Pro-Inflammatory Responses in Human Bronchial Epithelial Cells Induced by Spores and Hyphal Fragments of Common Damp Indoor Molds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. Preparation of Mold Samples
2.4. Cytokine/Chemokine Release
2.5. Gene Expression
2.6. Microscopic Examination of Germinative Potential
2.7. Cytotoxicity
2.8. Statistical Analysis
3. Results
3.1. Cytokine/Chemokine Release after Exposure to the Different Mold Species
3.2. Role of Experimental Conditions
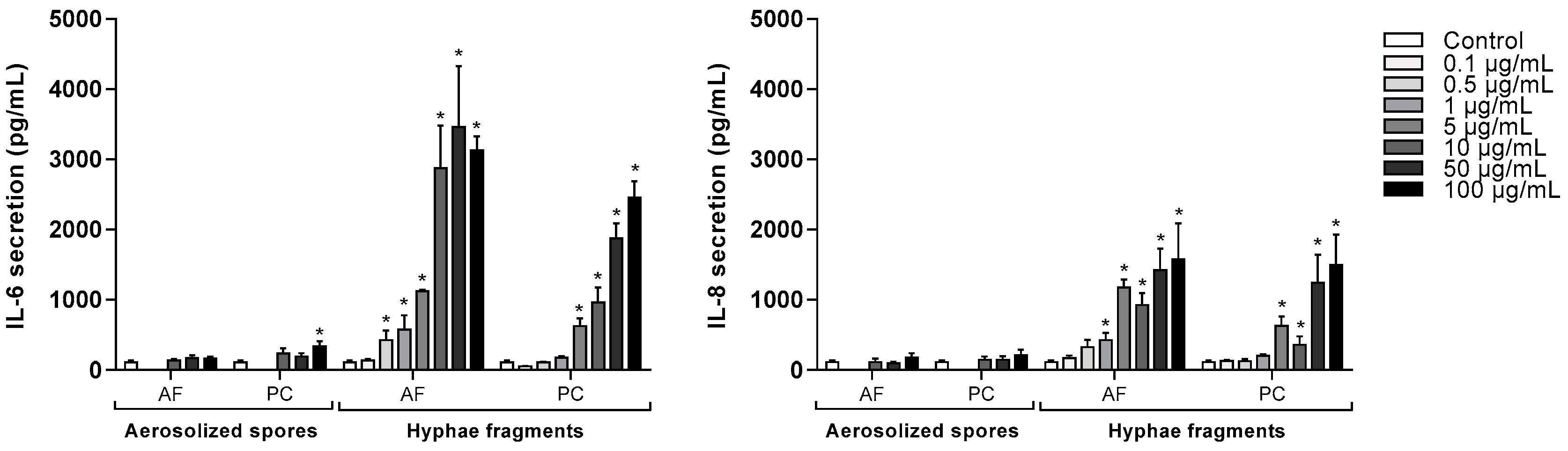
3.3. Concentration-Dependent Release of IL-6 and IL-8 after Exposure to A. fumigatus and P. chrysogenum
3.4. Gene Expression after Exposure of A. fumigatus and P. chrysogenum
3.5. The Role of TLR2 and TLR4 in A. fumigatus and P. chrysogenum Hyphae-Induced Release of IL-6 and IL-8
3.6. Effects of Untreated A. fumigatus Spores on the Gene Expression and Release of IL-6 and IL-8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Indoor Air Quality: Dampness and Mould; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W. Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C.; Sondergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef]
- Gorny, R.L.; Reponen, T.; Willeke, K.; Schmechel, D.; Robine, E.; Boissier, M.; Grinshpun, S.A. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 2002, 68, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Afanou, K.A.; Straumfors, A.; Skogstad, A.; Nilsen, T.; Synnes, O.; Skaar, I.; Hjeljord, L.; Tronsmo, A.; Green, B.J.; Eduard, W. Submicronic Fungal Bioaerosols: High-Resolution Microscopic Characterization and Quantification. Appl. Environ. Microbiol. 2014, 80, 7122–7130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afanou, K.A.; Straumfors, A.; Skogstad, A.; Skaar, I.; Hjeljord, L.; Skare, O.; Green, B.J.; Tronsmo, A.; Eduard, W. Profile and Morphology of Fungal Aerosols Characterized by Field Emission Scanning Electron Microscopy (FESEM). Aerosol Sci. Technol. 2015, 49, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Mensah-Attipoe, J.; Saari, S.; Veijalainen, A.M.; Pasanen, P.; Keskinen, J.; Leskinen, J.T.; Reponen, T. Release and characteristics of fungal fragments in various conditions. Sci. Total Environ. 2016, 547, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Margalit, A.; Kavanagh, K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol. Rev. 2015, 39, 670–687. [Google Scholar] [CrossRef] [Green Version]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pahtz, V.; Shopova, I.; Koster-Eiserfunke, N.; Kruger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2015, 37, 141–152. [Google Scholar] [CrossRef]
- Bhushan, B.; Homma, T.; Norton, J.E.; Sha, Q.; Siebert, J.; Gupta, D.S.; Schroeder, J.W., Jr.; Schleimer, R.P. Suppression of epithelial signal transducer and activator of transcription 1 activation by extracts of Aspergillus fumigatus. Am. J. Respir. Cell Mol. Biol. 2015, 53, 87–95. [Google Scholar] [CrossRef]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellanger, A.P.; Millon, L.; Khoufache, K.; Rivollet, D.; Bieche, I.; Laurendeau, I.; Vidaud, M.; Botterel, F.; Bretagne, S. Aspergillus fumigatus germ tube growth and not conidia ingestion induces expression of inflammatory mediator genes in the human lung epithelial cell line A549. J. Med. Microbiol. 