The Association between Health Conditions in World Trade Center Responders and Sleep-Related Quality of Life and Sleep Complaints
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- Sleep-related Quality of life (QOL) was assessed using the Functional Outcomes of Sleep Questionnaire (FOSQ) [18]. The FOSQ is a validated 30-item questionnaire that is used to assess the impact of disorders of excessive sleepiness on activity of daily living. It consists of 5 subscales evaluating activity level, vigilance, intimacy, general productivity and social outcomes and has a possible combined score that ranges from 5 (poor) to 20 (excellent). A score < 17 is taken to indicate poor sleep-related quality of life.
- (2)
- Excessive daytime sleepiness was evaluated with the Epworth Sleep Scale (ESS), a well-validated questionnaire that asks the subject to rate the likelihood of falling asleep in 8 commonly encountered situations [19]. Possible scores range from 0 (the least sleepy) to 24 (the most sleepy). A score of > 10 defines presence of excessive daytime sleepiness.
- (3)
- A questionnaire regarding sleep and snoring was administered that included questions regarding bed and wake up times, duration of sleep (hours/night), frequency of difficulty falling and maintaining sleep per week, and overall quality of sleep. Quality of sleep was rated on a 4-point scale (1: excellent, 2: good, 3: fair, and 4: poor); poor sleep quality was defined as ≥ 3. Sleep onset insomnia was defined as present when the subject reported having trouble falling asleep at least 1–2 times a week, and sleep maintenance insomnia was recorded when a subject reported waking up early and not being able to go back to sleep at least 1–2 days per week.
- (4)
- Home monitoring for OSA: Subjects wore an ARESTM Unicorder (SleepMed, Inc., West Palm Beach, FL, USA) at home for 2 nights, with a pre-addressed mailer to return the device to the sleep lab. The ARESTM Unicorder is a home sleep test device that has been validated against full in-lab polysomnography [20,21], shows high sensitivity for OSA (0.98) and is routinely used in clinical practice for home monitoring of OSA. It is worn on the forehead and does not require additional wires to external devices. It measures oxygen saturation and pulse rate from reflectance oximetry, airflow from a nasal cannula/pressure transducer, snoring via acoustic microphone and head movement actigraphy and head position from accelerometers. The ARESTM respiratory data were analyzed as follows: Data from the monitor were autoscored and then manually edited by a single trained sleep technician and reviewed by a sleep expert. Apneas were scored when there was a reduction in airflow to less than 10% of baseline for >10 s. Hypopneas 4% were scored for >30% reduction in airflow associated with 4% or more decrease in oxygen saturation. Hypopneas with arousals (HypopneasArsl) were scored for visible reduction (>30% reduction) in airflow associated with surrogates of arousal (head movement, changes in snoring, or changes in pulse rate combined with disappearance of inspiratory flow limitation and a marked (>150%) increase in flow amplitude) at the end of the event, but <4% decrease in oxygen saturation [22]. AHI4% was calculated as Apneas+Hypopneas4% divided by total valid recording time in hours (h). The Respiratory Disturbance Index (RDI) was calculated as Apneas + Hypopneas 4% + HypopneasArsl divided by total valid recording time. Using these metrics, we define OSA as present when AHI4% ≥ 5/h or when RDI ≥ 15/h. When OSA is present by this definition, severity was graded by the AHI4%: mild (AHI4% < 15/h), moderate (AHI4% ≥ 15/h but < 30/h) or severe (AHI4% ≥ 30/h) [23].
- (5)
- Chronic rhinosinusitsis (CRS): CRS was defined based on epidemiological criteria used in the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) [24,25]. Subjects were considered CRS+ if two or more of the following symptoms were reported as present for >8 weeks: (1) nasal blockade/obstruction (2) nasal discharge, (3) facial pain and (4) reduction in or loss of smell. At least one symptom had to be nasal blockade or discharge.
- (6)
- In addition, we quantified the subjects’ perception of nasal congestion of each individual nostril by asking them to close one nostril while in the supine position and rate the level of congestion in the free nostril on a scale from 1 to 10, 1 being totally blocked/congested and 10 being totally open/uncongested. The lowest score (denoting greater congestion) of the two self-reported values from the left and right nostril was used for analysis.
