Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium vivax) in Moscow Megacity Region
Abstract
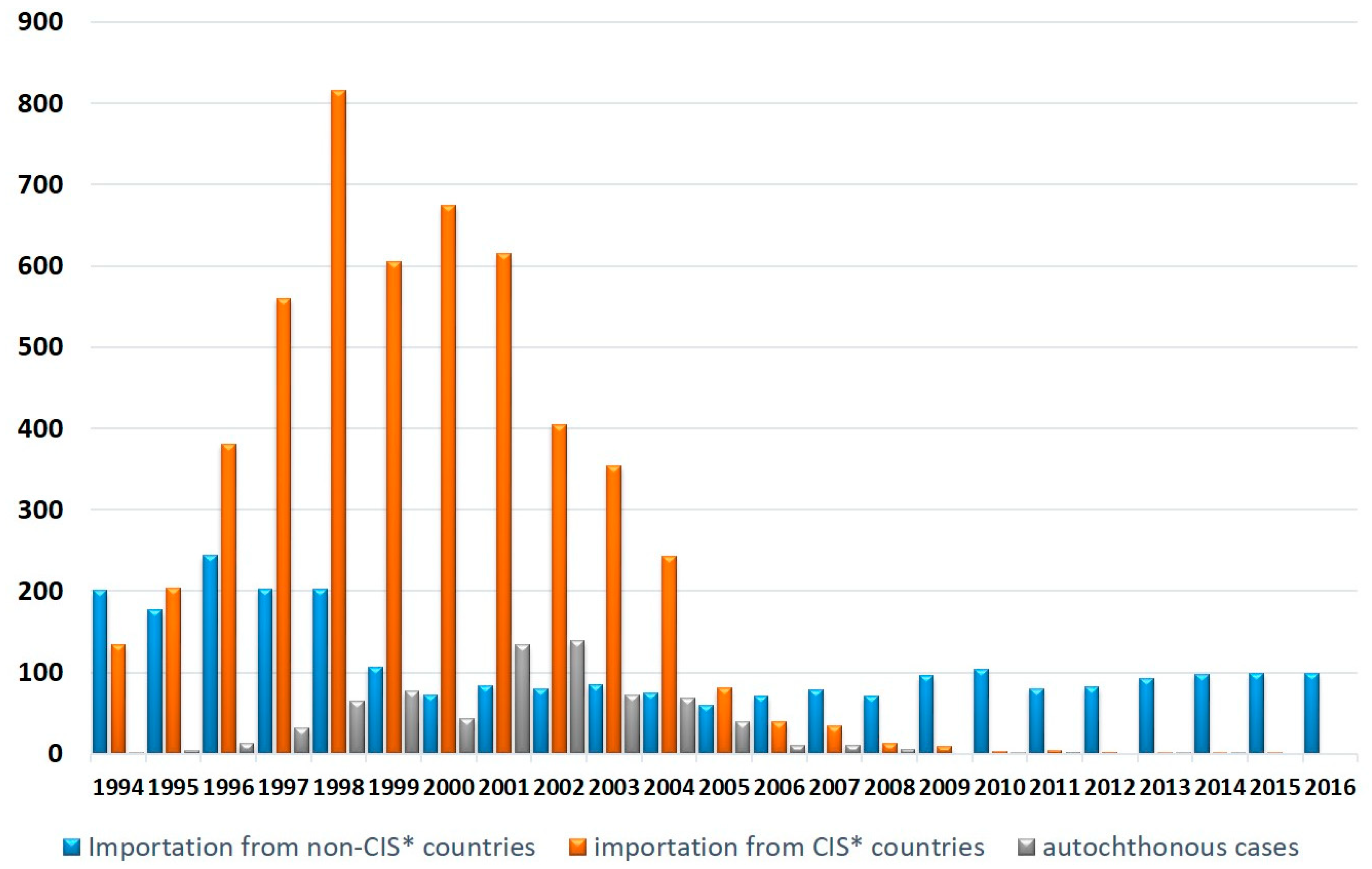
:1. Introduction
2. Materials and Methods
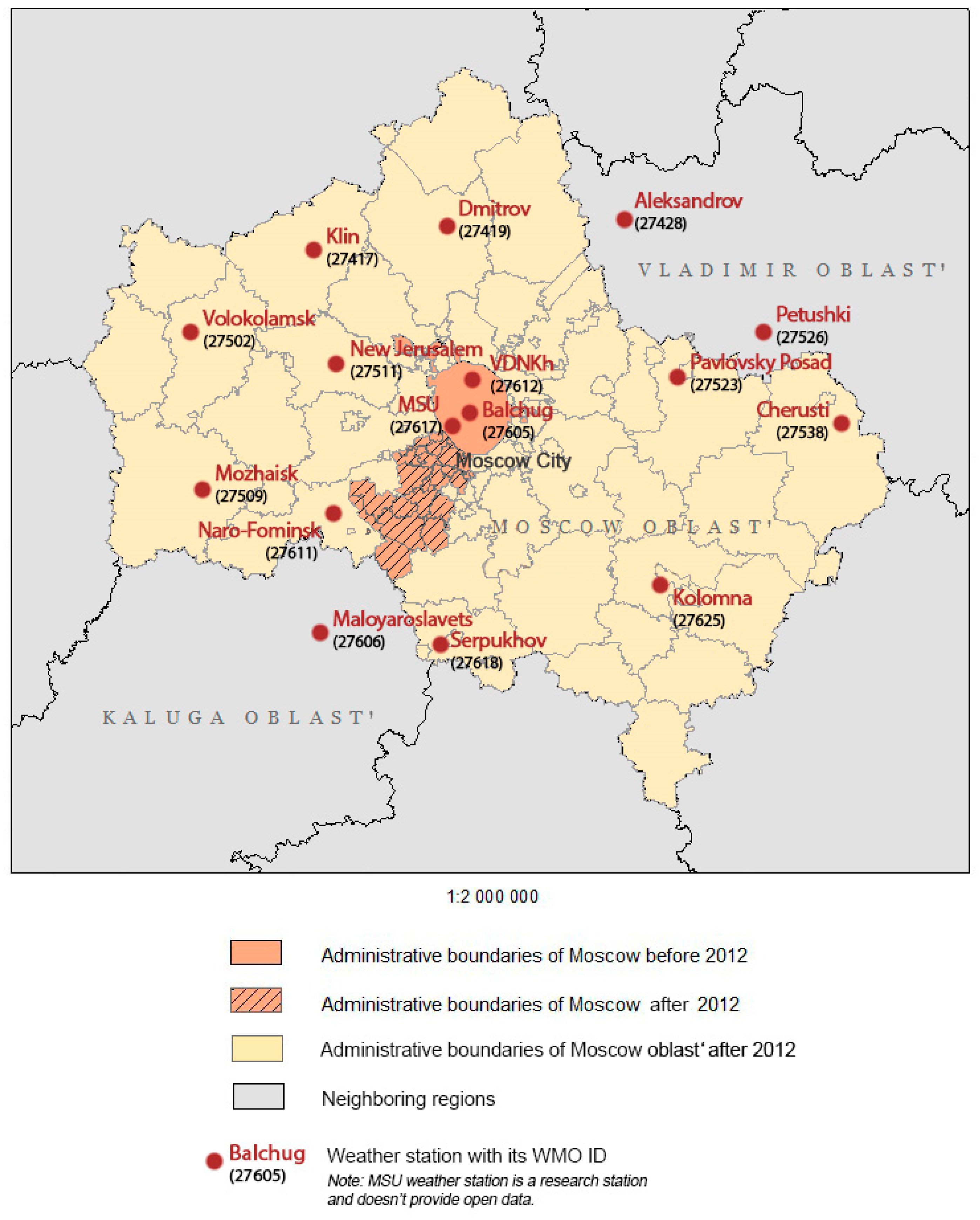
2.1. Study Area
2.2. Meteorological Data
2.3. Quantitative Indicators of Climatic Favorability for Malaria Transmission
- Season of manifestations: the period of the year during which most malaria manifestations start. It begins before the activity of mosquitoes, since a significant proportion of vivax malaria cases occurs nine to 12 months after the inoculation due to the long latency period [36];
