1. Introduction
Red mud is an insoluble residue produced by aluminum oxide (alumina) refining process with the characteristics of very fine particle size and high alkalinity [
1]. In 2015, 274 million tons of bauxite ore were mined globally for alumina production, with Australia (33%), China (20%) and Brazil (16%) being the leading bauxite producers in the world [
2]. Approximately 60 million tons of red mud is produced each year globally, with 6 million tons of red mud occurring in China. However, approximately 85% of the red mud is stored in the on-site reservoirs near the alumina refining plant. The reservoir is constructed with dams for containment purposes, and the red mud is treated by a natural settlement process [
3].
Currently, manufacturers often adopt the chemical ore dressing process for alumina refining. This process uses the alkali method (sodium hydroxide or sodium carbonates) to produce alumina from bauxite and can be divided into the Bayer process, the sintering method, and the combined method [
4,
5]. The main components of the red mud are hematite (Fe
2O
3), calcite (CaCO
3), cancrinite (Na
6CaAl
6Si
6(CO
3)O
24·2H
2O), hydrogarnet (Ca
3AlFe(SiO
4)(OH)
8), tricalcium aluminate (Ca
3Al
2(OH)
12), and sodalite ((Na
6Al
6Si
6O
24)·(2NaX or Na
2X)) [
4,
6]. Red mud has strong alkalinity and salinity, with heavy metal elements, including arsenic (As), lead (Pb), zinc (Zn), copper (Cu), nickel (Ni), chromium (Cr), and vanadium (V). The salinity and alkalinity of red mud can affect plant growth and lead to deterioration of soil quality. The leakage or spill of red mud releases oxyanionic trace elements such as Cr, molybdenum (Mo), and V. These contaminants have been reported to be highly soluble under high pH conditions (material pH of red mud) in leaching tests [
6,
7], suggesting that red mud could be a potential sink of contaminants [
8].
Red mud typically occupies land spaces for storage and further generates potential threats to the surrounding environment. Unlined or inappropriately lined red mud reservoirs could induce the leakage of leachate (i.e., bauxite liquor), leading soil swampiness and groundwater pollution [
9,
10]. Additionally, occasional dam failures damage infrastructure and contaminate the environment. For example, the catastrophic dam failure of the Ajkai Timfoldgyar Zrt red mud reservoir that occurred in Hungary released of 0.7 to 1 million m
3 of caustic red mud into the Torna River, which resulted in the loss of lives and seriously contaminated soil and water [
6,
9,
10,
11].
Overall, the environmental risks are closely related to the soda content, alkalinity, and heavy metal content in the red mud. However, there is still a lack of knowledge regarding the mineralogy and chemical composition within red mud and its leachates in China. The objective of this study is to understand the potential contamination by red mud and its associated leachates from red mud management facilities. The chemical compositions of red mud from different resources and production process are summarized. As red mud leachate has essential effects on the environment, the leaching ability of red mud and its environmental impacts were studied through the analysis of the concentration of elements in leachates. Fresh red mud and its leachates were sampled from the major alumina manufacturers in China. The main chemical parameters, including pH, electrical conductivity (EC), redox potential (ORP), and elemental concentration, were evaluated through laboratory analytical methods.
2. Materials and Methods
2.1. Resources and Distribution of Bauxite
Bauxite is the primary aluminum ore composed of one or more aluminum hydroxides minerals, including gibbsite (Al(OH)
3), boehmite (γ-AlO(OH)), diaspore (α-AlO(OH)), and impurities such as quartz (SiO
2), hematite (α-Fe
2O
3), and rutile (TiO
2) [
4,
12,
13]. Bauxite resources in China are primarily distributed among seven provinces: Shanxi, Henan, Guangxi, Guizhou, Yunnan, Chongqing, and Shandong. Diaspore is the primary type of bauxite in China and contains 37.4~74.0% of Al
2O
3 and 3.5~32.2% of SiO
2 [
4,
14,
15,
16,
17,
18]. The average Al
2O
3 content of bauxites in Guangxi varies between 52.3% and 62.4%, and the average Fe
2O
3 and SiO
2 contents range between 15.0~24.5% and 3.5~8.3% (
Table 1). However, the SiO
2 contents (7.5~32.2%) in bauxite from Henan, Shandong, Shanxi, and Guizhou are higher than those from Guangxi, which result in a lower Al/Si ratio (1.2~9.4) than that of Guangxi bauxite (6.3~17.8).