2009, 58, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, M.; Percier, P.; De Prins, S.; Huygen, K.; Potemberg, G.; Muraille, E.; Romano, M.; Michel, O.; Denis, O. Investigation of inflammatory and allergic responses to common mold species: Results from in vitro experiments, from a mouse model of asthma, and from a group of asthmatic patients. Indoor Air 2017, 27, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Osherov, N. Interaction of the pathogenic mold Aspergillus fumigatus with lung epithelial cells. Front. Microbiol. 2012, 3, 346. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.A.; Culibrk, L.; Moore, M.M.; Tebbutt, S.J. Interactions of Aspergillus fumigatus Conidia with Airway Epithelial Cells: A Critical Review. Front. Microbiol. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Wasylnka, J.A.; Moore, M.M. Uptake of Aspergillus fumigatus Conidia by phagocytic and nonphagocytic cells in vitro: Quantitation using strains expressing green fluorescent protein. Infect. Immun. 2002, 70, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Filler, S.G.; Sheppard, D.C. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006, 2, e129. [Google Scholar] [CrossRef] [PubMed]
- Escobar, N.; Ordonez, S.R.; Wosten, H.A.; Haas, P.J.; de Cock, H.; Haagsman, H.P. Hide, Keep Quiet, and Keep Low: Properties That Make Aspergillus fumigatus a Successful Lung Pathogen. Front. Microbiol. 2016, 7, 438. [Google Scholar] [CrossRef]
- Takazono, T.; Sheppard, D.C. Aspergillus in chronic lung disease: Modeling what goes on in the airways. Med. Mycol. 2017, 55, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Philippe, B.; Ibrahim-Granet, O.; Prevost, M.C.; Gougerot-Pocidalo, M.A.; Sanchez Perez, M.; Van der Meeren, A.; Latge, J.P. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 2003, 71, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar]
- Benndorf, D.; Muller, A.; Bock, K.; Manuwald, O.; Herbarth, O.; von Bergen, M. Identification of spore allergens from the indoor mould Aspergillus versicolor. Allergy 2008, 63, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; McCray, P.B., Jr.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015, 45, 1150–1162. [Google Scholar] [CrossRef] [Green Version]
- Huttunen, K.; Hyvarinen, A.; Nevalainen, A.; Komulainen, H.; Hirvonen, M.R. Production of proinflammatory mediators by indoor air bacteria and fungal spores in mouse and human cell lines. Environ. Health Perspect. 2003, 111, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Tovey, E.R.; Sercombe, J.K.; Blachere, F.M.; Beezhold, D.H.; Schmechel, D. Airborne fungal fragments and allergenicity. Med. Mycol. 2006, 44 (Suppl. 1), S245–S255. [Google Scholar] [CrossRef]
- Becker, K.L.; Ifrim, D.C.; Quintin, J.; Netea, M.G.; van de Veerdonk, F.L. Antifungal innate immunity: Recognition and inflammatory networks. Semin. Immunopathol. 2015, 37, 107–116. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Williams, P.B.; Barnes, C.S. Innate Immune Responses to Fungal Allergens. Curr. Allergy Asthma Rep. 2016, 16, 62. [Google Scholar] [CrossRef]