- Season of effective temperatures (SET): the period of the year during which ADTs consistently keep above the parasite development threshold (+16 °C for P. vivax);
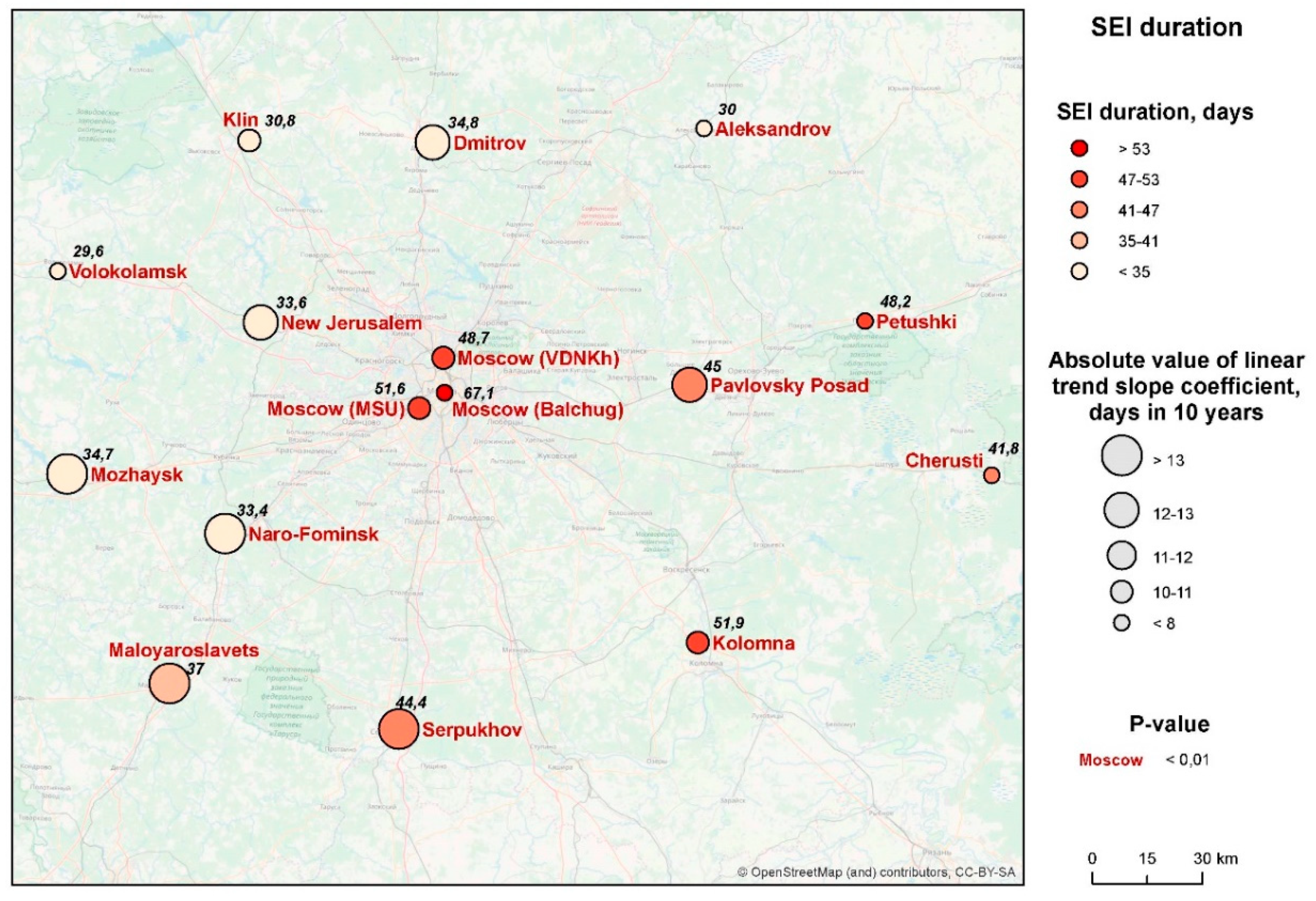
- Season of effective infectiveness of mosquitoes (SEI): the period during which a mosquito infected on a person can generate mature sporozoites. It starts at the same moment as SET, but stops earlier; this is because mosquitoes that contracted parasites nearer to the end of the SEI do not have enough time at a favorable temperature to generate fully developed parasites (the infection of mosquitoes is not effective in this case).
