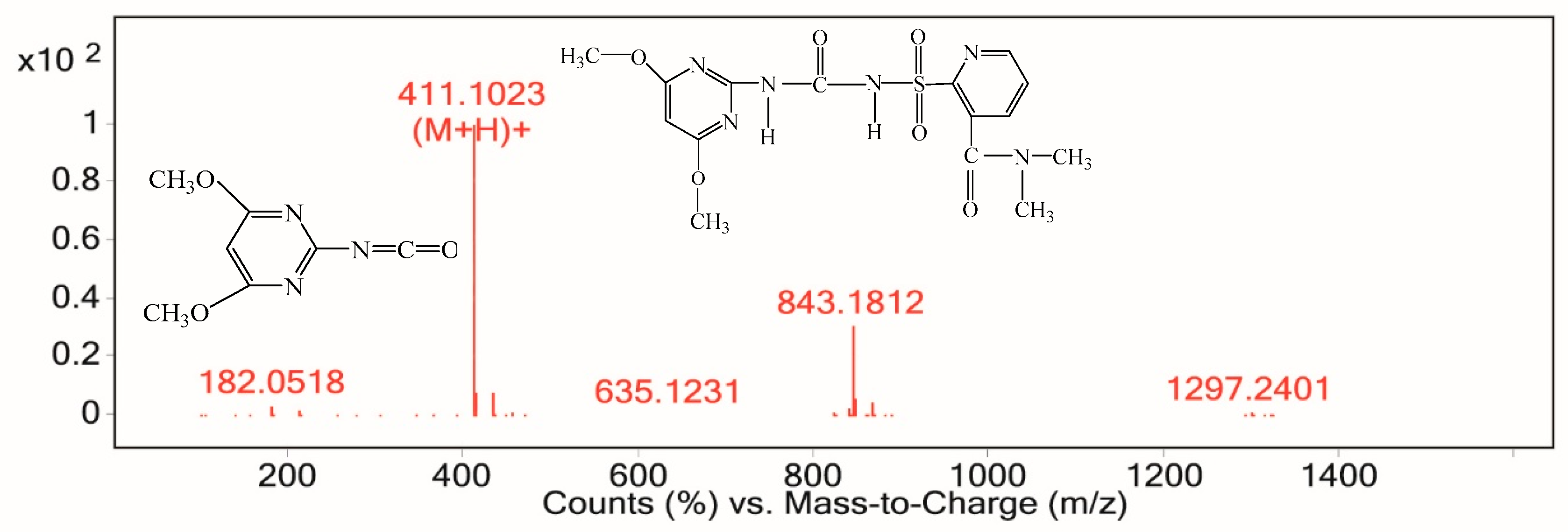
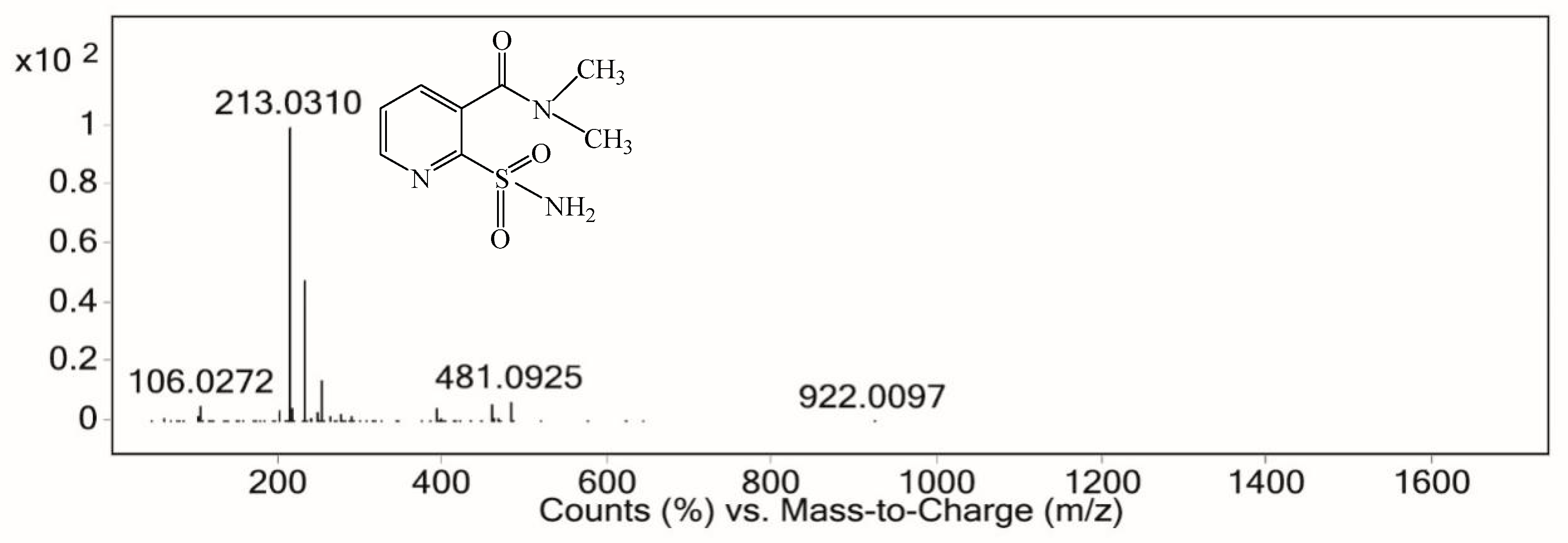
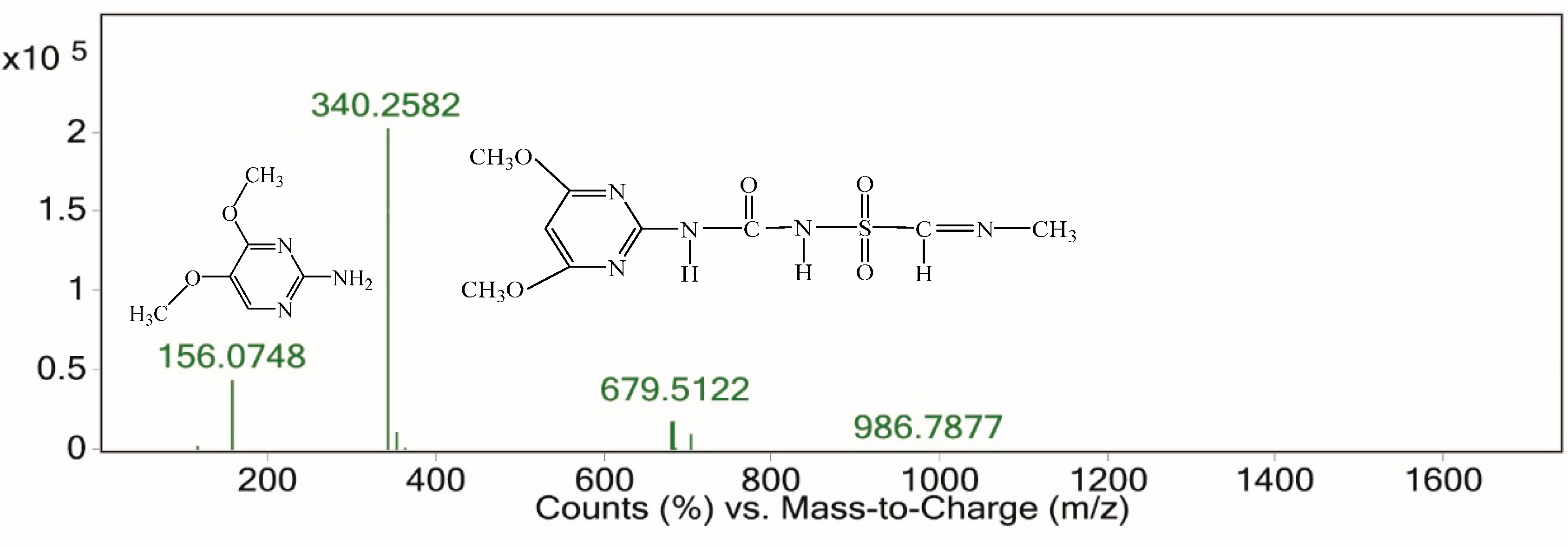
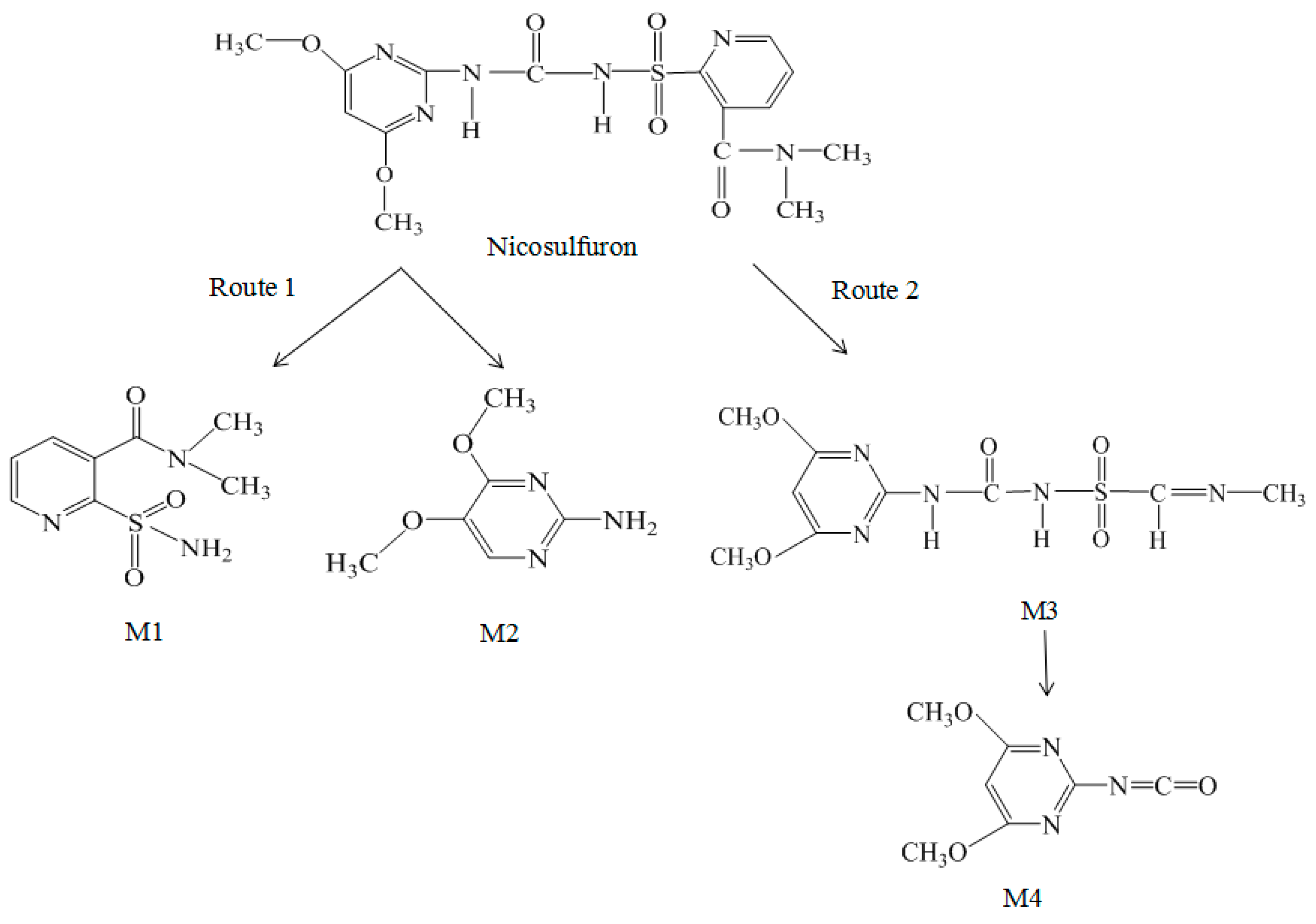
3.7.2. Electrochemical Degradation Mechanism of Nicosulfuron
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benzi, M.; Robotti, E.; Gianotti, V. HPLC-DAD-MSn to investigate the photodegradation pathway of nicosulfuron in aqueous solution. Anal. Bioanal. Chem. 2011, 399, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef] [PubMed]
- Jesus Lerma-Garcia, M.; Simo-Alfonso, E.F.; Zougagh, M.; Rios, A. Use of gold nanoparticle-coated sorbent materials for the selective preconcentration of sulfonylurea herbicides in water samples and determination by capillary liquid chromatography. Talanta J. 2013, 105, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Overview the Global Market of Sulfonylurea and Imidazoline Ketones Herbicides[DB/OL]. Available online: www.agrichem.cn/news/2014/1/15/201411510334746965.shtml (accessed on 15 January 2014).