- (7)
- Comorbid conditions: Information on comorbid Anxiety/Panic, Depression, GERD and PTSD were obtained from a combination of self-report of physician diagnosis (GERD) obtained in our WTCSNORE questionnaire, standardized questionnaires (Depression, Panic disorder, PTSD) and from the certified conditions listed in WTC Health Program General Responder Data Center (GERD, Depression, Anxiety and Panic Disorder and PTSD) for each subject using the annual visit closest in time to the sleep study. If any of the sources used identified the presence of a co-morbid condition the subject was coded as positive for that condition. Depression was assessed by the patient health questionnaire (PHQ), PTSD (PTSD symptom checklist), panic (PHQ) and anxiety from the GAD-7 [26,27,28,29].
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| BMI | Body Mass Index |
| H | Hour/s |
| CI | Confidence Interval |
| OR | Odds Ratio |
| CRS | Chronic Rhinosinusitis |
| GERD | Gastroesophageal Reflux Disorder |
| PTSD | Post Traumatic Stress Disorder |
| WTC | World Trade Center |
| FOSQ | Functional Outcomes of Sleep Questionnaire |
| QOL | Quality of Life |
| AHI4% | Apnea Hypopnea (with 4% O2 desaturation) Index |
| RDI | Respiratory Disturbance Index |
| SDB | Sleep Disordered Breathing |
| PAP | Positive Airway Pressure |
References
- Lioy, P.J.; Georgopoulos, P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann. N. Y. Acad. Sci. 2006, 1076, 54–79. [Google Scholar] [CrossRef]
- Ahuja, S.; Zhu, Z.; Shao, Y.; Berger, K.I.; Reibman, J.; Ahmed, O. Obstructive Sleep Apnea in Community Members Exposed to World Trade Center Dust and Fumes. J. Clin. Sleep Med. 2018, 14, 735–743. [Google Scholar] [CrossRef]
- Weakley, J.; Hall, C.B.; Liu, X.; Zeig-Owens, R.; Webber, M.P.; Schwartz, T.; Prezant, D. The effect of World Trade Center exposure on the latency of chronic rhinosinusitis diagnoses in New York City firefighters: 2001–2011. Occup. Environ. Med. 2016, 73, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.M.; Kornblith, E.S.; Li, J.; Adams, S.W.; Gocheva, V.V.; Schwarzer, R.; Cone, J.E. Police officers who responded to 9/11: Comorbidity of PTSD, depression, and anxiety 10–11 years later. Am. J. Ind. Med. 2016, 59, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Sunderram, J.; Weintraub, M.; Black, K.; Alimokhtari, S.; Twumasi, A.; Sanders, H.; Udasin, I.; Harrison, D.; Chitkara, N.; de la Hoz, R.E.; et al. Chronic Rhinosinusitis Is an Independent Risk Factor for OSA in World Trade Center Responders. Chest 2019, 155, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Steffen, A.D.; Van Dongen, H.P.A.; Pack, F.M.; Strakovsky, I.; Staley, B.; Dinges, D.F.; Maislin, G.; Pack, A.I.; Weaver, T.E. Determinants of sleepiness in obstructive sleep apnea. Sleep 2018, 41. [Google Scholar] [CrossRef] [Green Version]
