Start-Up of a Biofilter in a Full-Scale Groundwater Treatment Plant for Iron and Manganese Removal
Abstract
:1. Introduction
2. Materials and Methods
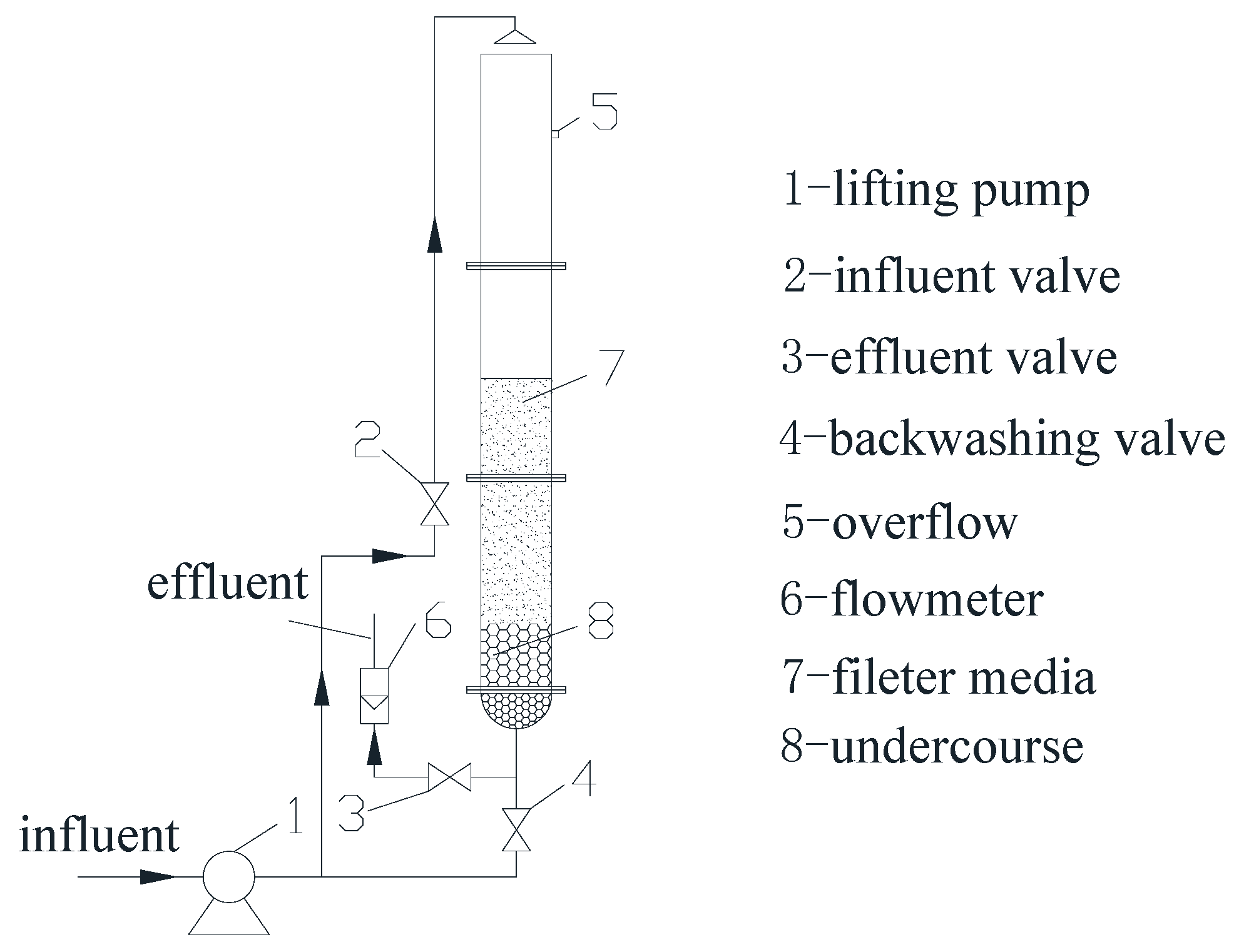
2.1. Water Treatment Plant and Groundwater