- Trigo, C.; Spokas, K.A.; Cox, L.; Koskinen, W.C. Influence of soil biochar aging on sorption of the herbicides MCPA, nicosulfuron, terbuthylazine, indaziflam, and fluoroethyldiaminotriazine. J. Agric. Food Chem. 2014, 62, 10855–10860. [Google Scholar] [CrossRef] [PubMed]
- Senguin, F.; Druart, J.C.; Le Cohu, R. Effects of atrazine and nicosulfuron on periphytic diatom communities in freshwater outdoor lentic mesocosms. Int. J. Limnol. 2001, 37, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Reemtsma, T.; Alder, L.; Banasiak, U. Emerging pesticide metabolites in groundwater and surface water as determined by the application of a multimethod for 150 pesticide metabolites. Water Res. 2013, 47, 5535–5545. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.N.; Kong, D.Y.; Lu, J.H.; Zhou, Q.S. Transformation of sulfonylurea herbicides in simulated drinking water treatment processes. Environ. Sci. Pollut. Res. 2015, 5, 3847–3855. [Google Scholar] [CrossRef]
- Petric, I.; Karpouzas, D.G.; Bru, D.; Udikovic-Kolic, N.; Kandeler, E.; Djuric, S.; Martin-Laurent, F. Nicosulfuron application in agricultural soils drives the selection towards NS-tolerant microorganisms harboring various levels of sensitivity to nicosulfuron. Environ. Sci. Pollut. Res. Int. 2016, 23, 4320–4333. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, W.; Hou, Z.; Wu, X.; Zhao, W.; Zhang, X.; Pan, H.; Zhang, S. Biodegradation of nicosulfuron by the bacterium Serratia marcescens N80. J. Environ. Sci. Health B 2012, 47, 153–160. [Google Scholar] [CrossRef]
- MacBean, C. The Pesticide Manual, 16th ed.; BCPC: Hampshire, UK, 2012; pp. 10–15. ISBN 9781901396867. [Google Scholar]
- European Food Safety Authority. Conclusion regarding the peer review of the pesticide risk assessment of the active substance nicosulfuron. EFSA J. 2008, 6, 1–91. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, X.X.; Xu, Y.J.; Han, L.J. Dissipation and residues of nicosulfuron in corn and soil under field conditions. Bull. Environ. Contam. Toxicol. 2010, 85, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.A.M.D.; Silva, D.V.; Guimarães, F.R.; Leal, P.L.; Moreira, F.M.D.S.; Silva, A.A.D. Biological attributes of soil cultivated with corn intercropped with Urochloa brizantha in different plant arrangements with and without herbicide application. Agric. Ecosyst. Environ. 2018, 254, 35–40. [Google Scholar] [CrossRef]
- Yang, Y.J.; Tao, B.; Zhang, W.H.; Zhang, J.L. Isolation and screening of microorganisms capable of degrading nicosulfuron in water. Front. Agric. China 2008, 2, 224–228. [Google Scholar] [CrossRef]
- Louis, C.; Florent, R.; Muriel, J.; Pascale, B.H.; Isabelle, B.; Joan, A. Biotransformation of herbicides by aquatic microbial communities associated to submerged leaves. Environ. Sci. Pollut. Res. 2017, 24, 3664–3674. [Google Scholar] [CrossRef]
- Ahonsi, M.O.; Berner, D.K.; Emechebe, A.M.; Lagoke, S.T. Effects of ALS-inhibitor herbicides, crop sequence, and fertilization on natural soil suppressiveness to Striga hermonthica. Agric. Ecosyst. Environ. 2004, 104, 453–463. [Google Scholar] [CrossRef]
