The Relationship between Eicosanoid Levels and Serum Levels of Metabolic and Hormonal Parameters Depending on the Presence of Metabolic Syndrome in Patients with Benign Prostatic Hyperplasia
Abstract
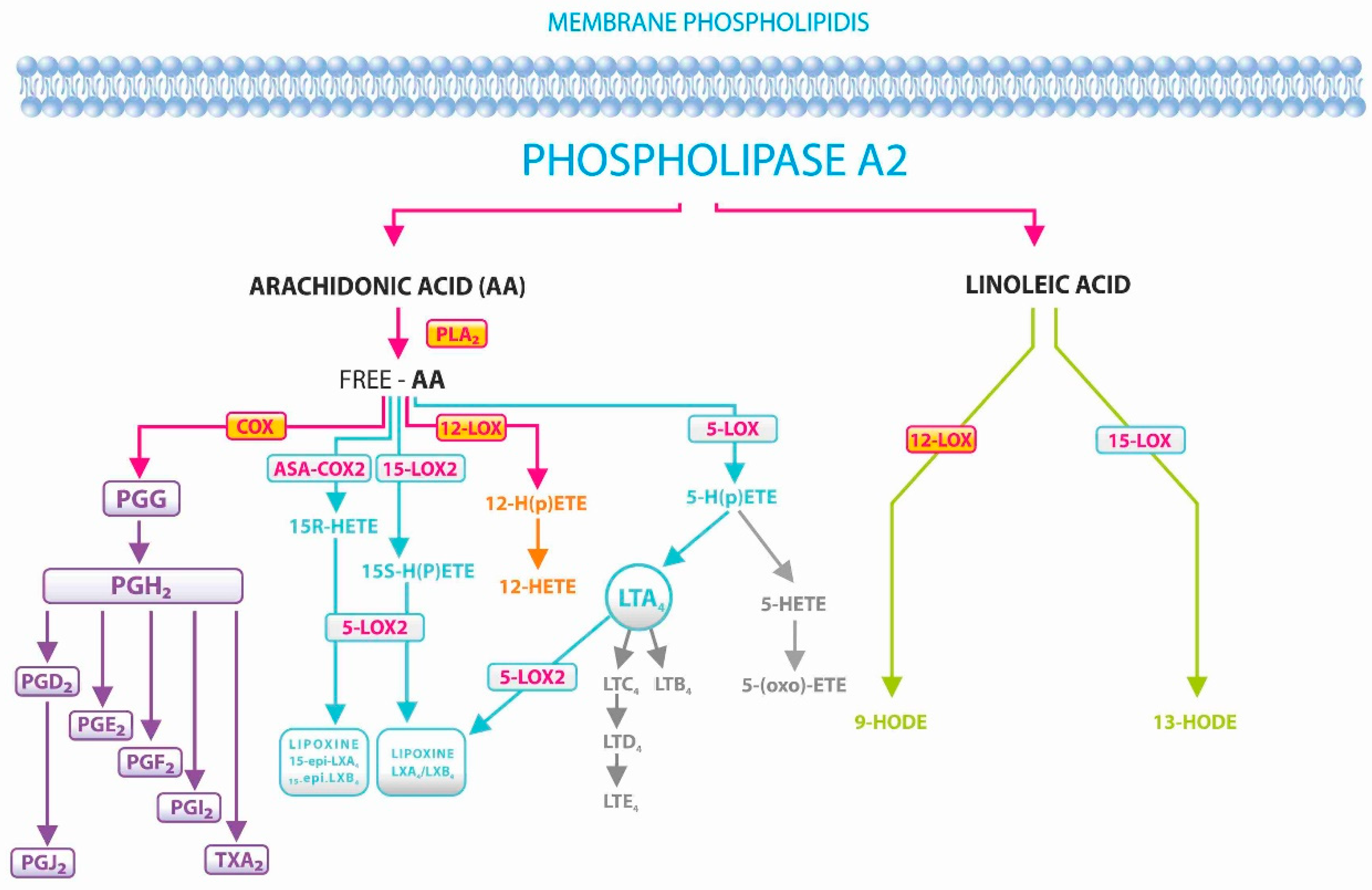
1. Background
2. Material and Methods
2.1. Patients
2.2. Clinical Examination
2.3. Blood Serum Analysis
2.4. Isolation of Fatty Acids
2.5. Analysis of Fatty Acid Methyl Esters
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Nunzio, C.; Aronson, W.; Freedland, S.J.; Giovannucci, E.; Parsons, J.K. The correlation between metabolic syndrome and prostatic diseases. Eur. Urol. 2012, 61, 560–570. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Vignozzi, L.; Salvi, M.; Serni, S.; De Nunzio, C. Metabolic syndrome and benign prostatic enlargement: A systematic review and meta-analysis. BJU Int. 2015, 115, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Rył, A.; Rotter, I.; Miazgowski, T.; Słojewski, M.; Dołęgowska, B.; Lubkowska, A.; Laszczyńska, M. Metabolic syndrome and benign prostatic hyperplasia: Association or coincidence? Diabetol. Metab. Syndr. 2015, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z. Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty AIDS. Postępy Higieny i Medycyny Doświadczalnej 2010, 64, 115–132. [Google Scholar] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Z.; Tang, X.; Dai, F.; Wei, J.; Sun, G. 5-Oxo-ETE from Nasal Epithelial Cells Upregulates Eosinophil Cation Protein by Eosinophils in Nasal Polyps in vitro. Int. Arch. Allergy Immunol. 2018, 177, 107–115. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef]
- Macica, C.; Balazy, M.; Falck, J.R.; Mioskowski, C.; Carroll, M.A. Characterization of cytochrome P-450-dependent arachidonic acid metabolism in rabbit intestine. Am. J. Physiol. 1993, 265, G735–G741. [Google Scholar] [CrossRef]
- Bednar, M.M.; Gross, C.E.; Russell, S.R.; Fuller, S.P.; Ahern, T.P.; Howard, D.B.; Falck, J.R.; Reddy, K.M.; Balazy, M. 16(R)-hydroxyeicosatetraenoic acid, a novel cytochrome P450 product of arachidonic acid, suppresses activation of human olymorphonuclear leukocytes and reduces intracranial pressure in arabbit model of thromboembolic stroke. Neurosurgery 2000, 47, 1410–1419. [Google Scholar] [CrossRef]
- Wittwer, J.; Hersberger, M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 67–77. [Google Scholar] [CrossRef]