2.2. Start-Up Process
2.3. Filter Column Test
2.4. Analysis Methods
2.5. Filter Inoculation
3. Results and Discussion
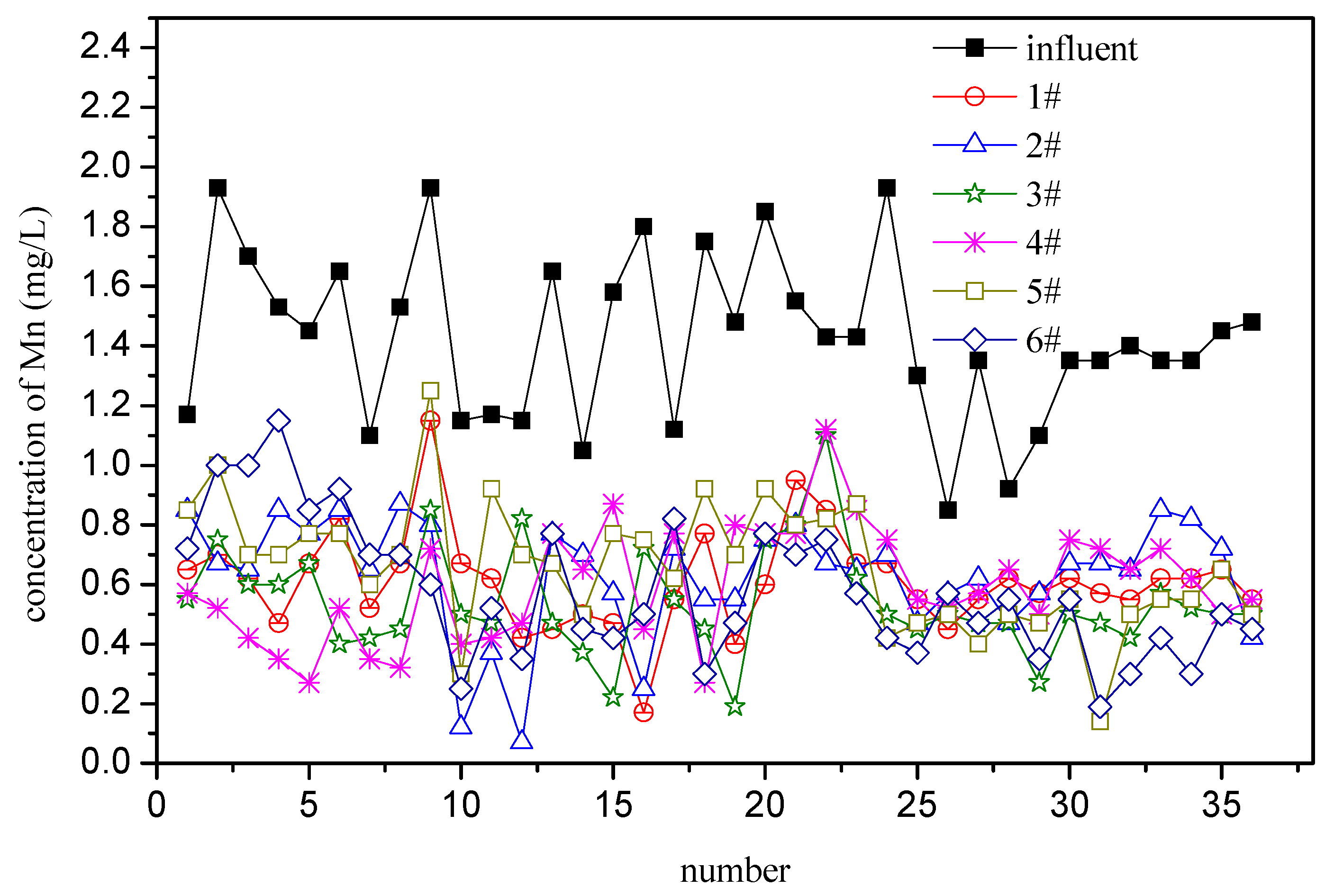
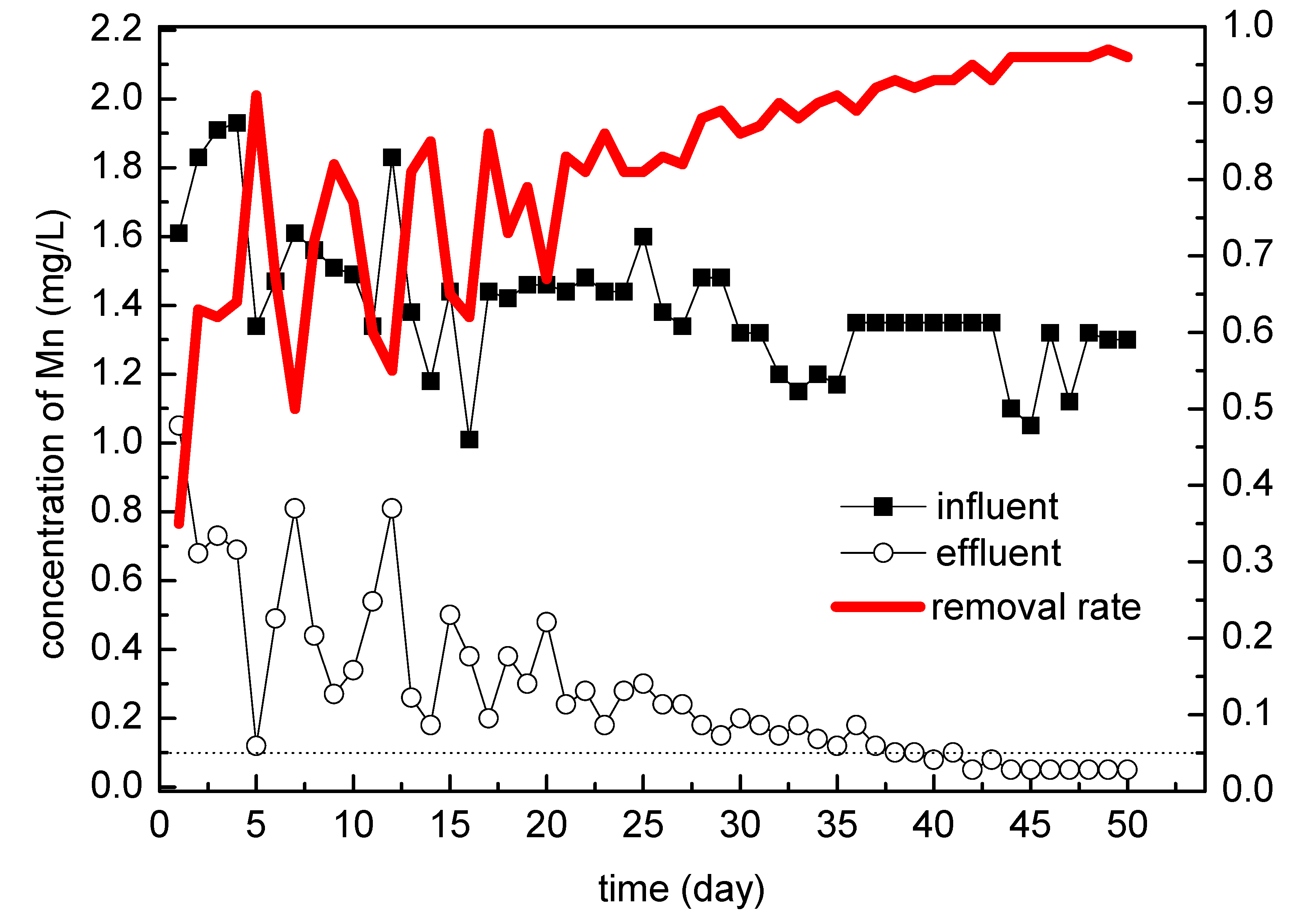
