Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Aerobic Denitrifying Bacteria
2.2. Bacterial Identification and Denitrification Gene Amplification
2.3. Effect of Carbon Source on Aerobic Denitrification
2.4. Assessment of Aerobic Denitrification Capability with Batch Experiments
2.5. Box–Behnken Design for Optimizing the Environmental Factors
2.6. Evaluation of the Nitrate Removal in Wastewater Treatment Plant Effluent by Strain GL6 under the Optimal Conditions
2.7. Analytical Methods
3. Results and Discussion
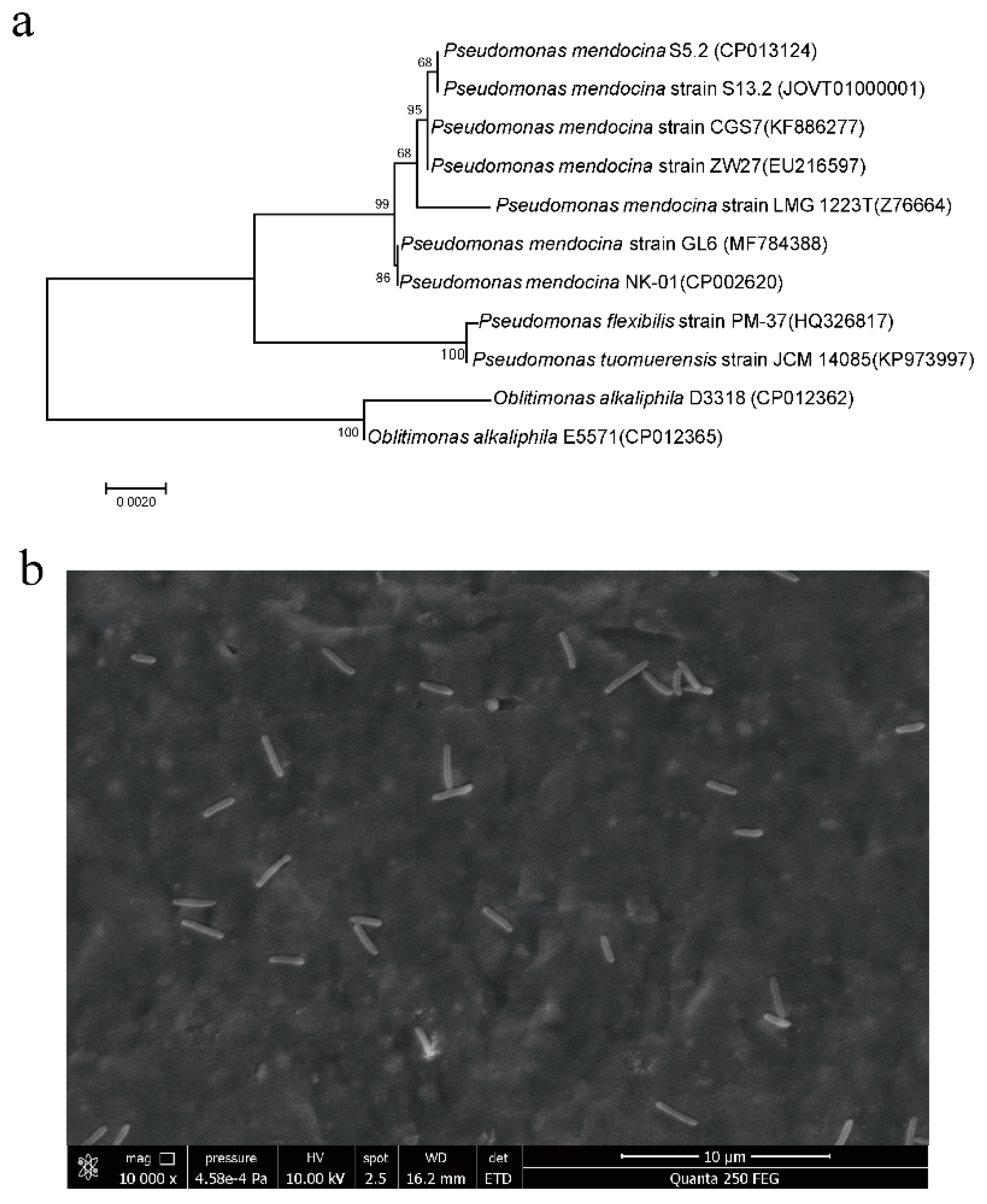
3.1. Isolation and Identification of Strain GL6
3.2. PCR Amplification of Denitrification Genes
3.3. Influence of Carbon Source on Denitrification
3.4. Growth and Aerobic Denitrification Performance of Strain GL6
3.5. Optimization of the Environmental Factors on the Aerobic Denitrification by Box–Behnken Design
3.6. Assessment of Strain GL6 for Application in Wastewater Treatment Plant Effluent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Zhou, W.L.; Wu, S.Q.; He, S.B.; Huang, J.C.; Zhang, X. Nitrogen removal by thiosulfate-driven denitrification and plant uptake in enhanced floating treatment wetland. Sci. Total Environ. 2018, 621, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Finlay, J.C.; Small, G.E.; Sterner, R.W. Human Influences on Nitrogen Removal in Lakes. Science 2013, 342, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Feng, C.P.; Wang, Q.H.; Yang, Y.N.; Zhang, Z.Y.; Sugiura, N. Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm-electrode reactor. J. Hazard. Mater. 2011, 192, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Wang, L.C.; Guo, X.Y.; Zhao, G.M.; Deng, J.C.; Zeng, C.F. Spatio-temporal differences in nitrogen reduction rates under biotic and abiotic processes in river water of the Taihu Basin, China. Int. J. Environ. Res. Public Health 2018, 15, 2568. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Chu, L.B. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Sahinkaya, E.; Dursun, N.; Kilic, A.; Demirel, S.; Uyanik, S.; Cinar, O. Simultaneous heterotrophic and sulfur-oxidizing autotrophic denitrification process for drinking water treatment: Control of sulfate production. Water Res. 2011, 45, 6661–6667. [Google Scholar] [CrossRef]
- Robertson, L.A.; van Niel, E.W.J.; Torremans, R.A.M.; Kuenen, J.G. Simultaneous nitrification and denitrification in aerobic chemostat cultures of Thiosphaera pantotropha. Appl. Environ. Microbiol. 1988, 54, 2812–2818. [Google Scholar]
- Guo, L.Y.; Chen, Q.K.; Fang, F.; Hu, Z.X.; Wu, J.; Miao, A.J.; Xiao, L.; Chen, X.F.; Yang, L.Y. Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. Bioresour. Technol. 2013, 142, 45–51. [Google Scholar] [CrossRef]
- Duan, J.M.; Fang, H.D.; Su, B.; Chen, J.F.; Lin, J.M. Characterization of a halophilic heterotrophic nitrification-aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef]
- Han, Y.H.; Zhang, W.X.; Lu, W.X.; Zhou, Z.H.; Zhuang, Z.G.; Li, M. Co-immobilization of Pseudomonas stutzeri YHA-13 and Alcaligenes sp. ZGED-12 with polyvinyl alcohol–alginate for removal of nitrogen and phosphorus from synthetic wastewater. Environ. Technol. 2014, 35, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Zhao, B.; An, Q.; Tian, M. Characteristics of a Novel Aerobic Denitrifying Bacterium, Enterobacter cloacae Strain HNR. Appl. Biochem. Biotechnol. 2016, 178, 947–959. [Google Scholar] [CrossRef]
- Chen, M.X.; Wang, W.C.; Feng, Y.; Zhu, X.H.; Zhou, H.Z.; Tan, Z.L.; Li, X.D. Impact resistance of different factors on ammonia removal by heterotrophic nitrification-aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef] [PubMed]