- Budhiraja, R.; Kushida, C.A.; Nichols, D.A.; Walsh, J.K.; Simon, R.D.; Gottlieb, D.J.; Quan, S.F. Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur. Respir. J. 2017, 50, 1700348. [Google Scholar] [CrossRef] [PubMed]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.M.; Calhoun, S.L.; Vela-Bueno, A.; Kales, A. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. J. Clin. Endocrinol. Metab. 2005, 90, 4510–4515. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Baldwin, C.M.; Resnick, H.E.; Gottlieb, D.J.; Nieto, F.J. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep 2005, 28, 472–477. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Whitney, C.W.; Bonekat, W.H.; Iber, C.; James, G.D.; Lebowitz, M.; Nieto, F.J.; Rosenberg, C.E. Relation of sleepiness to respiratory disturbance index: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 1999, 159, 502–507. [Google Scholar] [CrossRef]
- Bengtsson, C.; Lindberg, E.; Jonsson, L.; Holmstrom, M.; Sundbom, F.; Hedner, J.; Malinovschi, A.; Middelveld, R.; Forsberg, B.; Janson, C. Chronic Rhinosinusitis Impairs Sleep Quality: Results of the GA2LEN Study. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Gregory, A.M.; Buysse, D.J.; Willis, T.A.; Rijsdijk, F.V.; Maughan, B.; Rowe, R.; Cartwright, S.; Barclay, N.L.; Eley, T.C. Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. J. Psychosom. Res. 2011, 71, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Westermeyer, J.; Khawaja, I.; Freerks, M.; Sutherland, R.J.; Engle, K.; Johnson, D.; Thuras, P.; Rossom, R.; Hurwitz, T. Correlates of daytime sleepiness in patients with posttraumatic stress disorder and sleep disturbance. Prim. Care Companion J. Clin. Psychiatry 2010, 12. [Google Scholar] [CrossRef]
- Lindam, A.; Jansson, C.; Nordenstedt, H.; Pedersen, N.L.; Lagergren, J. A population-based study of gastroesophageal reflux disease and sleep problems in elderly twins. PLoS ONE 2012, 7, e48602. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Ge, S.; Gu, Y.; Ding, X.; Zhang, Y.; Ye, J.; Zhang, L. Allergic and Non-Allergic Rhinitis Are Common in Obstructive Sleep Apnea but Not Associated With Disease Severity. J. Clin. Sleep Med. 2017, 13, 959–966. [Google Scholar] [CrossRef]
- Pan, M.L.; Tsao, H.M.; Hsu, C.C.; Wu, K.M.; Hsu, T.S.; Wu, Y.T.; Hu, G.C. Bidirectional association between obstructive sleep apnea and depression: A population-based longitudinal study. Medicine (Baltim.) 2016, 95, e4833. [Google Scholar] [CrossRef]