The Al/Si ratio determines the alumina processing procedure. In general, Guangxi aluminum mines are bauxite deposits with a high Al/Si ratio (6.3-17.8). This type of bauxite can be utilized to extract alumina using the Bayer process. Shanxi, Shandong, Henan, Guizhou, and Sichuan aluminum mines, which account for over 98% of the reserves in China, are mainly bauxite deposits with a relatively low Al/Si ratio (3.3–9.4). These types of ores are middle/low-grade diaspore bauxites. They are usually challenging and energy consuming to process for alumina. Therefore, alternative methods modified from the traditional Bayer process, i.e., the sintering process and the Bayer-sintering combined process, have been developed for alumina refining [
4].
2.2. The Sampling of Red Mud and Leachate
In this study, samples of red mud and leachates from five management facilities were collected from three provinces (i.e., Guangxi, Shangdong, and Henan) in China (
Figure 1). The details of the sampling are summarized in
Table 2. Fresh red mud samples GX-A1 and GX-A2, and leachate samples GX-A2-L and GX-A2-L were collected from the same manufacturer but two adjacent reservoirs (A1 and A2, respectively) in Pingguo County, Guangxi Province. The average annual temperature of Pingguo County is 21.5 °C, and the annual precipitation reaches 1500 mm. Fresh red mud sample GX-B and leachate sample GX-B-L were collected in Jingxi County, Guangxi Province. The average annual temperature of Jingxi County is 19.1 °C, and the annual precipitation reaches 1636 mm. Red mud sample SD-A and its leachate sample SD-A-L were collected in Zibo, Shandong. The average annual temperature of Zibo is 13.5 °C, and the annual precipitation reaches 650 mm. Red mud sample HN-A and its leachate sample HN-A-L were collected in Xingyang, Henan Province. The average annual temperature is 14.3 °C, and the annual precipitation reaches 645 mm. Fresh red mud was sampled after pressure filtration, and the leachate was collected from the drainage pipe of the red mud reservoir.
2.3. Bulk Chemical Analysis
Specimens were analyzed using X-ray fluorescence (XRF) quantitative analysis (Shimadzu XRF-1800, Kyoto, Japan). The analytical method for silicate rocks was used, and specimens were analyzed in duplicates. Five grams of each sample was air-dried for 24 h and ground to <0.075 mm with agate mortar and pestle. Loss on ignition tests were performed before XRF analysis by sintering samples at 900 °C using a muffle furnace (YSD-5-12T, Yaoshi Instrument Equipment Ltd., Shanghai, China). The LOI is used as an indicator for organic contents in the samples. XRF tests were conducted by fusing 0.9 g of calcined powder (i.e., specimen after LOI) with 1 g of NH4NO3 oxidizer and 9.0 g of lithium borate flux (50%/50% Li2B4O7-LiBO2) at 1050 °C into a flat molten glass disk. The specimens were then analyzed by XRF spectrometry.
2.4. Mineralogical Analysis
Quantitative X-ray diffraction analysis (Rigaku D/MAX-2005 X-ray diffractometer, Tokyo, Japan) was performed on the red mud samples to determine the major mineral phases. Specimens were placed in a desiccator for 24 h and ground to 0.075 mm with agate mortar and pestle. Cu Kα radiation was used, and each sample was placed in a 2-mm deep sample holder and scanned at 0.02° intervals between 5° to 50° 2θ with a 2 s dwell time. Samples were scanned within 48 h after desiccation to minimize crystal dehydration.