- Plato, A.; Hardison, S.E.; Brown, G.D. Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 2015, 37, 97–106. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Park, S.J.; Mehrad, B. Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 2009, 22, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Øya, E.; Afanou, A.K.J.; Malla, N.; Uhlig, S.; Rolen, E.; Skaar, I.; Straumfors, A.; Winberg, J.O.; Bang, B.E.; Schwarze, P.E.; et al. Characterization and pro-inflammatory responses of spore and hyphae samples from various mold species. Indoor Air 2018, 28, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Grytting, V.S.; Olderbø, B.P.; Holme, J.A.; Samuelsen, J.T.; Solhaug, A.; Becher, R.; Bølling, A.K. Di-n-butyl phthalate modifies PMA-induced macrophage differentiation of THP-1 monocytes via PPARgamma. Toxicol. In Vitro 2018, 54, 168–177. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Skuland, T.; Ovrevik, J.; Lag, M.; Refsnes, M. Role of size and surface area for pro-inflammatory responses to silica nanoparticles in epithelial lung cells: Importance of exposure conditions. Toxicol. In Vitro 2014, 28, 146–155. [Google Scholar] [CrossRef]
- Chai, L.Y.; Netea, M.G.; Vonk, A.G.; Kullberg, B.J. Fungal strategies for overcoming host innate immune response. Med. Mycol. 2009, 47, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reponen, T.; Seo, S.C.; Grimsley, F.; Lee, T.; Crawford, C.; Grinshpun, S.A. Fungal Fragments in Moldy Houses: A Field Study in Homes in New Orleans and Southern Ohio. Atmos. Environ. 2007, 41, 8140–8149. [Google Scholar] [CrossRef] [Green Version]
- Beisswenger, C.; Hess, C.; Bals, R. Aspergillus fumigatus conidia induce interferon-beta signalling in respiratory epithelial cells. Eur. Respir. J. 2012, 39, 411–418. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1alpha and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Øya, E.; Zegeye, F.D.; Bølling, A.K.; Øvstebø, R.; Afanou, A.K.J.; Øvrevik, J.; Refsnes, M.; Holme, J.A. Hyphae fragments from A. fumigatus sensitize lung cells to silica particles (Min-U-Sil): Increased release of IL-1beta. Toxicol. In Vitro 2018, 55, 1–10. [Google Scholar]
- Afonina, I.S.; Muller, C.; Martin, S.J.; Beyaert, R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity 2015, 42, 991–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardestani, S.; Li, B.; Deskins, D.L.; Wu, H.; Massion, P.P.; Young, P.P. Membrane versus soluble isoforms of TNF-alpha exert opposing effects on tumor growth and survival of tumor-associated myeloid cells. Cancer Res. 2013, 73, 3938–3950. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Aimanianda, V.; Wang, X.; Gresnigt, M.S.; Ammerdorffer, A.; Jacobs, C.W.; Gazendam, R.P.; Joosten, L.A.; Netea, M.G.; Latge, J.P.; et al. Aspergillus Cell Wall Chitin Induces Anti- and Proinflammatory Cytokines in Human PBMCs via the Fc-gamma Receptor/Syk/PI3K Pathway. mBio 2016, 7, e01823-15. [Google Scholar] [CrossRef]
- Mambula, S.S.; Sau, K.; Henneke, P.; Golenbock, D.T.; Levitz, S.M. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J. Biol. Chem. 2002, 277, 39320–39326. [Google Scholar] [CrossRef]