- You, C.R.; Oh, J.H.; Seo, M.; Lee, H.Y.; Joo, H.; Jung, S.H.; Lee, S.H.; Choi, M.G. Association Between Non-erosive Reflux Disease and High Risk of Obstructive Sleep Apnea in Korean Population. J. Neurogastroenterol. Motil. 2014, 20, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Weaver, T.E.; Laizner, A.M.; Evans, L.K.; Maislin, G.; Chugh, D.K.; Lyon, K.; Smith, P.L.; Schwartz, A.R.; Redline, S.; Pack, A.I.; et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997, 20, 835–843. [Google Scholar]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Ayappa, I.; Norman, R.G.; Seelall, V.; Rapoport, D.M. Validation of a self-applied unattended monitor for sleep disordered breathing. J. Clin. Sleep Med. 2008, 4, 26–37. [Google Scholar]
- Westbrook, P.R.; Levendowski, D.J.; Cvetinovic, M.; Zavora, T.; Velimirovic, V.; Henninger, D.; Nicholson, D. Description and validation of the apnea risk evaluation system: A novel method to diagnose sleep apnea-hypopnea in the home. Chest 2005, 128, 2166–2175. [Google Scholar] [CrossRef]
- Ayappa, I.; Norman, R.G.; Krieger, A.C.; Rosen, A.; O’Malley R, L.; Rapoport, D.M. Non-Invasive detection of respiratory effort-related arousals (REras) by a nasal cannula/pressure transducer system. Sleep 2000, 23, 763–771. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012, 23, 1–298. [Google Scholar]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999, 282, 1737–1744. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Lowe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Blanchard, E.B.; Jones-Alexander, J.; Buckley, T.C.; Forneris, C.A. Psychometric properties of the PTSD Checklist (PCL). Behav. Res. Ther. 1996, 34, 669–673. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.F.; Chan, C.S.; Dement, W.C.; Gevins, A.; Goodwin, J.L.; Gottlieb, D.J.; Green, S.; Guilleminault, C.; Hirshkowitz, M.; Hyde, P.R.; et al. The association between obstructive sleep apnea and neurocognitive performance—The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2011, 34, 303–314. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Alt, J.A.; Smith, T.L. Chronic rhinosinusitis and sleep: A contemporary review. Int. Forum Allergy Rhinol. 2013, 3, 941–949. [Google Scholar] [CrossRef]
- Alt, J.A.; Smith, T.L.; Mace, J.C.; Soler, Z.M. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope 2013, 123, 2364–2370. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, M.; Schleimer, R.P.; Keshavarzian, A. Sleep disruption in chronic rhinosinusitis. Expert Rev. Anti-Infect. Ther. 2017, 15, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Ohayon, M.M.; Shapiro, C.M. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr. Psychiatry 2000, 41, 469–478. [Google Scholar] [CrossRef]