- Malaria transmission season: the period during which mosquitoes with mature sporozoites can infect humans. It starts later than the SEI by the lag equal to the duration of the period for development of a single generation of sporozoites, which may take three to four weeks at the beginning of the warm period.
3. Results
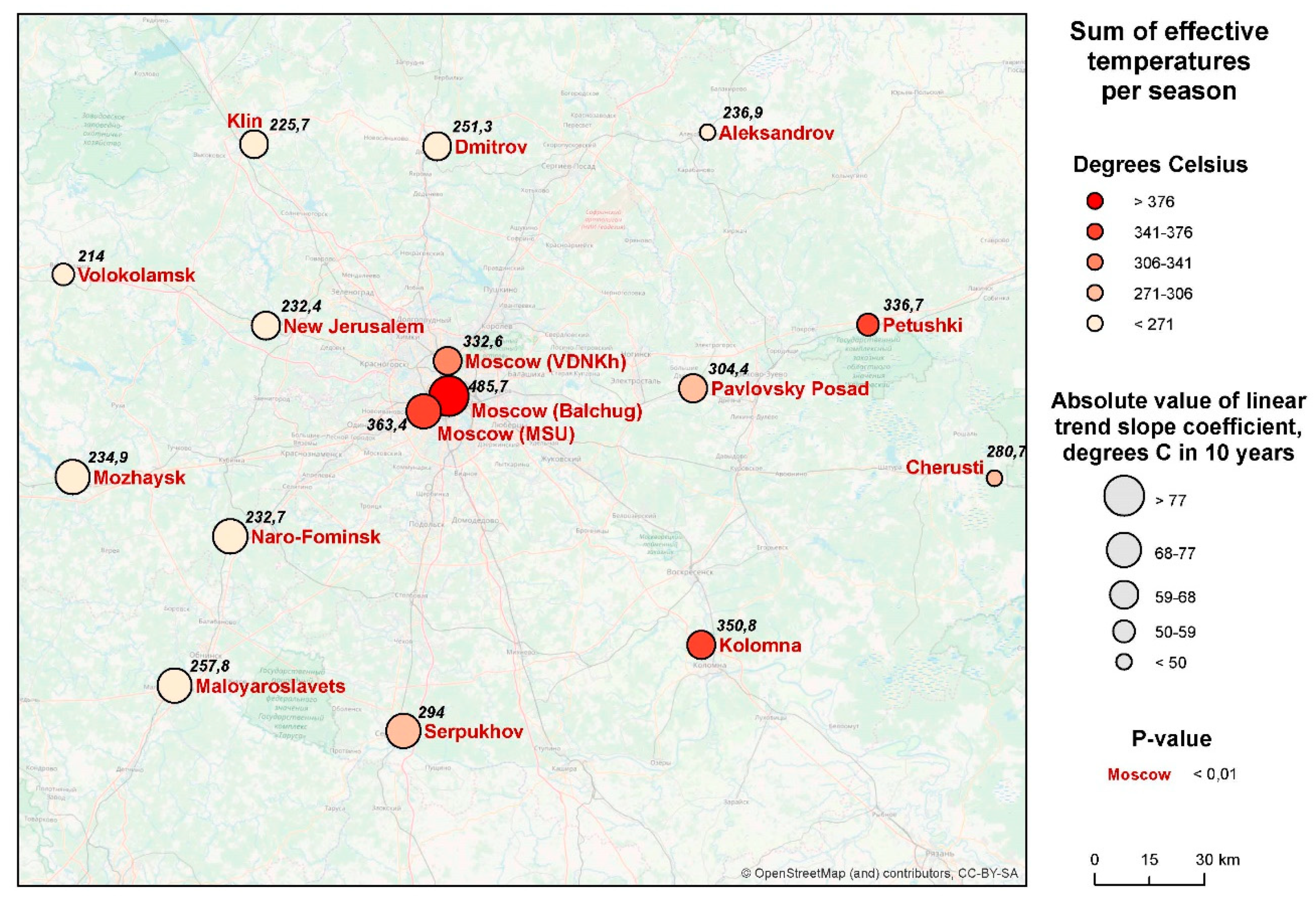
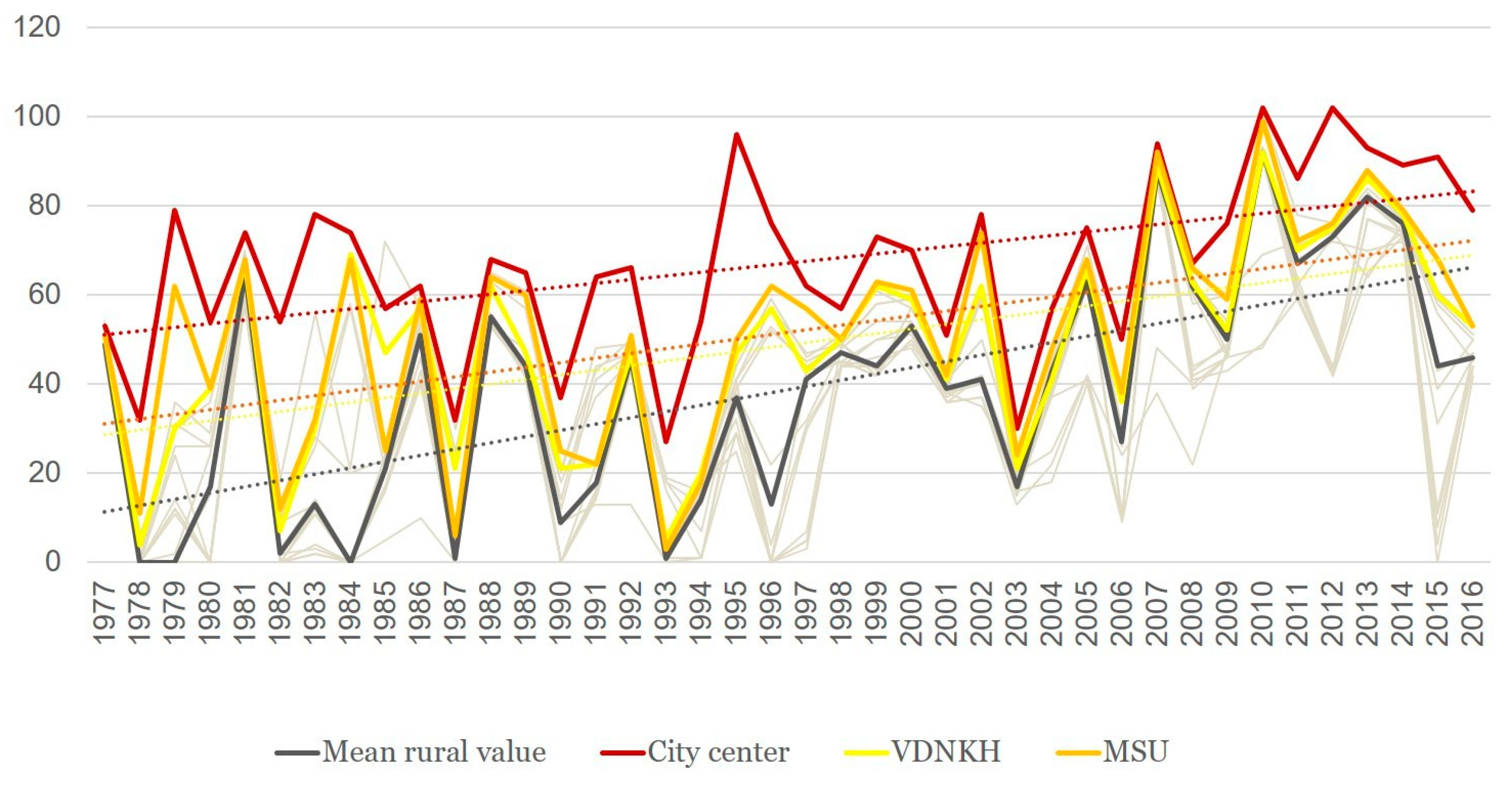
3.1. Sums of Effective Temperatures (ET)
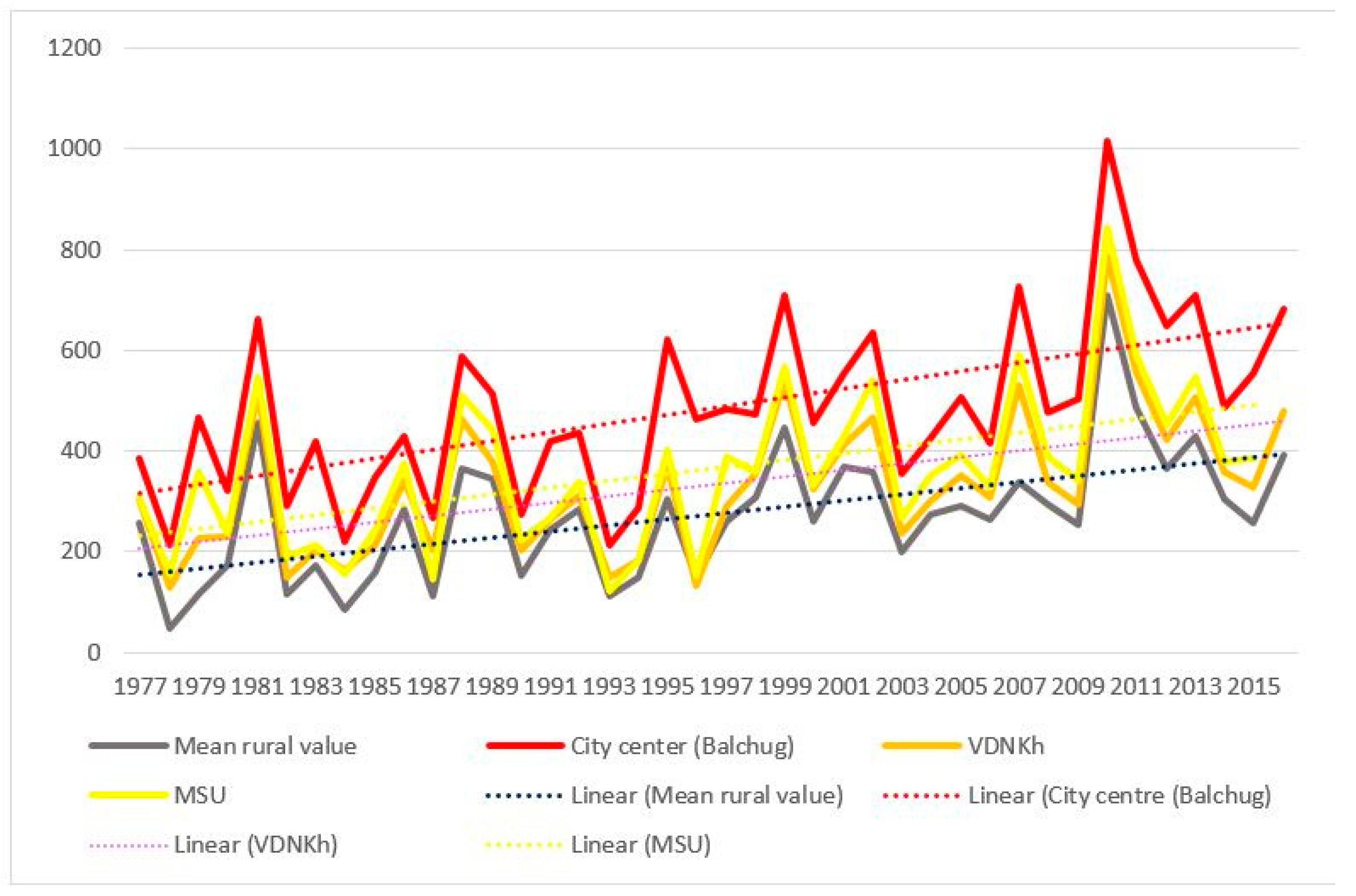
- Sums of ETs for the city center remain well above the average rural values every year, whereas the values for the park zones stay in between the two.