- Bojic, L.A.; McLaren, D.G.; Harms, A.C.; Hankemeier, T.; Dane, A.; Wang, S.P.; Rosa, R.; Previs, S.F.; Johns, D.G.; Castro-Perez, J.M. Quantitative profiling of oxylipins in plasma and atherosclerotic plaques of hypercholesterolemic rabbits. Anal. Bioanal. Chem. 2016, 408, 97–105. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Itoh, L.; Fairall, K.; Amin, Y.; Inaba, A.; Szanto, B.L.; Balint, L.; Nagy, L.; Yamamoto, K.; Schwabe, J.W. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008, 15, 924–931. [Google Scholar] [CrossRef]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. IDF Epidemiology Task Force Consensus Group. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Wesołowski, P.; Wańkowicz, Z. Insulin resistance diagnostic methods and clinical outcomes. Nefrol. Dial. Pol. 2011, 15, 243–246. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation andpurification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Kim, O.Y.; Lim, H.H.; Lee, M.J.; Kim, J.Y.; Lee, J.H. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 366–374. [Google Scholar] [CrossRef]
- Huang, X.; Sjögren, P.; Ärnlöv, J.; Cederholm, T.; Lind, L.; Stenvinkel, P.; Lindholm, B.; Risérus, U.; Carrero, J.J. Serum fatty acid patterns, insulin sensitivity and the metabolic syndrome in individuals with chronic kidney disease. J. Intern. Med. 2014, 275, 71–83. [Google Scholar] [CrossRef]
- Maciejewska, D.; Ossowski, P.; Drozd, A.; Ryterska, K.; Jamioł-Milc, D.; Banaszczak, M.; Kaczorowska, M.; Sabinicz, A.; Raszeja-Wyszomirska, J.; Stachowska, E. Metabolites of arachidonic acid and linoleic acid in early stages of non-alcoholic fatty liver disease—A pilot study. Prostaglandins Other. Lipid Mediat. 2015, 121, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, M.J.; Swift, L.L.; Fazio, S.; Linton, M.F.; Raggi, P.; Solus, J.F.; Oeser, A.; Bian, A.; Gebretsadik, T.; Shintani, A.; et al. Free fatty acids are associated with metabolic syndrome and insulin resistance but not inflammation in systemic lupus erythematosus. Lupus 2013, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mamalakis, G.; Kafatos, A.; Kalogeropoulos, N.; Andrikopoulos, N.; Daskalopulos, G.; Kranidis, A. Prostate cancer vs. hyperplasia: Relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukot. Essent Fatty Acids 2002, 66, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Careaga, V.P.; Sacca, P.A.; Mazza, O.N.; Scorticati, C.; Vitagliano, G.; Fletcher, S.J.; Maier, M.S.; Calvo, J.C. Fatty acid composition of human periprostatic adipose tissue from argentine patients and its relationship to prostate cancer and benign prostatic hyperplasia. Res. Cancer Tumor. 2015, 4, 1–6. [Google Scholar]
- Giskeødegård, G.F.; Hansen, A.F.; Bertilsson, H.; Gonzalez, S.V.; Kristiansen, K.A.; Bruheim, P.; Mjøs, S.A.; Angelsen, A.; Bathen, T.F.; Tessem, M.B. Metabolic markers in blood can separate prostate cancer from benign prostatic hyperplasia. Br. J. Cancer 2015, 113, 1712–1729. [Google Scholar] [CrossRef]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.P.; Montoliu, I.; Collin, S.; Scherer, M.; Guy, P.; Tavazzi, I.; Thorimbert, A.; Moco, S.; Rothney, M.P.; Ergun, D.L.; et al. Topographical body fat distribution links to amino acid and lipid metabolism in healthy obese women. PLoS ONE 2013, 8, 73445. [Google Scholar] [CrossRef]