- Jenkins, M.M.; Colvonen, P.J.; Norman, S.B.; Afari, N.; Allard, C.B.; Drummond, S.P. Prevalence and Mental Health Correlates of Insomnia in First-Encounter Veterans with and without Military Sexual Trauma. Sleep 2015, 38, 1547–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef] [PubMed]
- de la Hoz, R.E.; Aurora, R.N.; Landsbergis, P.; Bienenfeld, L.A.; Afilaka, A.A.; Herbert, R. Snoring and obstructive sleep apnea among former World Trade Center rescue workers and volunteers. J. Occup. Environ. Med. 2010, 52, 29–32. [Google Scholar] [CrossRef]
- Haba-Rubio, J.; Marques-Vidal, P.; Andries, D.; Tobback, N.; Preisig, M.; Vollenweider, P.; Waeber, G.; Luca, G.; Tafti, M.; Heinzer, R. Objective sleep structure and cardiovascular risk factors in the general population: The HypnoLaus Study. Sleep 2015, 38, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Wisnivesky, J.P.; Teitelbaum, S.L.; Todd, A.C.; Boffetta, P.; Crane, M.; Crowley, L.; de la Hoz, R.E.; Dellenbaugh, C.; Harrison, D.; Herbert, R.; et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: A cohort study. Lancet 2011, 378, 888–897. [Google Scholar] [CrossRef]
| Variable | N of Valid Data | Summary |
|---|---|---|
| Age (years, Mean ± SD) | 626 | 52.8 ± 8.6 |
| BMI (kg/m2, Mean ± SD) | 626 | 29.9 ± 5.5 |
| Female (%) | 626 | 109 (17.3%) |
| Sleep Duration (h, Mean ± SD) ≥7 h (%) 6–6.99 h (%) <6 h (%) | 588 | 6.4 ± 1.3 42.6% 30.9% 26.5% |
| Snoring (Yes, %) | 626 | 312 (49.8%) |
| Quality of Life (FOSQ) (Mean ± SD) Good, ≥17 (%) Poor, <17 (%) | 566 | 17.4 ± 2.6 62.0% 38.0% |
| Sleepiness (ESS, Mean ± SD) Sleepy, >10 (%) Not Sleepy, ≤10 (%) | 620 | 8.3 ± 4.8 31.3% 68.7% |
| Poor Sleep Quality (Yes, %) | 623 | 441 (70.8%) |
| Sleep Onset Insomnia (Yes, %) | 609 | 296 (48.6%) |
| Sleep Maintenance Insomnia (Yes, %) | 622 | 116 (18.7%) |
| OSA + (%) Mild Moderate Severe | 592 | 443 (74.8%) 274 (46.3%) 112 (18.9%) 57 (9.6%) |
| CRS + (%) | 626 | 295 (47.1%) |
| Depression + (%) | 612 | 118 (19.3%) |
| PTSD + (%) | 612 | 144 (23.5%) |
| Anxiety and Panic Disorder + (%) | 612 | 144 (23.5%) |
| GERD + (%) | 564 | 293 (52%) |
| Age | Female | BMI kg/m2 | Sleep Duration (h) | Sleepiness (ESS) | Quality of Life (FOSQ) | AHI4%/h | RDI/h | Perception of Nasal Congestion at time of Visit | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | N (%) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Median (Q1, Q3) | Median (Q1, Q3) | Mean ± SD | ||
| OSA+ OSA- | N = 443 N = 149 | 53.5 ± 8.3 ‡ 50.3 ± 8.8 | 58 (13.1%) ‡ 44 (29.5%) | 30.7 ± 5.5 ‡ 27.5 ± 4.8 | 6.4 ± 1.3 6.4 ± 1.3 | 8.4 ± 4.9 8.0 ± 4.5 | 17.4 ± 2.7 17.6 ± 2.5 | 11 (6, 19) ‡ 2 (1, 3) | 26 (18, 37) ‡ 9 (8, 12) | 6.8 ± 2.2 6.8 ± 2.2 |