3.1. Full-Scale Filter Test
3.1.1. Initial Intermittent Operation Stage
3.1.2. Continuous Operation Stage
3.1.3. Speed-Up Stage
3.2. Pilot-ScaleFilter Column Test
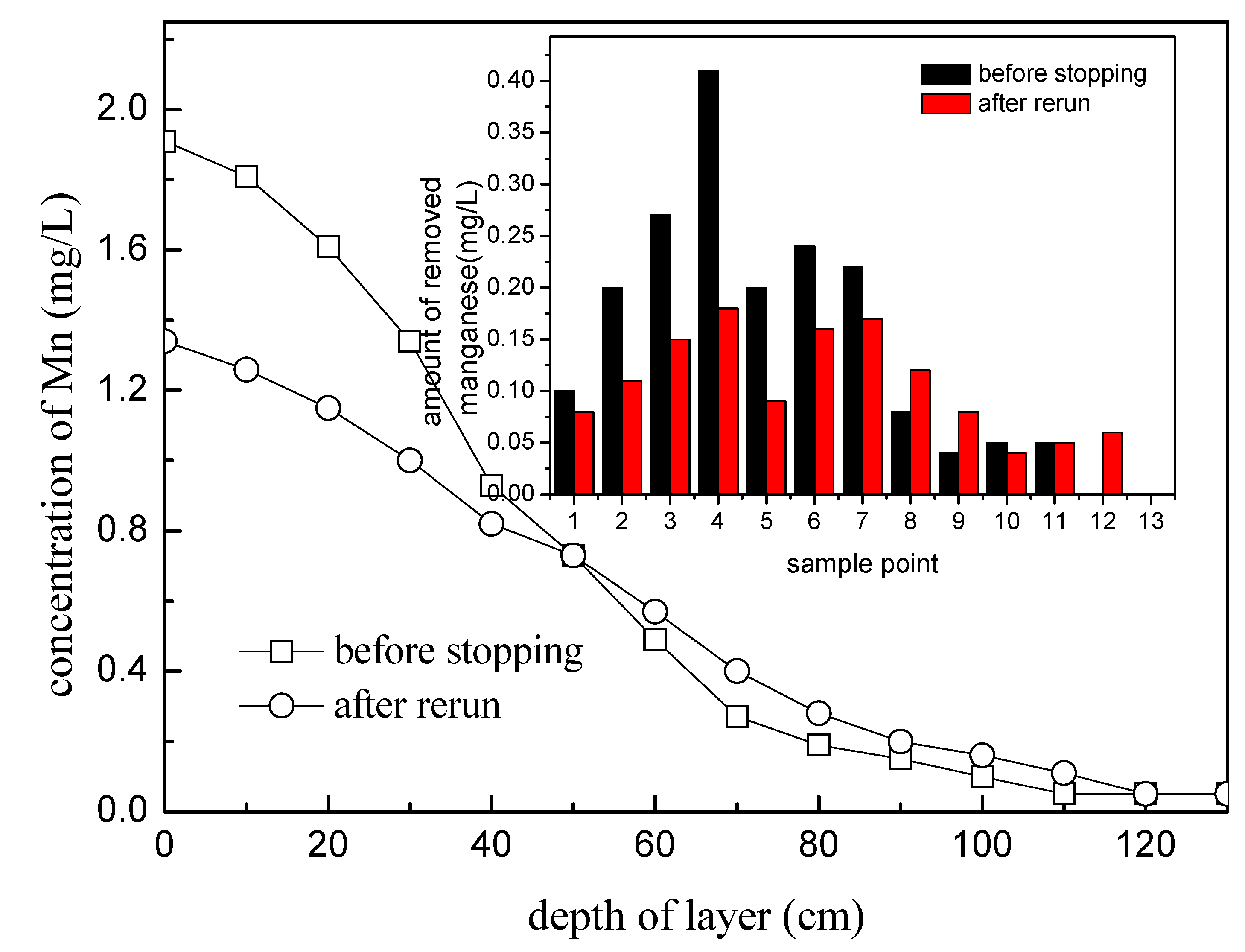
3.2.1. Start-Up of the Filter Column
3.2.2. Suspension and Rerun of the Filter Column
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Standards for Drinking Water Quality (GB5749-2006); Ministry of Health of the PRC: Beijing, China, 2007.