- Freitas, M.A.M.; Valadao, S.D.V.; Souza, M.F.; Silva, A.A.; Saraiva, D.T.; Freitas, M.M.; Cecon, P.R.; Feerreira, L.R. Levels of nutrients and grain yield of maize intercropped with signalgrass (Brachiaria) in different arrangements of plants. Planta Daninha 2015, 33, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Sabadie, J. Nicosulfuron: Alcoholysis, chemical hydrolysis, and degradation on various minerals. J. Agric. Food Chem. 2002, 50, 526–531. [Google Scholar] [CrossRef]
- Lu, X.H.; Kang, Z.H.; Tao, B.; Wang, Y.N.; Dong, J.G.; Zhang, J.L. Degradation of nicosulfuron by Bacillus subtilis YB1 and Aspergillus niger YF1. Appl. Biochem. Microbiol. 2012, 48, 510–515. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, X.; Yang, Q.; Wang, C.; Wang, Z. Determination of some sulfonylurea herbicides in soil by a novel liquid-phase microextraction combined with sweeping micellar electrokinetic chromatography. Anal. Bioanal. Chem. 2011, 401, 1071–1081. [Google Scholar] [CrossRef]
- Song, J.L.; Gu, J.G.; Zhai, Y.; Ruan, Z.Y.; Shi, Y.H.; Yan, Y.C. Biodegradation of nicosulfuron by a novel fungal strain, LZM1, with potential for biocontrolling corn diseases. Bioresour. Technol. 2013, 140, 243–248. [Google Scholar] [CrossRef]
- Zhao, W.S.; Qiu, L.H.; Guo, Q.G.; Li, S.Z.; Ma, P. Research progress on microbial degradation of nicosulfuron. Chin. J. Pestic. Sci. 2016, 18, 676–685. [Google Scholar] [CrossRef]
- Yuan, M.M.; Xu, H.; Yan, W. Advances in the study of electrochemical oxidation reactor for organic compounds. Environ. Eng. 2018, 36, 1–6. [Google Scholar] [CrossRef]
- Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination 2006, 190, 201–211. [Google Scholar] [CrossRef]
- Zhou, N.N.; Zhang, W.; Zhao, J.L.; Li, K.; Chen, Y.W.; Shen, S.B. DSA anode electric catalytic oxidation technology and research progress of phenol containing wastewater treatment. Mod. Chem. Ind. 2017, 37, 29–32. [Google Scholar] [CrossRef]
- Zhang, Z.X. Techniques of Titanium Electrodes, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2003; pp. 56–68. ISBN 9787502432232. [Google Scholar]
- Nonato, T.C.M.; Alves, A.A.D.; Broock, W.F.; Dalsasso, R.L.; Sens, M.L. The optimization of the electroflotation process using DSA® electrodes for treating the simulated effluent of produced water from oil production. Desalin. Water Treat. 2017, 70, 139–144. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Kwangseok, M.; Daewon, P. The characteristics of hydrogen production according to electrode materials in alkaline water electrolysis. J. Energy Eng. 2015, 24, 34–39. [Google Scholar] [CrossRef]
- SánchezPadilla, N.M.; Montemayor, S.M.; Rodríguez Varela, F.J. An easy route to synthesize novel Fe3O4@Pt core-shell nanostructures with high electrocatalytic activity. J. New Mater. Electr. Syst. 2012, 15, 171. [Google Scholar] [CrossRef]
- Zafar, M.S.; Tausif, M.; Zia, U.H.; Ashraf, M.; Hussain, S. New development of anodic electro-catalyst for chlor-alkali industry. Port. Electrochim. Acta 2016, 34, 257–266. [Google Scholar] [CrossRef]
- Trieu, V.; Schley, B.; Natter, H.; Kintrup, J.; Bulan, A.; Hempelmann, R. RuO2-based anodes with tailored surface morphology for improved chlorine electro-activity. Electrochim. Acta 2012, 78, 188–194. [Google Scholar] [CrossRef]