| CRS+ CRS- | N = 295 N = 331 | 52.9 ± 8.8 52.7 ± 8.4 | 46 (15.7%) 46 (18.8%) | 30.3 ± 5.3 * 29.6 ± 5.7 | 6.3 ± 1.4 * 6.5 ± 1.2 | 9.2 ± 5.1 ‡ 7.4 ± 4.5 | 16.7 ± 3.0 ‡ 18.1 ± 2.1 | 9 (4, 17) * 6 (3, 15) | 22 (15, 35) 19 (12, 32) | 6.2 ± 2.2 ‡ 7.3 ± 2.1 |
| GERD+ GERD- | N = 293 N = 271 | 52.6 ± 8.0 52.9 ± 9.2 | 45 (15.4%) 54 (20%) | 30.9 ± 6.1 ‡ 28.8 ± 4.9 | 6.4 ± 1.3 6.4 ± 1.3 | 9.0 ± 5.0 Ɨ 7.7 ± 4.7 | 16.9 ± 2.9 ‡ 18.0 ± 2.3 | 8 (4, 17) 7 (3, 14.5) | 22 (15, 34) 20 (12, 32) | 6.3 ± 2.2 ‡ 7.4 ± 2.0 |
| PTSD+ PTSD- | N = 144 N = 468 | 51.8 ± 8.0 53.1 ± 8.8 | 25 (17.4%) 82 (17.5%) | 31.3 ± 6.2 Ɨ 29.5 ± 5.3 | 6.1 ± 1.5 * 6.5 ± 1.3 | 10.8 ± 5.3 ‡ 7.5 ± 4.4 | 15.3 ± 3.4 ‡ 18.1 ± 2.0 | 8 (4, 16) 7 (3, 16) | 23 (14, 35) 20 (13, 33) | 6.0 ± 2.2 ‡ 7.0 ± 2.2 |
| Anxiety/Panic+ Anxiety/Panic- | N = 144 N = 468 | 51.9 ± 8.3 53.1 ± 8.7 | 24 (16.7%) 83 (17.7%) | 30.9 ± 5.8 * 29.6 ± 5.4 | 6.0 ± 1.4 ‡ 6.5 ± 1.3 | 10.6 ± 5.2 ‡ 7.5 ± 4.4 | 15.6 ± 3.4 ‡ 18.0 ± 2.1 | 8 (4, 17) 7 (3, 15) | 21 (14, 33) 20 (13, 33) | 6.2 ± 2.3 Ɨ 7.0 ± 2.2 |
| Depression+ Depression- | N = 118 N = 494 | 52.9 ± 8.3 52.8 ± 8.7 | 22 (18.6%) 85 (17.2%) | 31.5 ± 6.8 * 29.5 ± 5.1 | 6.0 ± 1.4 Ɨ 6.5 ± 1.3 | 10.7 ± 5.4 ‡ 7.7 ± 4.5 | 15.4 ± 3.4 ‡ 17.9 ± 2.2 | 8 (4, 15) 7 (3, 16) | 21 (13, 33) 20 (14, 33) | 5.9 ± 2.3 ‡ 7.0 ± 2.1 |
| Poor Sleep-Related Quality of Life | Sleepiness | Poor Sleep Quality | Sleep Onset Insomnia | Sleep Maintenance Insomnia | ||
|---|---|---|---|---|---|---|
| FOSQ < 17 | ESS > 10 | Yes | Present | Present | ||
| OSA+ OSA- | N = 443 N = 149 | 172 (38.8%) 52 (34.9%) | 136 (31.0%) 45 (30.2%) | 315 (71.3%) 99 (66.4%) | 207 (48.0%) 71 (48.6%) | 81 (18.3%) 25 (16.9%) |
| CRS + CRS- | N = 295 N = 331 | 146 (49.5%) ‡ 91 (27.5%) | 116 (39.9%) ‡ 77 (23.5%) | 232 (79.5%) ‡ 208 (63.0%) | 162 (56.8%) ‡ 133 (41.2%) | 76 (26.0%) ‡ 40 (12.12%) |
| GERD+ GERD- | N = 293 N = 271 | 132 (45.0%) Ɨ 85 (31.4%) | 111 (37.9%) Ɨ 67 (25.1%) | 231 (78.8%) ‡ 171 (63.3%) | 153 (53.5%) Ɨ 114 (43.2%) | 60 (20.6%) 45 (16.7%) |
| PTSD+ PTSD- | N = 144 N = 468 | 92 (63.9%) ‡ 138 (29.5%) | 73 (51.0%) ‡ 117 (25.2%) | 123 (85.4%) ‡ 310 (66.4%) | 110 (78.0%) ‡ 184 (40.4%) | 52 (36.1%) ‡ 62 (13.3%) |
| Anxiety/Panic+ Anxiety/Panic- | N = 144 N = 468 | 85 (59.0%) ‡ 145 (31.0%) | 68 (47.6%) ‡ 122 (26.2%) | 123 (86.0%) ‡ 310 (66.2%) | 93 (66.4%) ‡ 201 (44.0%) | 47 (32.6%) ‡ 67 (14.4%) |
| Depression+ Depression- | N = 118 N = 494 | 76 (64.4%) ‡ 154 (31.2%) | 61 (52.1%) ‡ 129 (26.3%) | 98 (83.8%) Ɨ 335 (67.8%) | 83 (72.8%) ‡ 211 (43.7%) | 39 (33.0%) ‡ 75 (15.2%) |
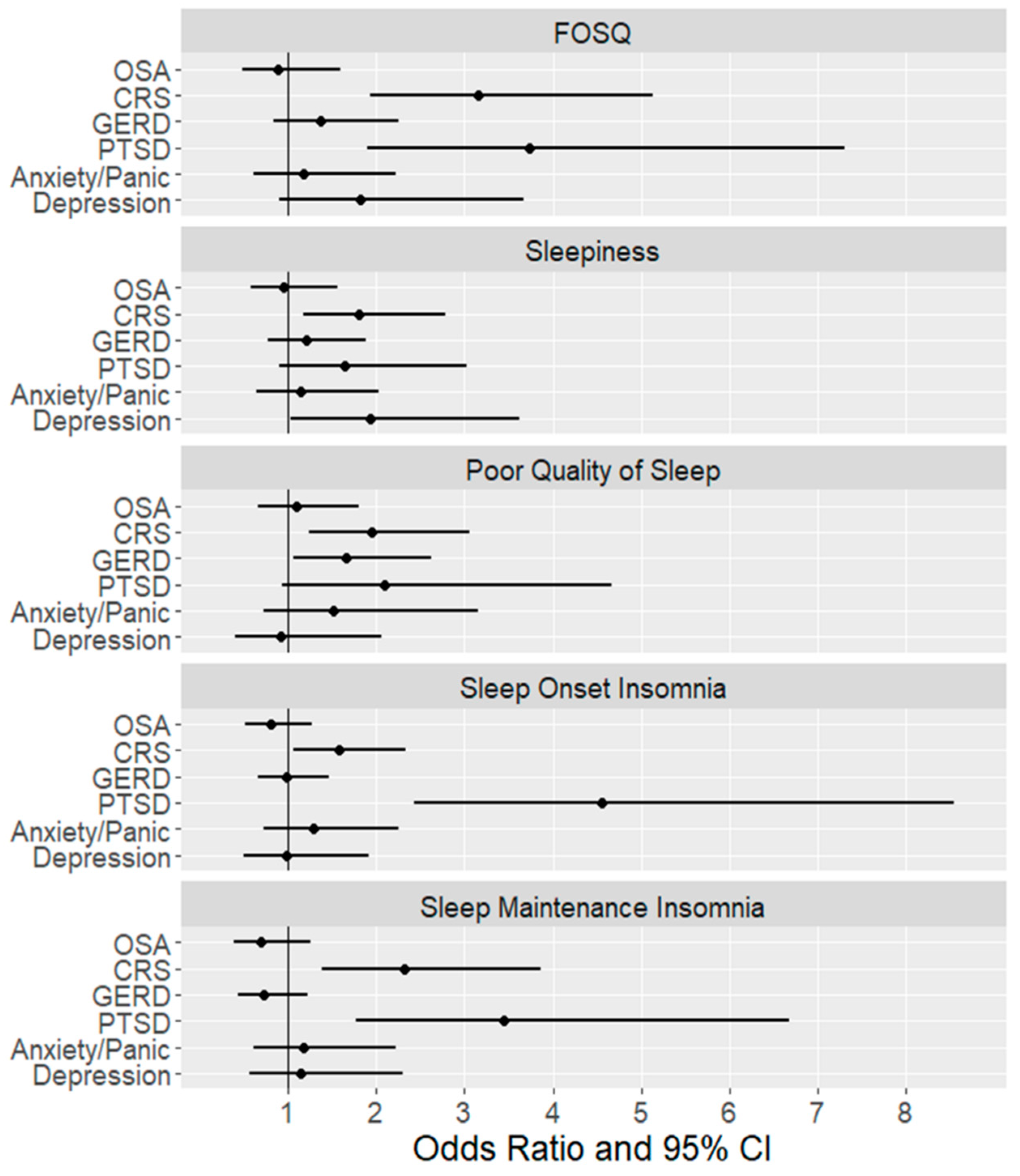