- Civardi, J.; Tompeck, M. Iron and Manganese Removal Handbook, 2nd ed.; AWWA: Denver, CO, USA, 2015. [Google Scholar]
- Dietrich, A.M. EPA Secondary Maximum Contaminant Levels: A Strategy for Drinking Water Quality and Consumer Acceptability; United States Environmental Protection Agency: Washington, DC, USA, 2015.
- Wong, J.M. Chlorination-filtration for iron and manganese removal. JAWWA 1984, 76, 76–79. [Google Scholar] [CrossRef]
- Knocke, W.R.; Hamon, J.R.; Thompson, C.P. Soluble manganese removal on oxide-coated filter media. JAWWA 1988, 80, 65–70. [Google Scholar] [CrossRef]
- Ellis, D.; Bouchard, C.; Lantagne, G. Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 2000, 130, 255–264. [Google Scholar] [CrossRef]
- Aziz, H.A.; Smith, P.G. Removal of manganese from water using crushed dolomite filtration technique. Water Res. 1996, 30, 489–492. [Google Scholar] [CrossRef]
- Frederick, W.P. Legislation/Regulation—Nitrate and Cancer: Is There a Link? JAWWA 1993, 85, 12–14. [Google Scholar]
- Dominguez, H.L.; Andrea, T.; Manuela, A. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Chu, W.H.; Sui, M.H. Comparison of drinking water treatment processes combinations for the minimization of subsequent disinfection by-products formation during chlorination and chloramination. Chem. Eng. J. 2018, 335, 352–361. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Biological treatment of Mn(II) and Fe(II) containing groundwater: Kinetic considerations and product characterization. Water Res. 2004, 38, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Petrusevski, B.; Schippers, J.C. Biological iron removal from groundwater: A review. J. Water Supply Res. Technol. Aqua. 2005, 54, 239–245. [Google Scholar] [CrossRef]
- Yang, H.; Li, D.; Zeng, H.P.; Zhang, J. Autotrophic nitrogen conversion process and microbial population distribution in biofilter that simultaneously removes Fe, Mn and ammonia from groundwater. Int. Biodeterior. Biodegrad. 2019, 135, 53–61. [Google Scholar] [CrossRef]
- Yang, H.; Li, D.; Zeng, H.P.; Zhang, J. Impact of Mn and ammonia on nitrogen conversion in biofilter coupling nitrification and ANAMMOX that simultaneously removes Fe, Mn and ammonia. Sci. Total Environ. 2019, 648, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Nuratiqah, M.; Hassimi, A.H.; Siti Rozaimah Sheikh, A. A review of biological aerated filters for iron and manganese ions removal in water treatment. J. Water Proc. Eng. 2018, 23, 1–12. [Google Scholar]
- Breda, I.L.; Ramsay, L.; Roslev, P. Manganese oxidation and bacterial diversity on different filter media coatings during the start- up of drinking water biofilters. J. Water Supply Res. Technol. Aqua. 2017, 66, 641–650. [Google Scholar] [CrossRef]
- Cai, Y.; Li, D.; Liang, Y.W.; Zeng, H.P. Operational parameters required for the start-up process of a biofilter to remove Fe, Mn, and NH3-N from low-temperature groundwater. Desalin. Water Treat. 2016, 57, 3588–3596. [Google Scholar] [CrossRef]
- Yang, H.; Li, D.; Zhang, J. Design of biological filter for iron and manganese removal from water. J. Environ. Sci. Health Part A 2004, 39, 1447–1454. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Wang, H.T.; Yang, H.; Wang, B.Z. Operational Performance of Biological Treatment Plant for Iron and Manganese Removal. J. Water Supply Res. Technol. Aqua. 2005, 54, 15–24. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Wang, H.T.; Chen, L.X.; Wang, H. Application of biological process to treat the groundwater with high concentration of iron and manganese. J. Water Supply Res. Technol. Aqua. 2006, 55, 313–320. [Google Scholar] [CrossRef]