- He, T.X.; Li, Z.L.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.X.; Zhou, Y.Q.; Li, J.B.; Wang, J.C. A 3DBER-S-EC process for simultaneous nitrogen and phosphorus removal from wastewater with low organic carbon content. J. Environ. Manag. 2018, 209, 57–64. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Han, Y.H.; Qiu, S.; Zeng, H.Y.; Ma, F.; Wang, J.; Qiu, Y.L.; An, X.D. Short-term effects of tourmaline on nitrogen removals and microbial communities in a sequencing bath reactor at low tempreatures. Int. J. Environ. Res. Public Health 2018, 15, 1280. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.A.; Madden, T.L. BLAST 2 SEQUENCES, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 1999, 174, 247–250. [Google Scholar] [CrossRef]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef]
- Kong, Q.X.; Wang, X.W.; Jin, M.; Shen, Z.Q.; Li, J.W. Development and application of a novel and effective screening method for aerobic denitrifying bacteria. FEMS Microbiol. Lett. 2006, 260, 150–155. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar]
- Michotey, V.; Méjean, V.; Bonin, P. Comparison of methods for quantification of cytochrome cd1-denitrifying bacteria in environmental marine samples. Appl. Environ. Microbiol. 2000, 66, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Braker, G.; Tiedje, J.M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Scala, D.J.; Kerkhof, L.J. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol. Lett. 1998, 162, 61–68. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B.; Strous, M.; Tegetmeyer, H.E. Microbial nitrate respiration—Genes, enzymes and environmental distribution. J. Biotechnol. 2011, 155, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.C.; Richardson, D.J.; Ferguson, S.J. Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha: The periplasmic enzyme catalyzes the first step in aerobic denitrification. FEBS Lett. 1990, 265, 85–87. [Google Scholar] [CrossRef]
- Padhi, S.K.; Tripathy, S.; Sen, R.; Mahapatra, A.S.; Mohanty, S.; Maiti, N.K. Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int. Biodeterior. Biodegrad. 2013, 78, 67–73. [Google Scholar] [CrossRef]
- He, D.; Zheng, M.S.; Ma, T.; Li, C.; Ni, J.R. Interaction of Cr(VI) reduction and denitrification by strain Pseudomonas aeruginosa PCN-2 under aerobic conditions. Bioresour. Technol. 2015, 185, 346–352. [Google Scholar] [CrossRef]
- Huang, T.L.; Guo, L.; Zhang, H.H.; Su, J.F.; Wen, G.; Zhang, K. Nitrogen-removal efficiency of a novel aerobic denitrifying bacterium, Pseudomonas stutzeri strain ZF31, isolated from a drinking-water reservoir. Bioresour. Technol. 2015, 196, 209–216. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar]
- Shapleigh, J.P.; Payne, W.J. Differentiation of c, d1 cytochrome and copper nitrite reductase production in denitrifiers. FEMS Microbiol. Lett. 1985, 26, 275–279. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; Li, Y.; Hao, H.H.; Chen, J.M. Characteristics of a novel thermophilic heterotrophic bacterium, Anoxybacillus contaminans HA, for nitrification-aerobic denitrification. Appl. Microbiol. Biotechnol. 2015, 99, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, Y.Q.; Liu, H.J.; Xi, C.W.; Song, L.Y. A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Zobellella taiwanensis DN-7, can remove high-strength ammonium. Appl. Microbiol. Biotechnol. 2016, 100, 4219–4229. [Google Scholar] [CrossRef]