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| OR 1 | 95% CI | OR 2 | 95% CI | OR 3 | 95% CI | ||
| Poor Quality of Life (FOSQ < 17) | OSA Severity | ||||||
| Mild OSA vs. No OSA | 1.21 | (0.80, 1.84) | 1.02 | (0.65, 1.59) | 0.87 | (0.51, 1.47) | |
| Moderate OSA vs. No OSA | 1 | (0.60, 1.67) | 0.8 | (0.46, 1.41) | 0.73 | (0.36, 1.45) | |
| Severe OSA vs. No OSA | 1.46 | (0.78, 2.72) | 0.98 | (0.50,1.95) | 1.09 | (0.48, 2.49) | |
| CRS | 2.58 ‡ | (1.85, 3.60) | 2.44 ‡ | (1.72, 3.46) | 2.48 ‡ | (1.62, 3.80) | |
| GERD | 1.79 Ɨ | (1.27, 2.53) | 1.80 Ɨ | (1.24, 2.60) | 1.14 | (0.74, 1.76) | |
| PTSD | 4.23 ‡ | (2.85, 6.27) | 3.93 ‡ | (2.60, 5.94) | 3.18 Ɨ | (1.72, 5.9) | |
| Anxiety/Panic | 3.21 ‡ | (2.18, 4.72) | 2.87 ‡ | (1.91, 4.29) | 1.18 | (0.66, 2.12) | |
| Depression | 3.99 ‡ | (2.62, 6.09) | 3.61 ‡ | (2.33, 5.59) | 1.62 | (0.85, 3.11) | |
| Sleepiness (ESS>10) | OSA Severity | ||||||
| Mild OSA vs. No OSA | 1 | (0.65, 1.54) | 1.07 | (0.67, 1.71) | 0.9 | (0.53, 1.52) | |
| Moderate OSA vs. No OSA | 1.08 | (0.63,1.84) | 1.2 | (0.67, 2.15) | 1.07 | (0.55, 2.08) | |
| Severe OSA vs. No OSA | 1.16 | (0.60, 2.22) | 1.09 | (0.53, 2.24) | 0.97 | (0.42, 2.22) | |
| CRS | 2.16 ‡ | (1.53,3.06) | 1.93 Ɨ | (1.34, 2.77) | 1.80 Ɨ | (1.17, 2.76) | |
| GERD | 1.82 Ɨ | (1.27, 2.62) | 1.73 Ɨ | (1.18, 2.55) | 1.2 | (0.78, 1.87) | |
| PTSD | 3.10 ‡ | (2.10, 4.58) | 2.71 ‡ | (1.80, 4.09) | 1.64 | (0.89, 3.02) | |
| Anxiety/Panic | 2.55 ‡ | (1.73, 3.76) | 2.03 Ɨ | (1.35, 3.06) | 1.12 | (0.63, 2.01) | |
| Depression | 3.06 ‡ | (2.02, 4.63) | 2.62 ‡ | (1.70, 4.05) | 1.99 | (1.05, 3.76) | |
| Poor Sleep Quality | OSA Severity | ||||||
| Mild OSA vs. No OSA | 1.16 | (0.75, 1.77) | 1.21 | (0.75, 1.94) | 1.05 | (0.62, 1.78) | |
| Moderate OSA vs. No OSA | 1.38 | (0.81,2.37) | 1.28 | (0.70, 2.36) | 1.04 | (0.51, 2.13) | |
| Severe OSA vs. No OSA | 1.55 | (0.78,3.10) | 1.39 | (0.64, 3.04) | 1.31 | (0.55, 3.15) | |
| CRS | 2.27 ‡ | (1.58,3.25) | 2.20 ‡ | (1.49,3.25) | 1.95 Ɨ | (1.24, 3.07) | |
| GERD | 2.16 ‡ | (1.48,3.14) | 2.21 ‡ | (1.46, 3.33) | 1.67 * | (1.06, 2.64) | |
| PTSD | 2.97 ‡ | (1.80,4.90) | 2.45 Ɨ | (1.44, 4.16) | 2.13 | (0.96, 4.76) | |
| Anxiety/Panic | 3.13 ‡ | (1.88,5.22) | 2.38 Ɨ | (1.40, 4.06) | 1.49 | (0.71, 3.13) | |
| Depression | 2.45 Ɨ | (1.45, 4.14) | 1.96 * | (1.13, 3.42) | 0.95 | (0.42, 2.14) | |
| Sleep Onset Insomnia | OSA Severity | ||||||
| Mild OSA vs. No OSA | 0.89 | (0.59, 1.33) | 0.79 | (0.52, 1.20) | 0.85 | (0.53, 1.38) | |
| Moderate OSA vs. No OSA | 1.13 | (0.69,1.86) | 0.94 | (0.56, 1.57) | 0.96 | (0.51, 1.79) | |
| Severe OSA vs. No OSA | 1.1 | (0.59,2.04) | 0.83 | (0.43, 1.61) | 1.05 | (0.49, 2.24) | |