- Parsons, J.K.; Sarma, A.V.; McVary, K.; Wei, J.T. Obesity and benign prostatic hyperplasia: Clinical connections, emerging etiological paradigms and future directions. J. Urol. 2013, 189, S102–S106. [Google Scholar] [CrossRef]
- Fowke, J.H.; Motley, S.S.; Cookson, M.S.; Concepcion, R.; Chang, S.S.; Wills, M.L.; Smith, J.A., Jr. The association between body size, prostate volume and prostate-specific antigen. Prostate Cancer Prostatic Dis. 2007, 10, 137–142. [Google Scholar] [CrossRef]
- Mosli, H.A.; Mosli, H.H. Influence of body mass index on Benign Prostatic Hyperplasia-related complications in patients undergoing prostatectomy. Springerplus 2013, 2, 537. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, J.R.; Rao, J.M.; Song, W.; Zhou, K.Q. Association between benign prostatic hyperplasia, body mass index, and metabolic syndrome in Chinese men. Asian J. Androl. 2015, 17, 826–830. [Google Scholar] [CrossRef]
- Weaver, J.R.; Holman, T.R.; Imai, Y.; Jadhav, A.; Kenyon, V.; Maloney, D.J.; Nadler, J.L.; Rai, G.; Simeonov, A.; Taylor-Fishwick, D.A. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol. Cell Endocrinol. 2012, 358, 88–95. [Google Scholar] [CrossRef]
- Wang, D.; Strandgaard, S.; Iversen, J.; Wilcox, C.S. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R195–R200. [Google Scholar] [CrossRef]
- Morgantini, C.; Meriwether, D.; Baldi, S.; Venturi, E.; Pinnola, S.; Wagner, A.C.; Fogelman, A.M.; Ferrannini, E.; Natali, A.; Reddy, S.T. HDL lipid composition is profoundly altered in patients with type 2 diabetes and atherosclerotic vascular disease. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 594–599. [Google Scholar] [CrossRef]
- Ross, D.J.; Hough, G.; Hama, S.; Aboulhosn, J.; Belperio, J.A.; Saggar, R.; Van Lenten, B.J.; Ardehali, A.; Eghbali, M.; Reddy, S.; et al. Proinflammatory highdensity lipoprotein results from oxidized lipid mediators in the pathogenesis of both idiopathic and associated types of pulmonary arterial hypertension. Pulm. Circ. 2015, 5, 640–648. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Maciejewska, D.; Drozd, A.; Ossowski, P.; Ryterska, K.; Jamioł-Milc, D.; Banaszczak, M.; Raszeja-Wyszomirska, J.; Kaczorowska, M.; Sabinicz, A.; Stachowska, E. Fatty acid changes help to better understand regression of nonalcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 301–310. [Google Scholar] [CrossRef]