- Li, D. The Study on the Theory and Application in Engineering for Biological Removal of Iron and Manganese. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 20 January 2005. (In Chinese). [Google Scholar]
- Li, X.; Chu, Z.; Liu, Y.; Zhu, M.; Yang, L.; Zhang, J. Molecular characterization of microbial populations in full-scale biofilters treating iron, manganese and ammonia containing groundwater in Harbin, China. Bioresour. Technol. 2013, 147, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, D.; Yang, H.; Chen, L.X.; Gao, J. Mechanism and Engineering Technology of Biological Manganese Fixation and Manganese Removal; China Architecture & Building Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Tamara, S.; Marinko, M.; Felicita, B.; Laszlo, S. Rapid startup of biofilters for removal of ammonium, iron and manganese from ground water. J. Water Supply Res. Technol. Aqua. 2004, 53, 509–518. [Google Scholar]
- Cai, Y.; Li, D.; Liang, Y.H.; Luo, Y.H.; Zeng, H.P.; Zhang, J. Effective start-up biofiltration method for Fe, Mn, and ammonia removal and bacterial community analysis. Bioresour. Technol. 2015, 176, 149–155. [Google Scholar] [CrossRef] [PubMed]
- SEPA (State Environmental Protection Administration). Monitoring and Analytical Methods for Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. (In Chinese)
- Edzwald, J.K. Water Quality & Treatment: A Handbook on Drinking Water, 6th ed.; American Water Works Association, McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Li, D.; Cao, R.H.; Yang, H.; Wang, Y.J.; Lv, S.S.; Zhang, J. Start-up and operation of biofilter coupled nitrification and CANON for the removal of iron, manganese and ammonia nitrogen. Huanjing Kexue 2018, 39, 1264–1271. (In Chinese) [Google Scholar] [PubMed]
- Cheng, Q.F. Competitive mechanism of ammonia, iron and manganese for dissolved oxygen using pilot-scale biofilter at different dissolved oxygen concentrations. Water Sci. Technol. Water Supply 2016, 16, 766–774. [Google Scholar] [CrossRef]





| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Temperature (°C) | 12–14 | Total dissolved solids (mg/L) | 240–260 |
| pH | 6.7–6.9 | DO (mg/L) | 0 |
| Turbidity (NTU) | 32.3–39.0 | Conductivity (Us/cm) | 268–280 |
| Mn2+ (mg/L) | 0.8–2.0 | Fe2+ (mg/L) | 0.15–0.20 |
| Stage | Frequency of Operation | Velocity | Time |
|---|---|---|---|
| Intermittent Operation | Once three days, 6 h at a time | 1.5 m/h | Two months |
| Once a day, 7 h at a time | 1.5 m/h | Two months | |
| Continuous operation | Continuous running | 1.5 m/h | Two months |
| speed-up | Continuous running | 1.5 m/h to 5 m/h step by step | One month |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Yin, C.; Zhang, J.; Li, D. Start-Up of a Biofilter in a Full-Scale Groundwater Treatment Plant for Iron and Manganese Removal. Int. J. Environ. Res. Public Health 2019, 16, 698. https://doi.org/10.3390/ijerph16050698
Zeng H, Yin C, Zhang J, Li D. Start-Up of a Biofilter in a Full-Scale Groundwater Treatment Plant for Iron and Manganese Removal. International Journal of Environmental Research and Public Health. 2019; 16(5):698. https://doi.org/10.3390/ijerph16050698
Chicago/Turabian StyleZeng, Huiping, Can Yin, Jie Zhang, and Dong Li. 2019. "Start-Up of a Biofilter in a Full-Scale Groundwater Treatment Plant for Iron and Manganese Removal" International Journal of Environmental Research and Public Health 16, no. 5: 698. https://doi.org/10.3390/ijerph16050698
APA StyleZeng, H., Yin, C., Zhang, J., & Li, D. (2019). Start-Up of a Biofilter in a Full-Scale Groundwater Treatment Plant for Iron and Manganese Removal. International Journal of Environmental Research and Public Health, 16(5), 698. https://doi.org/10.3390/ijerph16050698