- Nagai, K.; Nagasawa, K.; Mitsushima, S. Oer activity of Ir-Ta-Zr composite anode as a counter electrode for electrohydrogenation of toluene. Electrocatalysis 2016, 7, 441–444. [Google Scholar] [CrossRef]
- Montiel, V.; Garcia-Garcia, V.; Gonzalez-Garcia, J.; Perez-Mallol, J.R.; Sanchez-Cano, G.; Aldaz, A. Viability of the electrochemical treatment of industrial effluents. J. New Mater. Electr. Syst. 2000, 3, 269–274. [Google Scholar]
- Rathinakumaran, K.; Meyyappan, R.M. Electrochemical degradation of dye effluents using mixed oxide coated DSA electrode-a kinetic study. Int. J. Chem. Sci. 2015, 13, 1401–1409. [Google Scholar]
- Herrada, R.A.; Acosta-Santoyo, G.; Sepúlveda-Guzmánc, S.; Enric, B.; Sirés, I.; Buotos, E. IrO2-Ta2O5|Ti electrodes prepared by electrodeposition from different Ir:Ta ratios for the degradation of polycyclic aromatic hydrocarbons. Electrochim. Acta 2018, 263, 353–361. [Google Scholar] [CrossRef]
- Cuevas, O.; Herrada, R.A.; Corona, J.L.; Olvera, M.G.; Sepúlveda-Guzmán, S.; Sirés, I.; Bustos, E. Assessment of IrO2-Ta2O5|Ti electrodes for the electrokinetic treatment of hydrocarbon-contaminated soil using different electrode arrays. Electrochim. Acta 2016, 208, 282–287. [Google Scholar] [CrossRef]
- Xu, Q.T.; Song, X.L.; Li, X. A novel high-performance Ti/ATONPs-MWCNTs electrode based on screen printing technique for degradation of C.I. Acid Red 73. Int. J. Electrochem. Sci. 2018, 13, 119–135. [Google Scholar] [CrossRef]
- Tehare, K.K.; Zate, M.K.; Navale, S.T.; Bhande, S.S.; Gaikwad, S.L.; Patil, S.A.; Gore, S.K.; Naushad, M.; Alfadul, S.M.; Mane, R.S. Electrochemical supercapacitors of cobalt hydroxide nanoplates grown on conducting cadmiumoxide base-electrodes. Arab. J. Chem. 2017, 10, 515–522. [Google Scholar] [CrossRef]
- Ekar, S.U.; Shekhar, G.; Khollam, Y.B.; Wani, P.N.; Jadkar, S.R.R.; Naushad, M.; Chaskar, M.G.; Jadhav, S.S.; Fadel, A.; Jadhav, V.V. Green synthesis and dye-sensitized solar cell application of rutile and anatase TiO2 nanorods. J. Solid State Electr. 2017, 21, 2713–2718. [Google Scholar] [CrossRef]
- Kong, D.S. Advances and some problems in electrocatalysis of DSA electrodes. Prog. Chem. 2009, 21, 1107–1117. [Google Scholar] [CrossRef]
- Kan, L.B.; Zhong, S.Q.; Wang, M. Preparation of modified titanium based DSA electrode and research progress in treatment of organic wastewater with the electrode. Contemp. Chem. Ind. 2015, 44, 2246–2249. [Google Scholar] [CrossRef]
- Wei, J.; Furrer, G.; Schulin, R. Kinetics of carbosulfan degradation in the aqueous phase in the presence of a cosolvent. J. Environ. Qual. 2000, 29, 1481–1487. [Google Scholar] [CrossRef]
- Wan, L.; Hou, Z.G.; Li, L.C.; Ma, X.L.; Ma, T.D.; Lu, Z.B. Effects of pH and initial concentration on hydrolysis of nicosulfron. J. Anhui Agric. Sci. 2011, 39, 15580–15581. [Google Scholar] [CrossRef]
- Zhao, W.S.; Wang, C.; Xu, L.; Zhao, C.Q.; Liang, H.W.; Qiu, L.H. Biodegradation of nicosulfuron by a novel Alcaligenes. faecalis strain ZWS11. J. Environ. Sci. 2015, 35, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, C.Z.; Lu, J.; Hu, C.J.; Peng, S.C.; Chen, T.H. Electro-catalytic degradation of bisphenol A with modified Co3O4/b-PbO2/Ti electrode. Electrochim. Acta 2014, 118, 169–175. [Google Scholar] [CrossRef]