- For the city center, temperatures allowed the full development of at least one generation of sporozoites every year. For individual rural stations, there were years when the temperatures remained below the threshold of 105 °C, i.e., the temperatures were insufficient to ensure the transmission of P. vivax. The average rural values moved below the threshold only twice, in 1978 and in 1984 (before 1977 not shown on the graph); there were regular cases of years when temperatures remained prohibitive to the development of the parasite.
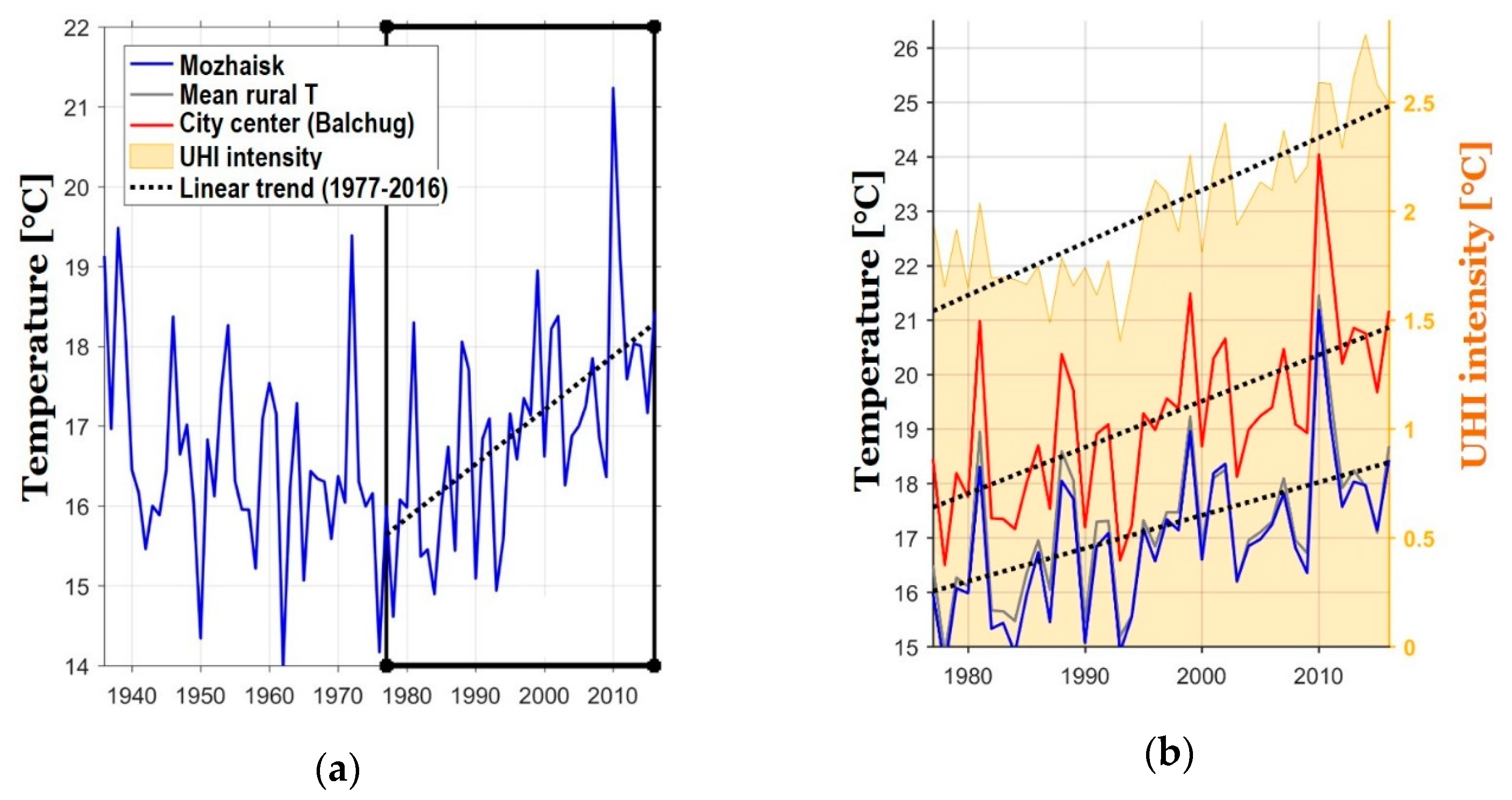
- There was a growing trend of sums of ETs, especially after 1997. For average rural values, the coefficient of the linear trend k equals 65 °C/10 years, which is statistically significant (R2 = 0.31, p < 0.01). This trend is further emphasized by the sums of ETs reaching below the threshold only once after 1997, in the year 2015, and in one place, i.e., in Volokolamsk (which is known as a “cold” station, where this indicator is always lower than in the other points).
- The growth rate of the sums of ET for the urban stations exceeded the growth rate in the rural stations: the trend slope coefficient and its significance for these two stations was greater than for the rural ones, revealing the intensification of the urban heat island.