- Tunaru, S.; Bonnavion, R.; Brandenburger, I.; Preussner, J.; Thomas, D.; Scholich, K.; Offermanns, S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 2018, 9, 177. [Google Scholar] [CrossRef]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef]
- Hughes-Fulford, M.; Li, C.F.; Boonyaratanakornkit, J.; Sayyah, S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006, 66, 1427–1433. [Google Scholar] [CrossRef]
- Ghosh, J.; Myers, C.E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Pidgeon, G.P.; Kandouz, M.; Meram, A.; Honn, K.V. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002, 62, 2721–2727. [Google Scholar] [PubMed]
- Jack, G.S.; Brash, A.R.; Olson, S.J.; Manning, S.; Coffey, C.S.; Smith, J.A., Jr.; Shappell, S.B. Reduced 15- lipoxygenase-2 immunostaining in prostate adenocarcinoma: Correlation with grade and expression in high-grade prostatic intraepithelial neoplasia. Hum. Pathol. 2000, 31, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Shappell, S.B.; Manning, S.; Boeglin, W.E.; Guan, Y.F.; Roberts, R.L.; Davis, L.; Olson, S.J.; Jack, G.S.; Coffey, C.S.; Wheeler, T.M.; et al. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia 2001, 3, 287–303. [Google Scholar] [CrossRef] [PubMed]
- His, L.C.; Wilson, L.C.; Eling, T.E. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate. Alteration in peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2002, 277, 40549–40556. [Google Scholar]
- Mammi, C.; Calanchini, M.; Antelmi, A.; Cinti, F.; Rosano, G.M.; Lenzi, A.; Caprio, M.; Fabbri, A. Androgens and adipose tissue in males: A complex and reciprocal interplay. Int. J. Endocrinol. 2012, 2012, 789653. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, J.K.; Cho, B. The role of androgen in the adipose tissue of males. World J. Mens Health 2013, 31, 136–140. [Google Scholar] [CrossRef]
| Variable | Patients with BPH and without MetS n = 99 | Patients with BPH and with MetS n = 52 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X | SD | Min | Max | X | SD | Min | Max | ||
| 16RS HETE (µg/mL) | 3.85 | 3.25 | 0.04 | 11.48 | 5.55 | 6.19 | 0.07 | 19.23 | 0.660 |
| 13S HODE (µg/mL) | 0.20 | 0.34 | 0.02 | 2.40 | 0.29 | 0.70 | 0.01 | 4.88 | 0.226 |
| 9S HODE (µg/mL) | 0.13 | 0.18 | 0.01 | 1.48 | 0.17 | 0.19 | 0.02 | 1.21 | 0.089 |
| 15S HETE (µg/mL) | 0.98 | 1.08 | 0.10 | 8.44 | 0.99 | 0.91 | 0.10 | 5.76 | 0.485 |
| 12S HETE (µg/mL) | 10.49 | 9.69 | 0.55 | 54.89 | 13.46 | 11.35 | 0.92 | 41.58 | 0.136 |
| 5 oxo ETE (µg/mL) | 0.66 | 0.48 | 0.06 | 2.33 | 0.59 | 0.31 | 0.29 | 1.13 | 0.751 |
| 5 HETE (µg/mL) | 0.15 | 0.12 | 0.02 | 0.66 | 0.18 | 0.15 | 0.02 | 0.81 | 0.186 |
| Variable | Abdominal Circumference below 94 cm n = 48 | Abdominal Circumference above 94 cm n = 97 | p | No Diabetes n = 112 | Diabetes n = 39 | p | ||||