- Kauffman, H.F.; Tomee, J.F.; van de Riet, M.A.; Timmerman, A.J.; Borger, P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000, 105, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.K.; Lu, X.; Li, X.; Sun, Q.Y.; Su, X.; Song, Y.; Sun, H.M.; Shi, Y. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2755–2764. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kita, H. Innate and adaptive immune responses to fungi in the airway. J. Allergy Clin. Immunol. 2018, 142, 353–363. [Google Scholar] [CrossRef]
- Gersuk, G.M.; Underhill, D.M.; Zhu, L.; Marr, K.A. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 2006, 176, 3717–3724. [Google Scholar] [CrossRef] [PubMed]
- Balloy, V.; Sallenave, J.M.; Wu, Y.; Touqui, L.; Latge, J.P.; Si-Tahar, M.; Chignard, M. Aspergillus fumigatus-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 MAPK, and ERK1/2 pathways and not by the toll-like receptor-MyD88 pathway. J. Biol. Chem. 2008, 283, 30513–30521. [Google Scholar] [CrossRef]
- Thorley, A.J.; Grandolfo, D.; Lim, E.; Goldstraw, P.; Young, A.; Tetley, T.D. Innate immune responses to bacterial ligands in the peripheral human lung--role of alveolar epithelial TLR expression and signalling. PLoS ONE 2011, 6, e21827. [Google Scholar] [CrossRef] [PubMed]
- Juarez, E.; Nunez, C.; Sada, E.; Ellner, J.J.; Schwander, S.K.; Torres, M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir. Res. 2010, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.W.; Normark, B.H.; Vandewalle, A.; Normark, S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J. Exp. Med. 2003, 198, 1225–1235. [Google Scholar] [CrossRef]
- Guillot, L.; Medjane, S.; Le-Barillec, K.; Balloy, V.; Danel, C.; Chignard, M.; Si-Tahar, M. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: Evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 2004, 279, 2712–2718. [Google Scholar] [CrossRef]
- Elson, G.; Dunn-Siegrist, I.; Daubeuf, B.; Pugin, J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 2007, 109, 1574–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øya, E.; Becher, R.; Ekeren, L.; Afanou, A.K.J.; Øvrevik, J.; Holme, J.A. Pro-Inflammatory Responses in Human Bronchial Epithelial Cells Induced by Spores and Hyphal Fragments of Common Damp Indoor Molds. Int. J. Environ. Res. Public Health 2019, 16, 1085. https://doi.org/10.3390/ijerph16061085
Øya E, Becher R, Ekeren L, Afanou AKJ, Øvrevik J, Holme JA. Pro-Inflammatory Responses in Human Bronchial Epithelial Cells Induced by Spores and Hyphal Fragments of Common Damp Indoor Molds. International Journal of Environmental Research and Public Health. 2019; 16(6):1085. https://doi.org/10.3390/ijerph16061085
Chicago/Turabian StyleØya, Elisabeth, Rune Becher, Leni Ekeren, Anani K.J. Afanou, Johan Øvrevik, and Jørn A. Holme. 2019. "Pro-Inflammatory Responses in Human Bronchial Epithelial Cells Induced by Spores and Hyphal Fragments of Common Damp Indoor Molds" International Journal of Environmental Research and Public Health 16, no. 6: 1085. https://doi.org/10.3390/ijerph16061085
APA StyleØya, E., Becher, R., Ekeren, L., Afanou, A. K. J., Øvrevik, J., & Holme, J. A. (2019). Pro-Inflammatory Responses in Human Bronchial Epithelial Cells Induced by Spores and Hyphal Fragments of Common Damp Indoor Molds. International Journal of Environmental Research and Public Health, 16(6), 1085. https://doi.org/10.3390/ijerph16061085