3.2. Duration of the Season of Effective Infectivity (SEI)
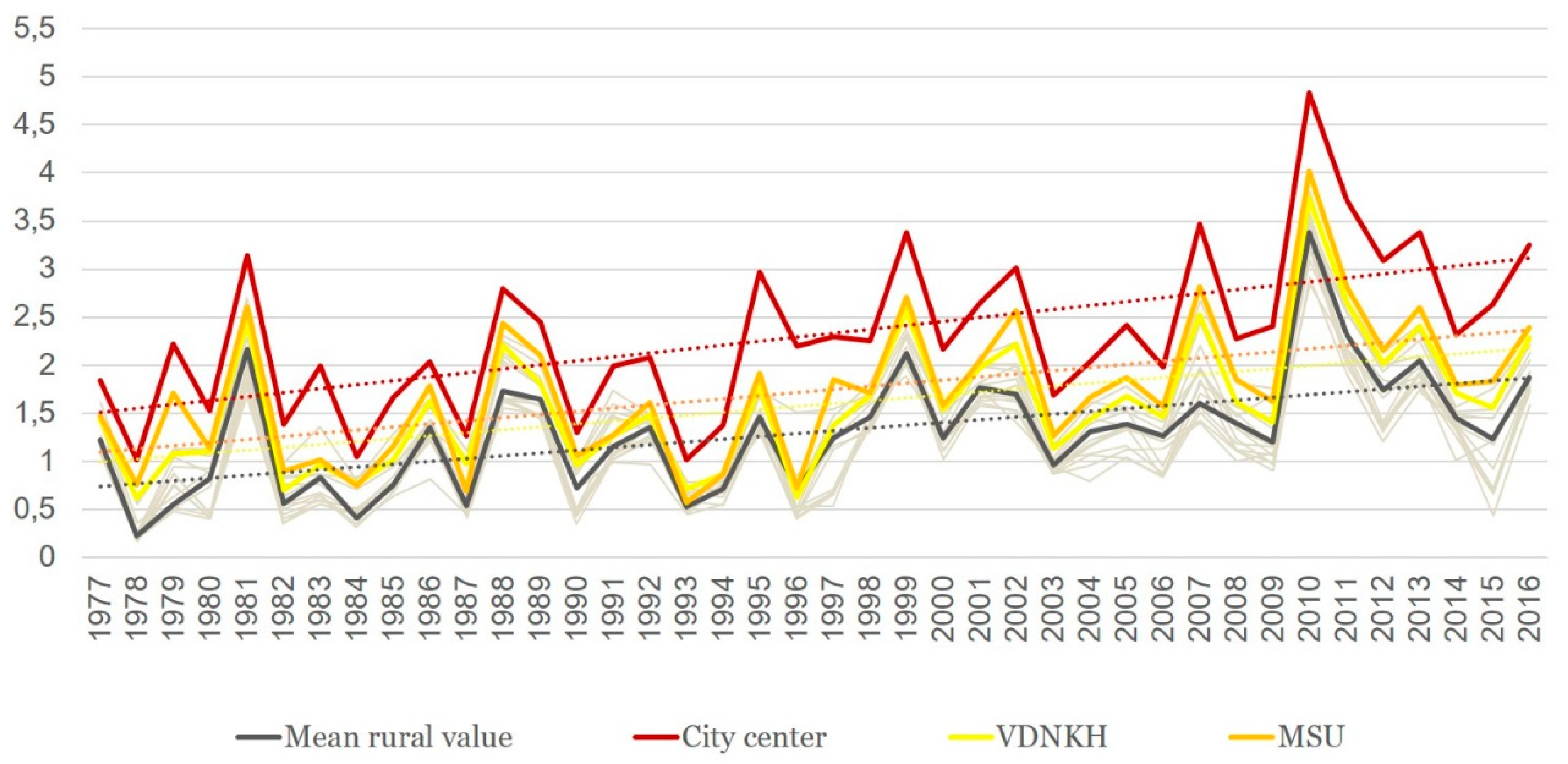
3.3. Index of Favorability (Kf)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: http://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1 (accessed on 26 December 2018).
- Beljaev, A.E.; Rybalka, V.M.; Lysenko, A.J.; Abrashkin-Zhuchkov, R.G.; Alekseeva, M.I.; Arsenyeva, L.P.; Babaeva, O.V.; Gorbunova, Y.P.; Dashkova, N.G.; Koshelev, B.A. Plasmodium vivax: Further observations on polymorphism in relation to the duration of exo-erythrocytic development. In Malaria Parasites of Mammals; Academy of sciences of the USSR Protozoology series, Issue 11; Nauka: Leningrad, USSR, 1986; pp. 40–157. (In Russian) [Google Scholar]
- WHO. Guidelines on Prevzention of the Reintroduction of Malaria; WHO Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2007; p. 49. [Google Scholar]
- Sergiev, V.P.; Baranova, A.M.; Orlov, V.S.; Mihajlov, L.G.; Kouznetsov, R.L.; Neujmin, N.I.; Arsenieva, L.P.; Shahova, M.A.; Glagoleva, L.A.; Osipova, M.M. Importation of malaria into the USSR from Afghanistan, 1981–1989. Bull. World Health Organ. 1993, 71, 385–388. [Google Scholar] [PubMed]
- Kondrashin, A.V.; Morozova, L.F.; Stepanova, E.V.; Turbabina, N.A.; Maksimova, M.S.; Morozov, E.N. On the epidemiology of Plasmodium vivax malaria: Past and present with special reference to the former USSR. Malar. J. 2018, 17, 346. [Google Scholar] [CrossRef] [PubMed]
- Dukhanina, N.N. Epidemiological Observations of Tropical Malaria in the North and the Establishment of Zones of its Spread. Ph.D. Thesis, Martsinovski Institute for Medical Parasitology and Tropical Medicine, Moscow, USSR, 1947. (In Russian). [Google Scholar]
- De Zulueta, J.; Ramsdale, C.D.; Coluzzi, M. Receptivity to malaria in Europe. Bull. World Health Organ. 1975, 52, 109–111. [Google Scholar] [PubMed]
- Dashkova, N.G.; Rasnitsyn, S.P. Review of Data on Susceptibility of Mosquitoes in USSR to imported strains of Malaria Parasites. Bull. World Health Organ. 1982, 60, 893–897. [Google Scholar]
- Beljaev, A.E. Determinants of malaria in the Middle East and North Africa. In The Contextual Determinants of Malaria; Casman, E.A., Dowlatabadi, H., Eds.; Resources for the Future Press: Washington, DC, USA, 2002; pp. 137–166. [Google Scholar]
- Beklemishev, V.N.; Vinogradskaya, O.N.; Derbeneva-Ukhova, V.P. The Textbook of Medical Entomology, Part 1; Medgiz: Moscow, USSR, 1949; p. 490. (In Russian) [Google Scholar]
- De Buck, A.; Swellengrebel, N.H.; Schoute, E. Studies on anophelism without malaria in the vicinity of Amsterdam. Bull. Entomol. Res. 1927, 17, 351–371. [Google Scholar] [CrossRef]
- Fantini, B. Anophelism without malaria: An ecological and epidemiological puzzle. Parassitologia 1994, 36, 83–106. [Google Scholar] [PubMed]