| X | SD | X | SD | X | SD | X | SD | |||
| 16RS HETE (µg/mL) | 4.10 | 5.24 | 4.57 | 4.28 | 0.607 | 4.00 | 3.54 | 6.52 | 7.64 | 0.776 |
| 13S HODE (µg/mL) | 0.16 | 0.33 | 0.26 | 0.56 | 0.006 * | 0.19 | 0.32 | 0.35 | 0.83 | 0.242 |
| 9S HODE (µg/mL) | 0.12 | 0.21 | 0.16 | 0.17 | 0.004 * | 0.14 | 0.18 | 0.17 | 0.21 | 0.291 |
| 15S HETE (µg/mL) | 0.72 | 0.78 | 1.11 | 1.10 | <0.001 * | 0.99 | 1.02 | 0.97 | 1.05 | 0.600 |
| 12S HETE (µg/mL) | 9.09 | 8.65 | 12.73 | 11.01 | 0.039 * | 10.92 | 9.21 | 13.49 | 13.46 | 0.703 |
| 5 oxo ETE (µg/mL) | 0.56 | 0.15 | 0.66 | 0.47 | 0.908 | 0.65 | 0.47 | 0.60 | 0.33 | 0.973 |
| 5 HETE (µg/mL) | 0.12 | 0.11 | 0.19 | 0.14 | 0.001 * | 0.16 | 0.12 | 0.17 | 0.16 | 0.952 |
| Variable | No hypercholesterolemia n = 100 | Hypercholesterolemia n = 51 | p | No hypertension n = 51 | Hypertension n = 100 | p | ||||
| X | SD | X | SD | X | SD | X | SD | |||
| 16RS HETE [µg/mL] | 4.25 | 4.03 | 5.35 | 6.61 | 0.995 | 4.16 | 3.09 | 4.67 | 5.45 | 0.634 |
| 13S HODE [µg/mL] | 0.22 | 0.37 | 0.26 | 0.71 | 0.377 | 0.18 | 0.32 | 0.25 | 0.57 | 0.501 |
| 9S HODE [µg/mL] | 0.16 | 0.22 | 0.12 | 0.07 | 0.613 | 0.14 | 0.21 | 0.15 | 0.17 | 0.512 |
| 15S HETE [µg/mL] | 1.04 | 1.18 | 0.87 | 0.53 | 0.739 | 1.00 | 0.86 | 0.97 | 1.11 | 0.364 |
| 12S HETE [µg/mL] | 11.23 | 10.55 | 12.24 | 10.18 | 0.714 | 10.74 | 10.41 | 11.97 | 10.45 | 0.353 |
| 5 oxo ETE [µg/mL] | 0.70 | 0.46 | 0.43 | 0.28 | 0.155 | 0.55 | 0.14 | 0.72 | 0.58 | 0.772 |
| 5 HETE [µg/mL] | 0.17 | 0.14 | 0.16 | 0.12 | 0.838 | 0.16 | 0.13 | 0.16 | 0.14 | 0.731 |
| Variable | Correlations in Patients with BPH and MetS | Correlations in Patients with BPH and without MetS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Body Weight | WC | TG | TCh | HDL | LDL | FPG | HOMA | Age | Body Weight | WC | TG | TCh | HDL | LDL | FPG | HOMA | ||
| 16RS HETE | P | 0.032 | −0.049 | −0.066 | 0.261 | −0.088 | −0.439 | −0.075 | −0.052 | −0.007 | −0.352 | 0.002 | 0.236 | 0.104 | −0.258 | −0.044 | −0.256 | −0.488 * | −0.166 |
| (p) | (0.913) | 0.867) | (0.823) | (0.368) | (0.764) | (0.116) | (0.800) | (0.861) | (0.982) | (0.078) | (0.991) | (0.245) | (0.612) | (0.203) | (0.832) | (0.206) | (0.011) | (0.429) | |
| 13S HODE | P | −0.064 | 0.113 | 0.210 | 0.183 | 0.210 | 0.165 | 0.054 | 0.182 | −0.052 | 0.213 * | −0.105 | 0.024 | −0.208 * | 0.117 | 0.174 | 0.106 | 0.045 | 0.004 |
| (p) | (0.657) | 0.430) | (0.139) | (0.198) | (0.139) | (0.248) | (0.707) | (0.200) | (0.728) | (0.039) | (0.312) | (0.817) | (0.044) | (0.262) | (0.094) | (0.313) | (0.664) | (0.973) | |
| 9S HODE | P | 0.098 | 0.005 | 0.114 | 0.672 * | 0.480 * | −0.156 | 0.184 | 0.516 * | 0.117 | 0.144 | −0.026 | 0.080 | −0.104 | −0.069 | 0.054 | −0.060 | 0.002 | −0.041 |