- Wang, X.; An, Q.; Zhao, B.; Guo, J.S.; Huang, Y.S.; Tian, M. Auto-aggregation properties of a novel aerobic denitrifier Enterobacter sp. strain FL. Appl. Microbiol. Biotechnol. 2018, 102, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cheng, D.Y.; Tan, P.; An, Q.; Guo, J.S. Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresour. Technol. 2018, 250, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeong, S.Y.; Yoon, S.J.; Cho, S.J.; Kim, Y.H.; Kim, M.J.; Ryu, E.Y.; Lee, S.J. Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J. Biosci. Bioeng. 2008, 106, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.Y.; Zhou, J.; Zhang, H.N. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Su, J.F.; Cheng, C.; Huang, T.L.; Ma, F.; Lu, J.S.; Shao, S.C. Characterization of coupling autotrophic denitrification with iron cycle bacterium Enterobacter sp. CC76 and its application of groundwater. J. Taiwan Inst. Chem. Eng. 2016, 66, 106–114. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.X.; Yang, T.; Lv, Y.K. Characterization of a microbial consortium capable of heterotrophic nitrifying under wide C/N range and its potential application in phenolic and coking wastewater. Biochem. Eng. J. 2017, 120, 33–40. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.X.; Liang, X.; Zhao, S.Q.; Wang, J.P.; Xia, Z.H. Nitrogen removal characteristics of a heterotrophic nitrifier Acinetobacter junii YB and its potential application for the treatment of high-strength nitrogenous wastewater. Bioresour. Technol. 2015, 193, 227–233. [Google Scholar] [CrossRef] [PubMed]





| Target Gene | Primer | Primer Sequence (5′→3′) | Product Size (bp) | Reference |
|---|---|---|---|---|
| napA | NAP1 | TCTGGACCATGGGCTTCAACCA | 876 | [20] |
| NAP2 | ACGACGACCGGCCAGCGCAG | |||
| nirK | nirK1F | GGMATGGTKCCSTGGCA | 514 | [21] |
| nirK5R | GCCTCGATCAGRTTRTGGTT | |||
| nirS | nirS cd3AF | GTSAACGTSAAGGARACSGG | 425 | [22] |
| nirS R3cd | GASTTCGGRTGSGTCTTGA | |||
| norB | cnorB2F | GACAAGNNNTACTGGTGGT | 389 | [23] |
| cnorB6R | GAANCCCCANACNCCNGC | |||
| nosZ | nosZ1527F | CGCTGTTCHTCGACAGYCA | 250 | [24] |
| nosZ1773R | ATRTCGATCARCTGBTCGTT |
| Substance | Initial TN (mg/L) | Final Nitrogen Concentration (mg/L) | Intracellular-N (%) | TN Removal (%) | |||
|---|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | NO2−-N | Organic-N | ||||
| Nitrate | 102.23 ± 0.21 | 1.82 ± 0.14 | 0.52 ± 0.29 | 0.02 ± 0.02 | 3.13 ± 0.65 | 20.63 ± 1.32 | ≈74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yan, C.; Shen, J.; Wei, R.; Gao, Y.; Miao, A.; Xiao, L.; Yang, L. Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. Int. J. Environ. Res. Public Health 2019, 16, 364. https://doi.org/10.3390/ijerph16030364
Zhang W, Yan C, Shen J, Wei R, Gao Y, Miao A, Xiao L, Yang L. Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. International Journal of Environmental Research and Public Health. 2019; 16(3):364. https://doi.org/10.3390/ijerph16030364
Chicago/Turabian StyleZhang, Wen, Cheng Yan, Jianing Shen, Ruping Wei, Yan Gao, Aijun Miao, Lin Xiao, and Liuyan Yang. 2019. "Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent" International Journal of Environmental Research and Public Health 16, no. 3: 364. https://doi.org/10.3390/ijerph16030364
APA StyleZhang, W., Yan, C., Shen, J., Wei, R., Gao, Y., Miao, A., Xiao, L., & Yang, L. (2019). Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. International Journal of Environmental Research and Public Health, 16(3), 364. https://doi.org/10.3390/ijerph16030364