- Molina Gómez, K.; Caicedo, M.A.; Gaitán, A.; Herrera-Varela, M.; Arce, M.I.; Vallejo, A.F.; Padilla, J.; Chaparro, P.; Pacheco, M.A.; Escalante, A.A.; et al. Characterizing the malaria rural-to-urban transmission interface: The importance of reactive case detection. PLoS Negl. Trop. Dis. 2017, 11, e0005780. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G.E. The Climate of the City; Gidrometeoizdat: Leningrad, USSR, 1983; p. 246. (In Russian) [Google Scholar]
- Oke, T.R.; Mills, G.; Christen, A.; Voogt, J.A. Urban Climates; Cambridge University Press: Cambridge, UK, 2017; p. 509. [Google Scholar]
- Layton, M.; Parise, M.E.; Campbell, C.C.; Advani, R.; Sexton, J.D.; Bosler, E.M.; Zucker, J.R. Mosquito-transmitted malaria in New York City, 1993. Lancet 1995, 346, 729–731. [Google Scholar] [CrossRef]
- Zucker, J.R. Changing patterns of autochthonous malaria transmission in the United States: A review of recent outbreaks. Emerg. Infect. Dis. 1996, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, A.J.; Kondrashin, A.V. Malariology; Otkrytye Sistemy: Moscow, Russia, 1999; 248p. (In Russian) [Google Scholar]
- Gorban, L.J.; Center for Hygiene and Epidemiology of the Perm, Krai, Perm, Russia. Personal communication, 2005.
- Mironova, V.A.; Beljaev, A.E. Migration processes and malaria in Russia. In Current Issues in the Epidemiology of Infectious Diseases; Shaposhnikov, A.A., Yushchenko, G.V., Eds.; RMAPO: Moscow, Russia, 2011; pp. 680–690. (In Russian) [Google Scholar]
- Kislov, A.V. (Ed.) Climate of Moscow in the Context of Global Warming; Publishing House of Moscow University: Moscow, Russia, 2017; p. 288. (In Russian) [Google Scholar]
- Lokoshchenko, M.A. Urban “heat island” in Moscow. Urban Clim. 2014, 10, 550–562. [Google Scholar] [CrossRef]
- Varentsov, M.; Wouters, H.; Platonov, V.; Konstantinov, P. Megacity-Induced Mesoclimatic Effects in the Lower Atmosphere: A Modeling Study for Multiple Summers over Moscow, Russia. Atmosphere 2018, 9, 50. [Google Scholar] [CrossRef]
- Lokoshchenko, M.A. Urban Heat Island and Urban Dry Island in Moscow and Their Centennial Changes. J. Appl. Meteorol. Climatol. 2017, 56, 2729–2745. [Google Scholar] [CrossRef]
- Kislov, A.V.; Varentsov, M.I.; Gorlach, I.A.; Alekseeva, L.I. “The Heat Island” of the Moscow agglomeration and the urban increase of global warming. Bull. Mosc. Univ. Geogr. 2017, 4, 12–19. (In Russian) [Google Scholar]
- FSSS. Available online: http://www.gks.ru/bgd/regl/B12_16/Main.htm (accessed on 6 November 2018).
- Tardivo, G.; Berti, A. A Dynamic method for gap filling in daily temperature datasets. J. Appl. Meteorol. Climatol. 2012, 51, 1079–1086. [Google Scholar] [CrossRef]
- Moshkovsky, S.D.; Rashina, M.G. Epidemiology and Medical Parasitology for Entomologists; Medgiz: Moscow, USSR, 1951; 455p. (In Russian) [Google Scholar]
- Moshkovsky, S.D. The Basic Patterns of Malaria Epidemiology; Academy of Sciences of the USSR: Moscow, USSR, 1950; 323p. (In Russian) [Google Scholar]
- WHO. Malaria Elimination. Guide for Participants. 2016. Available online: http://www.who.int/malaria/publications/atoz/9789241549424_eng.pdf (accessed on 27 October 2018).