| (p) | (0.488) | 0.974) | (0.421) | (0.001) | (0.001) | (0.270) | (0.197) | (0.001) | (0.423) | (0.166) | (0.801) | (0.441) | (0.320) | (0.506) | (0.605) | (0.570) | (0.987) | (0.700) | |
| 15S HETE | P | 0.079 | −0.038 | 0.018 | 0.573 * | 0.414 * | 0.072 | 0.030 | 0.539 * | 0.232 | 0.140 | 0.031 | 0.232 * | −0.100 | 0.022 | 0.081 | 0.025 | −0.023 | −0.010 |
| (p) | (0.583) | 0.792) | (0.899) | (0.001) | (0.003) | (0.614) | (0.834) | (0.001) | (0.112) | (0.181) | (0.771) | (0.025) | (0.339) | (0.837) | (0.440) | (0.810) | (0.826) | (0.923) | |
| 12S HETE | P | 0.214 | −0.097 | −0.070 | 0.318 * | 0.113 | 0.011 | −0.100 | 0.099 | −0.128 | 0.149 | 0.007 | 0.111 | −0.008 | −0.097 | −0.151 | −0.039 | −0.105 | −0.112 |
| (p) | (0.127) | 0.495) | (0.621) | (0.022) | (0.423) | (0.939) | (0.486) | (0.486) | (0.381) | (0.153) | (0.945) | (0.291) | (0.937) | (0.357) | (0.149) | (0.712) | (0.317) | (0.291) | |
| 5 HETE | P | 0.180 | 0.029 | 0.135 | 0.543 * | 0.272 | −0.154 | −0.038 | 0.336 * | 0.110 | 0.182 | 0.077 | 0.251 * | −0.031 | −0.005 | −0.030 | 0.022 | 0.011 | −0.021 |
| (p) | (0.205) | 0.840) | (0.346) | (0.001) | (0.054) | (0.279) | (0.791) | (0.016) | (0.457) | (0.083) | (0.466) | (0.016) | (0.769) | (0.966) | (0.779) | (0.833) | (0.921) | (0.845) | |
| Variable | Correlations in Patients with BPH and MetS | Correlations in Patients with BPH and without MetS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHEAS | E2 | SHBG | LH | TT | TF | IGF1 | I | DHEAS | E2 | SHBG | LH | TT | TF | IGF1 | I | ||
| 16RS HETE | P | −0.074 | 0.163 | −0.117 | 0.480 | −0.202 | −0.029 | 0.140 | −0.236 | 0.105 | 0.025 | 0.045 | −0.125 | −0.054 | −0.021 | −0.130 | −0.048 |
| (p) | (0.801) | (0.577) | (0.691) | (0.082) | (0.489) | (0.921) | (0.632) | (0.437) | (0.610) | (0.904) | (0.827) | (0.541) | (0.793) | (0.919) | (0.528) | (0.827) | |
| 13S HODE | P | 0.028 | 0.046 | 0.135 | 0.002 | 0.447 * | 0.259 | 0.164 | −0.167 | −0.013 | −0.058 | 0.073 | 0.088 | −0.114 | −0.092 | 0.117 | −0.019 |
| (p) | (0.846) | (0.751) | (0.346) | (0.990) | (0.001) | (0.066) | (0.250) | (0.302) | (0.899) | (0.578) | (0.486) | (0.401) | (0.275) | (0.379) | (0.263) | (0.865) | |
| 9S HODE | P | 0.050 | −0.061 | 0.072 | 0.126 | 0.059 | 0.275 * | 0.059 | −0.053 | −0.009 | −0.062 | 0.104 | −0.054 | −0.035 | −0.046 | 0.093 | −0.061 |
| (p) | (0.724) | (0.668) | (0.614) | (0.372) | (0.677) | (0.048) | (0.677) | (0.743) | (0.935) | (0.550) | (0.317) | (0.607) | (0.739) | (0.662) | (0.373) | (0.594) | |
| 15S HETE | P | 0.007 | −0.095 | −0.102 | 0.020 | −0.054 | 0.253 | −0.037 | 0.046 | −0.024 | −0.061 | 0.097 | −0.082 | 0.004 | −0.049 | 0.070 | −0.048 |