- Ruan, Z.Y.; Zhou, S.; Jiang, S.H.; Sun, L.; Zhai, Y.; Wang, Y.W. Isolation and characterization of a novel cinosulfuron degrading Kurthia sp. from a methanogenic microbial consortium. Bioresour. Technol. 2013, 147, 477–483. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, B.; Dong, J.G.; Zhang, J.L. Isolation, identification and characterization of an Bacillus subtilis strain capable of degrading nicosulfuron. Chin. J. Pestic. Sci. 2014, 16, 330–336. [Google Scholar] [CrossRef]
- Al-Osta, A.; Jadhav, V.V.; Saad, N.A.; Mane, R.S.; Naushad, M.; Hui, K.N.; Han, S.H. Diameter-dependent electrochemical supercapacitive properties of anodized titantium oixde nanotubes. Scripta Mater. 2015, 104, 60–63. [Google Scholar] [CrossRef]
- Zate, M.K.; Jadhav, V.V.; Gore, S.K.; Shendkar, J.H.; Ekar, S.U.; Al-Osta, A.; Naushad, M.; Mane, R.S. Structural, morphological and electrochemical supercapacitive properties of sprayed manganese ferrite thin film electrode. J. Anal. Appl. Pyrol. 2016, 122, 224–229. [Google Scholar] [CrossRef]
- Ansari, A.; Nematollahi, D. A comprehensive study on the electrocatalytic degradation, electrochemical behavior and degradation mechanism of malachite green using electrodeposited nanostructured β-PbO2 electrodes. Water Res. 2018, 144, 262–473. [Google Scholar] [CrossRef]
- Chen, J.M.; Xia, Y.J.; Dai, Q.Z. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochim. Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- Dang, J.J.; Yang, D.C.; Zhang, Z.; Wang, Z.M.; Zhang, J.L. Degradation of nicosulfuron by Bacillus cereus and toxicity of the degradation product 2-amino-4,6-dimethoxy pyrimidine to environmental organisms. Chin. J. Pestic. Sci. 2018, 20, 652–660. [Google Scholar] [CrossRef]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Batisson, I.; Besse-Hoggan, P. Identification of sulfonylurea biodegradation pathways enabled by a novel Nicosulfuron transforming strain Pseudomonas fluorescens SG-1: Toxicity assessment and effect of formulation. J. Hazard. Mater. 2017, 324, 184–193. [Google Scholar] [CrossRef] [PubMed]




| Name | Nicosulfuron |
|---|---|
| Chemical structure |  |
| Molecular Formula | C15H18N6O6S |
| CAS Number | 111991-09-4 |
| Molecular mass | 410.41 g/mol |
| Solubility | acetonitrile 2.3%, acetone 1.8%, ethanol 0.45%, water 12% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Zhang, X.; Chen, F.; Man, X.; Jiang, W. Study on Electrochemical Degradation of Nicosulfuron by IrO2-Based DSA Electrodes: Performance, Kinetics, and Degradation Mechanism. Int. J. Environ. Res. Public Health 2019, 16, 343. https://doi.org/10.3390/ijerph16030343
Zhao R, Zhang X, Chen F, Man X, Jiang W. Study on Electrochemical Degradation of Nicosulfuron by IrO2-Based DSA Electrodes: Performance, Kinetics, and Degradation Mechanism. International Journal of Environmental Research and Public Health. 2019; 16(3):343. https://doi.org/10.3390/ijerph16030343
Chicago/Turabian StyleZhao, Rui, Xuan Zhang, Fanli Chen, Xiaobing Man, and Wenqiang Jiang. 2019. "Study on Electrochemical Degradation of Nicosulfuron by IrO2-Based DSA Electrodes: Performance, Kinetics, and Degradation Mechanism" International Journal of Environmental Research and Public Health 16, no. 3: 343. https://doi.org/10.3390/ijerph16030343