- Sainz-Elipe, S.; Latorre, J.M.; Escosa, R.; Masià, M.; Fuentes, M.V.; Mas-Coma, S.; Bargues, M.D. Malaria resurgence risk in southern Europe: Climate assessment in an historically endemic area of rice fields at the Mediterranean shore of Spain. Malar. J. 2010, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Smith, D.L.; Patil, A.P.; Tatem, A.J.; Snow, R.W.; Hay, S.I. Climate change and the global malaria recession. Nature 2010, 465, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Benali, A.; Nunes, J.P.; Freitas, F.B.; Sousa, C.A.; Novo, M.T.; Lourenço, P.M.; Almeida, A.P.G. Satellite-derived estimation of environmental suitability for malaria vector development in Portugal. Remote Sens. Environ. 2014, 145, 116–130. [Google Scholar] [CrossRef]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of climate change on global malaria distribution. Proc. Natl. Acad. Sci. USA 2014, 111, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Capinha, C.; Rocha, J.; Sousa, C. Mapping Risk of Malaria Transmission in Mainland Portugal Using a Mathematical Modelling Approach. PLoS ONE 2016, 11, e0164788. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, A.J.; Beljaev, A.E.; Rybalka, V.M. Population studies of Plasmodium vivax. 1. The theory of polymorphism of sporozoites and epidemiological phenomena of tertian malaria. Bull. World Health Organ. 1977, 55, 541–549. [Google Scholar] [PubMed]
- Lindsay, S.W.; Hole, D.G.; Hutchinson, R.A.; Richards, S.A.; Willis, S.G. Assessing the future threat from vivax malaria in the United Kingdom using two markedly different modelling approaches. Malar. J. 2010, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Semyonov, S.M.; Gelver, E.S.; Yasyukevich, V.V. Temperature conditions for development of two species of malaria pathogens in the vector organism in Russia in the 20th century. Dokl. Biol. Sci. 2002, 387, 523–528. (In Russian) [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109 (Suppl. 1), 141–161. [Google Scholar] [PubMed]
- Kuhn, K.G.; Campbell-Lendrum, D.H.; Armstrong, B.; Davies, C.R. Malaria in Britain: Past, present, and future. Proc. Natl. Acad. Sci. USA 2003, 100, 9997–10001. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Sudre, B.; Rossi, M.; Van Bortel, W.; Danis, K.; Baka, A.; Vakalis, N.; Semenza, J.C. Mapping Environmental Suitability for Malaria Transmission, Greece. Emerg. Infect. Dis. 2013, 19, 786. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, M.; Kovats, R.S.; Livermore, M.T.J.; Martens, P. Climate change and malaria: Analysis of the SRES climate and socio-economic scenarios. Glob. Environ. Chang. 2004, 14, 87–99. [Google Scholar] [CrossRef]
- Mironova, V.A. Climate change trends and malaria in the Moscow region. Med. Parasitol. Parazitarnye Bolezni 2006, 4, 20–25. (In Russian) [Google Scholar]
- Oganov, L.I. Malaria in the Moscow Region and its Seasonal Periodicity. Ph.D. Thesis, Martsinovski Institute for Medical Parasitology and Tropical Medicine, Moscow, USSR, 1947. (In Russian). [Google Scholar]
- Shipitsina, N.K. The season of malaria transmission and malarious zones of the Soviet Union. In Problems of Medical Parasitology and Prevention of Infectious Diseases; Moshkovsky, S.D., Ed.; Martsinovski Institute of Medical Parasitology and Tropical Medicine: Moscow, USSR, 1964; pp. 258–278. (In Russian) [Google Scholar]
- Sergiev, V.P.; Baranova, A.M.; Majori, G.; Ezhov, M.N. Malaria in Europe. 1970–2000. A Fresh Look; WHO: Geneva, Switzerland, 2004; p. 220. (In Russian) [Google Scholar]
- Ejov, M.N.; Sergiev, V.P.; Baranova, A.M.; Kurdova-Mintcheva, R.; Emiroglu, N.; Gasimov, E. Malaria in the WHO European Region. On the Road to Elimination, 2000–2015; WHO EURO: Copenhagen, Denmark, 2017; p. 154. (In Russian) [Google Scholar]
- Townroe, S.; Callaghan, A. British Container Breeding Mosquitoes: The Impact of Urbanisation and Climate Change on Community Composition and Phenology. PLoS ONE 2014, 9, e95325. [Google Scholar] [CrossRef] [PubMed]
- Hundessa, S.; Williams, G.; Li, S.; Liu, L.; Cao, W.; Ren, H.; Guo, J.; Gasparrini, A.; Ebi, K.; Zhang, W.; et al. Projecting potential spatial and temporal changes in the distribution of Plasmodium vivax and Plasmodium falciparum malaria in China with climate change. Sci. Total Environ. 2018, 627, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Yasyukevich, V.V.; Titkina, S.N.; Semenov, S.M.; Davidovich, E.A.; Yasyukevich, N.V. Climate change in the second half of the 20th—the beginning of the 21st centuries and related changes of climate-driven ranges of the main malaria vectors in Russia and bordering countries. Prikladnaya Entomol. 2013, 4, 24–30. (In Russian) [Google Scholar]
- Zhao, X.; Smith, D.L.; Tatem, A.J. Exploring the spatiotemporal drivers of malaria elimination in Europe. Malar. J. 2016, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Guerra, C.A.; Moyes, C.L.; Elyazar, I.R.; Gething, P.W.; Hay, S.I.; Tatem, A.J. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar. J. 2012, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Tatem, A.J.; Gething, P.W.; Smith, D.L.; Hay, S.I. Urbanization and the global malaria recession. Malar. J. 2013, 12, 133. [Google Scholar] [CrossRef] [PubMed]



| Index of Favorability of Thermal Conditions (Kf) | Transmission | Epidemiological Outcomes when an Imported Case Appears |
|---|---|---|
| <0.5 | No transmission | Occurrence of secondary (introduced) cases is impossible. |
| 0.5–1 | Unfavorable (shortened) season, a sporadic transmission | Occurrence of a few introduced cases is possible; however, these would not produce a new generation of tertiary cases. |
| ≥1 | Favorable season, an established transmission | In addition to a few introduced cases at the beginning of the season, a second generation of tertiary cases may appear (indigenous cases), thus producing an epidemic. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironova, V.; Shartova, N.; Beljaev, A.; Varentsov, M.; Grishchenko, M. Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium vivax) in Moscow Megacity Region. Int. J. Environ. Res. Public Health 2019, 16, 694. https://doi.org/10.3390/ijerph16050694
Mironova V, Shartova N, Beljaev A, Varentsov M, Grishchenko M. Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium vivax) in Moscow Megacity Region. International Journal of Environmental Research and Public Health. 2019; 16(5):694. https://doi.org/10.3390/ijerph16050694
Chicago/Turabian StyleMironova, Varvara, Natalia Shartova, Andrei Beljaev, Mikhail Varentsov, and Mikhail Grishchenko. 2019. "Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium vivax) in Moscow Megacity Region" International Journal of Environmental Research and Public Health 16, no. 5: 694. https://doi.org/10.3390/ijerph16050694
APA StyleMironova, V., Shartova, N., Beljaev, A., Varentsov, M., & Grishchenko, M. (2019). Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium vivax) in Moscow Megacity Region. International Journal of Environmental Research and Public Health, 16(5), 694. https://doi.org/10.3390/ijerph16050694