| (p) | (0.962) | (0.505) | (0.478) | (0.890) | (0.709) | (0.074) | (0.796) | (0.776) | (0.822) | (0.561) | (0.356) | (0.433) | (0.966) | (0.643) | (0.508) | (0.676) | |
| 12S HETE | P | −0.161 | −0.110 | 0.068 | 0.139 | 0.099 | 0.060 | 0.116 | −0.239 | −0.033 | 0.004 | 0.121 | −0.164 | 0.069 | −0.058 | −0.022 | −0.124 |
| (p) | (0.253) | (0.439) | (0.632) | (0.324) | (0.487) | (0.672) | (0.414) | (0.132) | (0.753) | (0.972) | (0.248) | (0.117) | (0.508) | (0.579) | (0.833) | (0.279) | |
| 5 HETE | P | −0.107 | −0.076 | −0.034 | 0.120 | 0.016 | 0.190 | 0.167 | −0.054 | 0.043 | −0.013 | 0.141 | −0.076 | 0.129 | −0.012 | 0.075 | −0.045 |
| (p) | (0.454) | (0.597) | (0.815) | (0.404) | (0.910) | (0.181) | (0.242) | (0.740) | (0.685) | (0.905) | (0.180) | (0.470) | (0.220) | (0.907) | (0.476) | (0.700) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzesiak, K.; Rył, A.; Stachowska, E.; Słojewski, M.; Rotter, I.; Ratajczak, W.; Sipak, O.; Piasecka, M.; Dołęgowska, B.; Laszczyńska, M. The Relationship between Eicosanoid Levels and Serum Levels of Metabolic and Hormonal Parameters Depending on the Presence of Metabolic Syndrome in Patients with Benign Prostatic Hyperplasia. Int. J. Environ. Res. Public Health 2019, 16, 1006. https://doi.org/10.3390/ijerph16061006
Grzesiak K, Rył A, Stachowska E, Słojewski M, Rotter I, Ratajczak W, Sipak O, Piasecka M, Dołęgowska B, Laszczyńska M. The Relationship between Eicosanoid Levels and Serum Levels of Metabolic and Hormonal Parameters Depending on the Presence of Metabolic Syndrome in Patients with Benign Prostatic Hyperplasia. International Journal of Environmental Research and Public Health. 2019; 16(6):1006. https://doi.org/10.3390/ijerph16061006
Chicago/Turabian StyleGrzesiak, Katarzyna, Aleksandra Rył, Ewa Stachowska, Marcin Słojewski, Iwona Rotter, Weronika Ratajczak, Olimpia Sipak, Małgorzata Piasecka, Barbara Dołęgowska, and Maria Laszczyńska. 2019. "The Relationship between Eicosanoid Levels and Serum Levels of Metabolic and Hormonal Parameters Depending on the Presence of Metabolic Syndrome in Patients with Benign Prostatic Hyperplasia" International Journal of Environmental Research and Public Health 16, no. 6: 1006. https://doi.org/10.3390/ijerph16061006
APA StyleGrzesiak, K., Rył, A., Stachowska, E., Słojewski, M., Rotter, I., Ratajczak, W., Sipak, O., Piasecka, M., Dołęgowska, B., & Laszczyńska, M. (2019). The Relationship between Eicosanoid Levels and Serum Levels of Metabolic and Hormonal Parameters Depending on the Presence of Metabolic Syndrome in Patients with Benign Prostatic Hyperplasia. International Journal of Environmental Research and Public Health, 16(6), 1006. https://doi.org/10.3390/ijerph16061006