2.5. Hydrochemical Analysis
pH, EC, and ORP of leachate samples were recorded immediately after sampling. Leachate samples were then filtered through a 0.45-μm filter paper and preserved with trace-grade nitric acid (HNO3). The elemental concentrations of silver (Ag), aluminum (Al), As, beryllium (Be), boron (B), barium (Ba), calcium (Ca), cadmium (Cd), cobalt (Co), Cr, Cu, iron (Fe), mercury (Hg), potassium (K), lithium (Li), magnesium (Mg), manganese (Mn), Mo, sodium (Na), Ni, Pb, antimony (Sb), selenium (Se), silicon (Si), strontium (Sr), titanium (Ti), thallium (Tl), V, and Zn were determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Agilent Technologies 700 Series, Santa Clara, CA, USA) at Chengdu University of Science and Technology. Anions, including chloride (Cl−), fluoride (F−), nitrate (NO3−), and sulfate (SO42−), were determined by ion chromatography (IC, Shimadzu HIC-SP, Kyoto, Japan) at Southwest Jiaotong University.
4. Discussion
The bauxite source and extraction processes have a strong impact on the chemical and mineralogical composition of the red mud. For example, the variation in Na
2O contents is partially due to the addition of NaOH. In the Bayer process, NaOH is used to dissolve the Al from Fe-rich aluminite bauxite [
40]. However, the sintering and Bayer-sintering combined methods are often adopted to refine alumina from insoluble diaspore or kaolinite type bauxite ores (usually, Al and Si-rich, but low in iron content). Additionally, to enhance the dissolution of alumina and reduce the consumption of alkali, lime is usually added in the high-temperature sintering process. CaCO
3 can deposit and crystallize by introducing lime (CaO) and carbon dioxide (CO
2) during alumina production. Therefore, the associated red mud from sintering or Bayer-sintering combined methods is usually Ca-rich but low in iron content.
Additionally, the content of iron oxide (Fe
2O
3) in the red mud produced by the Bayer process is higher than that in the red mud produced by the sintering process. In the Bayer process, while extracting alumina from the bauxite using aqueous NaOH, Fe
2O
3 is left in the residue. Therefore, the red mud usually presents relatively high contents of Fe
2O
3 [
41]. Red mud from the sintering process usually contains elevated CaO contents, while red mud produced by the Bayer process contains elevated Na
2O and Al
2O
3 contents (
Figure 2). This difference is due to the addition of limestone in lieu of NaOH (or Na
2CO
3) in the sintering process. The aluminum recovery from the bauxite ore in the Bayer process is lower than in the sintering alumina process [
42]. Therefore, Bayer red mud usually contains more alumina (Al
2O
3) than red mud from the sintering process.
A series of saline deposits or colloid products, such as CaCO
3, Na
2CO
3, NaHCO
3, Na
2SiO
3, and NaAlO
2, are formed during desiccation. However, during the wetting process, these deposits can be easily soluble and cause salinization pollution to the land. The high concentration of alkaline substances (usually pH >12) is the primary reason for the classification of residue as a special industrial material in some countries, such as Australia [
43]. The alkaline mineral composition of red mud from refining processes consists of natron-decahydrate (Na
2CO
3·10H
2O), calcite (CaCO
3), hydrogarnet (Ca
3Al
2(SiO
4)
2(OH)
4), sodalite (Na
8(Al
6Si
6O
24)Cl
2), cancrinite (Na
6Ca
2((CO
3)
2Al
6Si
6O
24)·2H
2O). The following dissolution reactions enable the red mud to become strongly alkaline:
The reactions indicate that NaOH, Na
2CO
3, NaHCO
3, and NaAl(OH)
4 are the major soluble alkalinity compounds in red mud. These compounds are formed mainly due to the addition of NaOH, CO
2, and CaO during the alumina production process [
4]. The main anions leading to the alkalinity of red mud in the leachate are: OH
−, CO
32−, HCO
3−, Al(OH)
4−, H
2SiO
42−, and H
3SiO
4−, most of which are the dissolution products of sodalite (Na
8(Al
6Si
6O
24)Cl
2), cancrinite (Na
6Ca
2((CO
3)
2Al
6Si
6O
24)·2H
2O), hydrogarnet (Ca
3Al
2(SiO
4)
2(OH)
4), and tricalcium aluminate (Ca
3Al
2O
6). However, the alkaline substances of red mud vary with the refining processes. The Bayer red mud is strongly alkaline [
9]. Compared with other processes, more caustic soda is added in the Bayer process, resulting in much higher contents in sodium monoxide (Na
2O) in the red mud, with approximately 9.7% being detected in the red mud sample of SD-A (
Table 2).