| CRS | 1.88 ‡ | (1.36, 2.60) | 1.85 Ɨ | (1.34, 2.55) | 1.63 * | (1.10, 2.41) | |
| GERD | 1.51 * | (1.08, 2.12) | 1.44 * | (1.02, 2.02) | 0.99 | (0.66, 1.47) | |
| PTSD | 5.25 ‡ | (3.38, 8.15) | 5.22 ‡ | (3.34, 8.14) | 4.69 ‡ | (2.49, 8.86) | |
| Anxiety/Panic | 2.52 ‡ | (1.70, 3.75) | 2.48 ‡ | (1.66, 3.70) | 1.26 | (0.71, 2.23) | |
| Depression | 3.45 ‡ | (2.20, 5.41) | 3.31 ‡ | (2.10, 5.20) | 0.98 | (0.50, 1.92) | |
| Sleep Maintenance Insomnia | OSA Severity | ||||||
| Mild OSA vs. No OSA | 1.1 | (0.65, 1.87) | 0.93 | (0.54, 1.60) | 0.85 | (0.46, 1.56) | |
| Moderate OSA vs. No OSA | 1.07 | (0.56, 2.04) | 0.8 | (0.41, 1.58) | 0.56 | (0.25, 1.26) | |
| Severe OSA vs. No OSA | 1.18 | (0.54, 2.58) | 0.78 | (0.34, 1.80) | 0.75 | (0.29, 1.95) | |
| CRS | 2.54 ‡ | (1.67, 3.87) | 2.49 ‡ | (1.63, 3.81) | 2.41 Ɨ | (1.44, 4.04) | |
| GERD | 1.29 | (0.84, 1.98) | 1.2 | (0.77, 1.85) | 0.74 | (0.44, 1.24) | |
| PTSD | 3.68 ‡ | (2.39, 5.68) | 3.62 ‡ | (2.33, 5.63) | 3.53 ‡ | (1.82, 6.88) | |
| Anxiety/Panic | 2.89 ‡ | (1.87, 4.45) | 2.85 ‡ | (1.83, 4.42) | 1.24 | (0.64, 2.37) | |
| Depression | 2.74 ‡ | (1.74, 4.33) | 2.57 ‡ | (1.62, 4.09) | 1.06 | (0.52, 2.16) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayappa, I.; Chen, Y.; Bagchi, N.; Sanders, H.; Black, K.; Twumasi, A.; Rapoport, D.M.; Lu, S.-E.; Sunderram, J. The Association between Health Conditions in World Trade Center Responders and Sleep-Related Quality of Life and Sleep Complaints. Int. J. Environ. Res. Public Health 2019, 16, 1229. https://doi.org/10.3390/ijerph16071229
Ayappa I, Chen Y, Bagchi N, Sanders H, Black K, Twumasi A, Rapoport DM, Lu S-E, Sunderram J. The Association between Health Conditions in World Trade Center Responders and Sleep-Related Quality of Life and Sleep Complaints. International Journal of Environmental Research and Public Health. 2019; 16(7):1229. https://doi.org/10.3390/ijerph16071229
Chicago/Turabian StyleAyappa, Indu, Yingfeng Chen, Nisha Bagchi, Haley Sanders, Kathleen Black, Akosua Twumasi, David M. Rapoport, Shou-En Lu, and Jag Sunderram. 2019. "The Association between Health Conditions in World Trade Center Responders and Sleep-Related Quality of Life and Sleep Complaints" International Journal of Environmental Research and Public Health 16, no. 7: 1229. https://doi.org/10.3390/ijerph16071229
APA StyleAyappa, I., Chen, Y., Bagchi, N., Sanders, H., Black, K., Twumasi, A., Rapoport, D. M., Lu, S.-E., & Sunderram, J. (2019). The Association between Health Conditions in World Trade Center Responders and Sleep-Related Quality of Life and Sleep Complaints. International Journal of Environmental Research and Public Health, 16(7), 1229. https://doi.org/10.3390/ijerph16071229