In general, the major elements (including Al, Ca, K, Na, Mg, Si, Cl−, F−, NO
32−, and SO
42−) all exceeded the recommended value of groundwater quality standards and could increase the risk of salinization of soil and water [
7,
9]. Al mainly exists as Al(OH)
4− under high pH (pH > 12) conditions, and the insoluble Al(OH)
3 in red mud can easily be converted into soluble Al(OH)
4−. NaOH is added during the refining process, therefore, a high concentration of Na is expected to be retained or dissolved in the leachate. In some cases, KOH is added during the refining process, such as in sample HN-A-L, and a higher content of K (1016.0 mg/L) in the leachate can be found than in leachates of other red muds (
Table 6). Na or K is the top components for salinization of soil and water [
9].
As one of the main anions found in the major elements of red mud leachate, Cl
− is derived from CaCl
2. CaCl
2 is used to precipitate soluble hydroxides, aluminates, and carbonate. By adding CaCl
2, soluble alkaline substances can be converted to calcite (CaCO
3), hydrocalumite (Ca
4Al
2(OH)
12·CO
3), and aluminohydrocalcite (CaAl
2(CO
3)
2(OH)
4·
3H
2O). The insoluble tricalcium aluminate (Ca(AlO
2)
2) existing in red mud will participate in forming soluble Al(OH)
4− ions [
44,
45,
46]. This precipitation process aims to lower the pH and alkalinity of the leachate before discharge. The F
− is mainly leached from the fluoride in red mud, derived from the bauxite ore. The F
− content in red mud leachate is more than 44 times higher than the recommended value of USEPA (GW) and China (GW) (
Figure 3,
Table 6). The long-term intake of water with high fluoride concentration may cause bone fluorosis, dental fluorosis, osteoporosis, resulting in serious human health issues.
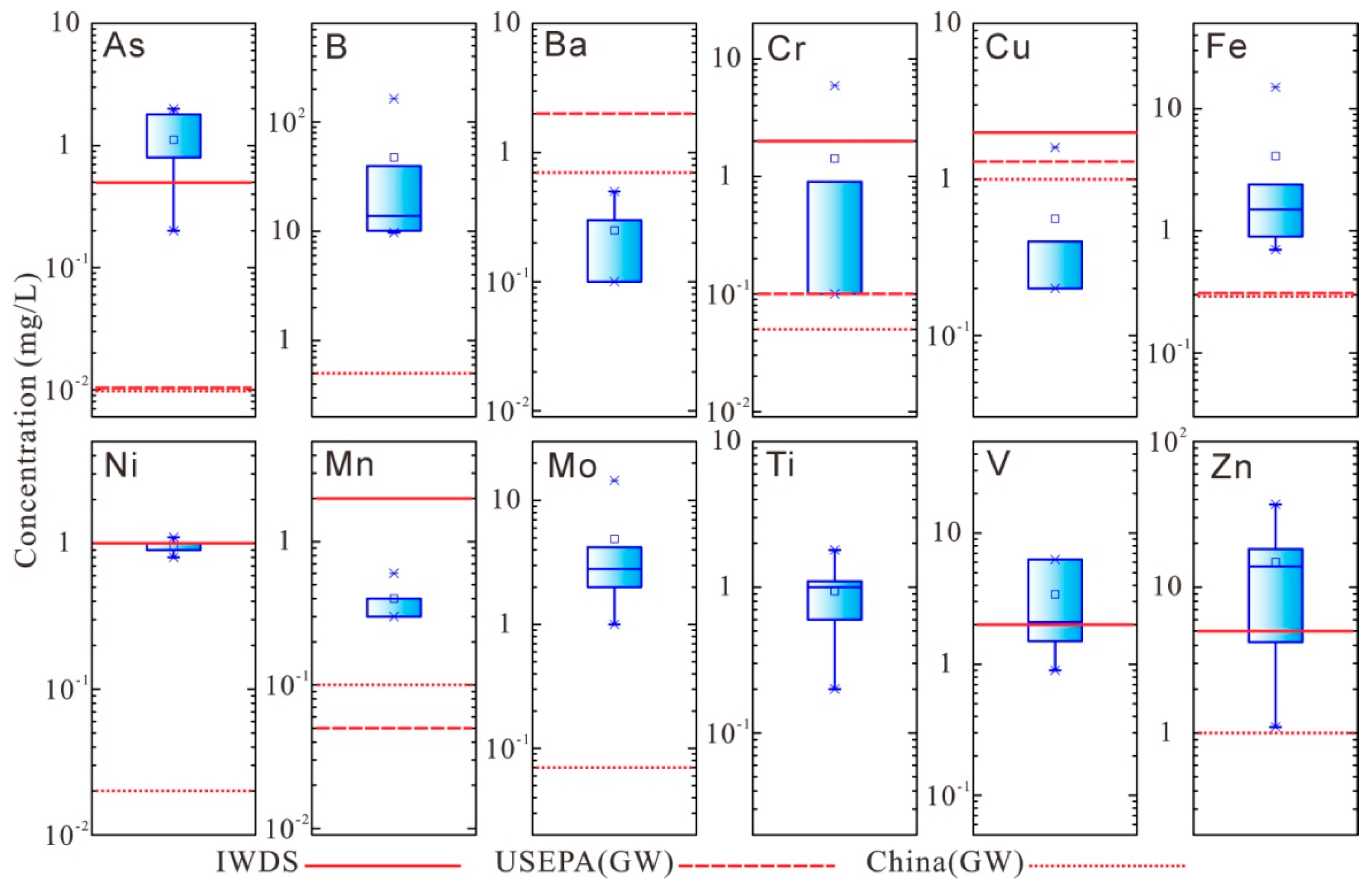
The concentrations of minor and trace elements, such as As, B, Cr, Fe, Ni, Mn, Mo, V, Zn, Ag, Be, Cd, Co, Hg, Pb, Sb, Se, and Tl, exceed the standards. The heavy metal elements, such as Cr, Fe, Ni, Mn, Mo, V, Zn, Ag, Co, Hg, and Pb, derived from the metal and its associated minerals in bauxite ore. These heavy metal elements could accumulate in soil and water. They increase ecological risks to crops, agricultural products, and groundwater, and also endanger human health through the food chain [
47]. The impacts of red mud and its associated leachate on the surrounding environment largely depend on the management of red mud. Measures to prevent the leakage or spill of red mud and its leachate should be considered in the design of red mud management facilities, especially in the design of the containment system (especially, the liner) of the red mud reservoirs. Therefore, further studies are needed to investigate the compatibility between red mud leachate and liner materials, as the chemical environment, especially high pH and salinity conditions, could affect the hydraulic behavior and strength of the liner materials [
48,
49,
50].